Abstract
Vaccination can significantly reduce worldwide morbidity and mortality to infectious diseases, thereby reducing the health burden as a result of microbial infections. Effective vaccines contain three components: a delivery system, an antigenic component of the pathogen, and an adjuvant. With the growing use of purely recombinant or synthetic antigens, there is a need to develop novel adjuvants that enhance the protective efficacy of a vaccine against infection. Using a structure–activity relationship (SAR) model, we describe here the synthesis of a novel TLR4 ligand adjuvant compound, BECC438, by bacterial enzymatic combinatorial chemistry (BECC). This compound was identified using an in vitro screening pipeline consisting of i) NFκB activation and cytokine production by immortalized cell lines, ii) cytokine production by primary human PBMCs, and iii) upregulation of surface costimulatory markers by primary human monocyte-derived dendritic cells. Using this SAR screening regimen, BECC438 was shown to produce an innate immune activation profile comparable to the well-characterized TLR4 agonist adjuvant compound, phosphorylated hexa-acyl disaccharide (PHAD). To evaluate the in vivo adjuvant activity of BECC438, we used the known protective Yersinia pestis (Yp) antigen, rF1-V, in a murine prime-boost vaccination schedule followed by lethal challenge. In addition to providing protection from lethal challenge, BECC438 stimulated production of higher levels of rF1-V-specific total IgG as compared to PHAD after both prime and boost vaccinations. Similar to PHAD, BECC438 elicited a balanced IgG1/IgG2c response, indicative of active TH2/TH1-driven immunity. These data demonstrate that the novel BECC-derived TLR4L adjuvant, BECC438, elicits cytokine profiles in vitro similar to PHAD, induces high antigen-specific immune titers and a TH1-associated IgG2c immune titer skew, and protects mice against a lethal Yp challenge.
Introduction
Infectious bacterial, viral, and parasitic diseases continue to be major causes of morbidity and mortality worldwide [1, 2]. The development of new and improved vaccines against infectious diseases remains a global priority. Modern subunit vaccine development requires the identification and formulation of protective antigens with an immunostimulatory adjuvant to strengthen the desired immune response against low- or non-stimulatory antigens. Identifying an appropriate adjuvant for each vaccine is critical to initiate an appropriate, protective immune response and reduce the global burden of disease.
Adjuvants licensed for use in human vaccines, such as aluminum hydroxide (Alhydrogel or alum) and oil-in-water (O/W) emulsions, are not universally effective, due in part to the requirement for repeated applications and/or the induction of immune responses that do not provide adequate protection against specific pathogens. Alhydrogel and O/W emulsions induce potent T helper 2 (TH2)-driven immune responses [3, 4], which are characterized by production of interleukin (IL)-4, IL-5, and IL-13. This cytokine profile drives the production of neutralizing IgG1 (murine) or IgG2 (human) and IgE antibodies, the latter of which promotes the degranulation of mast cells and eosinophils. A TH2 response is most effective against extracellular pathogens but may not provide strong, long-lasting protection against intracellular pathogens.
In contrast, the Toll-like receptor 4 (TLR4) agonist 3-O-deacyl-4-monophosphoryl lipid A (MPL) and related synthetic versions such as PHAD (phosphorylated hexa-acyl disaccharide), drive T helper 1 (TH1) immune responses[5], which promote strong cell-mediated immunity. TH1 responses drive production of the inflammatory cytokines IFN-γ and TNF-α, maturation of cytotoxic CD8+ T cells, and production of IgG2a/c (murine) [6] or IgG1(human), resulting in increased neutralization and/or opsonization and antibody-dependent cellular cytotoxicity.
The TLR4 complex, which consists of TLR4, lymphocyte antigen 96 (myeloid differentiation 2 [MD-2]), and CD14 is an innate immune receptor complex that is classically activated by recognition of the Gram-negative membrane component, lipopolysaccharide (LPS) and its constituent, lipid A [7]. The activation of cytokines produced by the downstream TLR4 signaling pathway is dependent on the structure of the lipid A–TLR4 complex [8–10] and determines the type of adaptive immune response elicited by vaccination. MPL, a TLR4 ligand (TLR4L) that drives TH1 immune responses, is chemically derived from purified Salmonella minnesota Re595 lipooligosaccharide (LOS) and is currently the only TLR4L licensed for use in humans as a component of the human papillomavirus vaccine, Cervarix (GlaxoSmithKline Biologicals), the hepatitis B vaccine, Fendrix (GlaxoSmithKline UK), and as part of the adjuvant system 04 (AS04) mixture of aluminum salts [11]. However, due to the product heterogeneity arising from the chemical degradation of the S. minnesota Re595 LOS, alternative technologies are required for synthesizing the next generation of TLR4 agonists.
To engineer novel TLR4Ls, we previously reported on the use of Bacterial Enzymatic Combinatorial Chemistry (BECC) to rapidly generate novel lipid A structures with adjuvant potential in an attenuated (biosafety level 2 [BSL2]) Yersinia pestis isolate. BECC involves expression or deletion of enzymes from the lipid A synthesis pathways in Gram-negative bacteria, allowing for direct isolation/purification of TLR4Ls from a bacterial pellet without requiring further modification. Previously reported BECC molecules demonstrated adjuvant-level immunostimulatory properties in mouse splenocytes, human primary blood mononuclear cells (PBMCs), and human monocyte-derived dendritic cells (DCs) [12]. Once vetted through our in vitro screening pipeline, individual BECC molecules are purified and their adjuvant potential evaluated in vivo using a murine Yersinia pestis (Yp) model. Yp is the causative agent of plague (or Black Death) and despite extensive effort, an effective Yp vaccine that does not cause adverse side effects or protect against all forms of plague does not exist[13]. The recombinant antigen (rF1-V), which is composed of the F1 capsular protein and the V virulence protein fused into a single protein, was used for these studies.
Here, we describe the novel BECC-derived TLR4L, BECC438, and demonstrate its adjuvant potential using a prime-boost vaccination schedule in the Yp model. We assess the adjuvant-like innate immune potential of BECC438 by assaying in vitro cytokine responses and evaluating its role in driving an in vivo TH1 immune response. We compare the adjuvant properties of BECC438 to that of PHAD (Avanti Polar Lipids, Alabaster, AL), which is capable of inducing a balanced Th1/Th2 response. However, the synthetic production and proprietary status of the molecule can make it cost prohibitive for use in development of novel vaccines, especially vaccines that will be most useful in underdeveloped countries where vaccine administration is not always profitable. In contrast, our BECC molecules can induce a similar or more balanced and robust immune response, and also be made quickly and inexpensively, which will allow for the rapid advancement of vaccine evaluation. Finally, we establish that in this vaccination model, BECC438 provides protection from a lethal Yp intraperitoneal challenge.
Materials and Methods
Bacterial enzymatic combinatorial chemistry mutagenesis
All bacterial strains/plasmids and associated maintenance and growth conditions are listed in Supplemental Table 1. To create mutant bacterial strains, Yp KIM6+ ΔmsbB [14] was mated to the Escherichia coli conjugation donor strain S17-1λpir, which contains the plasmid, pCVD442-pagPYpRep [12]. Merodiploid conjugates were selected on Yersinia Selective Agar Base (YSAB) (Remel, Inc., San Diego, CA) supplemented with 100 μg/mL carbenicillin. Single colonies were grown overnight in brain heart infusion (BHI) broth and dilutions were plated on Congo Red medium with 7.5% sucrose to select against sacB on the integrated plasmid. Sucrose-resistant colonies were then grown on YSAB with and without carbenicillin. Sucrose-resistant and carbenicillin-sensitive colonies were isolated for sequencing to confirm the presence of the repaired pagP gene (manuscript in preparation).
LOS was isolated from the mutants and purified, and its structure was confirmed by mass spectrometry and gas chromatography. The LOS molecules were then screened in vitro as previously reported using immortalized human cell lines THP-1 (ATCC, Manassas, VA) and HEK-Blue (Invivogen, San Diego, CA), and human primary cells (AllCells, Alameda, CA) for NFκB activation, cytokine production, and costimulatory marker upregulation [12]. Briefly, for cytokine studies peripheral blood mononuclear cells (PBMC) from three different donors were purchased from AllCells (www.allcells.com) and cultured for 36 hours with concentrations indicated of agonists. Supernatants were then collected and assayed for presence of proteins of interest by Luminex assay (EMD Millipore, Burlington, MA). Co-stimulatory marker surface expression assays were contracted to and completed by AllCells using standard protocols. Briefly, ex vivo human monocytes were differentiated to monocyte-derived dendritic cells (mDC) by culture with GM-CSF and IL-4 for 7 days. mDC were then stimulated for 24 hours with concentrations indicated of agonists, stained for indicated markers, and marker level determined by flow cytometry on and LSRII (BD Biosciences, Franklin Lakes, NJ). Luminex and flow cytometry experiments were done independently, as such two different sets of donors were used for each set of experiments.
Murine immunization, and Yp challenge
Eight-to-twelve-week-old female C57BL/6J mice were obtained from University of Maryland, Baltimore (UMB) Veterinary Animal Resources (Baltimore, MD). All animal experiments were approved by the UMB Institutional Animal Care and Use Committee, protocol #312006. Mice were immunized intramuscularly in the right caudal thigh with a 50 μL volume and up to 200 μL of blood were harvested from the right or left saphenous vein twice at 2-week intervals. Clotted whole blood was centrifuged, and sera aliquoted and stored at -80°C for immune titer determination via enzyme-linked immunosorbent assay (ELISA).
To create the vaccine inoculum, adjuvant molecule was resuspended at the indicated concentrations and incubated in a bath sonicator for 15 minutes to promote micelle formation. Antigen was then added to the vaccine solution and allowed to adsorb for 2 hours at room temperature. This unformulated component vaccine was created fresh for each vaccination and used immediately after adsorption.
For challenge, Yp CO92 Δpgm pCD+ (provided by James Bliska, SUNY-Stony Brook, NY) was grown at 26°C on BHI agar plates for single colony isolation. Single colonies were used to inoculate 5 mL of BHI broth supplemented with 1 mM magnesium chloride, followed by overnight growth at 26°C, 220 rpm. Cultures were diluted 1:40 and subcultured using the same growth conditions to a final optical density at 600 nm (OD600) of 0.25. The culture was serially diluted in phosphate-buffered saline (PBS) with 0.05% Triton X-100 (Sigma-Aldrich, St, Louis, MO). Plates were incubated at 26°C and colony-forming units (CFUs) were enumerated after growth for 48 hours on LB plates supplemented with 60 mM sodium pyruvate and 1 mM magnesium chloride. Mice were challenged intraperitoneally with an empirically determined ~20 × LD50 of Yp CO92 Δpgm pCD+ (98 ± 16 or 101 ± 9 CFU) with 1 μg of ferrous iron from iron (II) sulfate heptahydrate (Sigma-Aldrich, St, Louis, MO). Mice were checked twice daily for at least 14 days and moribund mice were euthanized.
rF1-V-Specific antibody titers by ELISA
Recombinant rF1-V fusion protein (Yersinia pestis F1-V Fusion Protein, Monomer-Enriched Antigen, Recombinant from Escherichia coli, NR-2561 [www.beiresources.org]) was obtained from the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH. rF1-V was diluted with cold, sterile-filtered 20 mM L-arginine, 10 mM NaCl, 1 mM L-cysteine solution (pH 9.9) and stored at −80°C. Nunc-Immuno MaxiSorp™ 96-well plates (Millipore Sigma, St. Louis, MO) were coated overnight at 4°C with 100 μL per well of a 1 μg/mL rF1-V protein solution in 0.1 M bicarbonate-carbonate buffer (pH 9.8 at 20°C). Plates were washed three times with PBS containing 0.05% Tween 20 (PBST) before blocking for 1 hour at 37°C with shaking at 90 rpm, with 100 μL per well of 5% non-fat milk (Quality Biological, Inc., Gaithersburg, MD) in PBS. Plates were washed six times with PBST before adding 100 μL per well of antibody diluted in 5% non-fat milk in PBST [15, 16]. All samples were run in duplicate and each plate had four blank wells and a 10-point standard curve generated from two-fold serial dilutions of the reference serum. Antigen-specific total IgG and IgG1 plates were incubated with primary and HRP-conjugated secondary antibodies (goat anti-mouse IgG, KPL-474-1802 [Kierkegaard and Perry Laboratories] at a 1: 2,000 dilution and goat anti-mouse IgG1, 1070-05 [SouthernBiotech, Birmingham, AL] at a 1: 4,000 dilution) for 60 min at 37°C and 90 rpm. Antigen-specific IgG2C and IgG2A plates were incubated with primary and HRP-conjugated secondary antibodies (goat anti-mouse IgG2C, 1077-05, and goat anti-mouse IgG2A, 1080-05 [SouthernBiotech, Birmingham, AL], both at a 1: 1,000 dilution) for 75 minutes at 37°C and 90 rpm. Next, each plate was washed six times with PBST. Room temperature BD OptEIA™ TMB substrate (BD Biosciences, San Jose, CA) was added, the plates were incubated for 15 min (total IgG and IgG1 plates) or 20 min (IgG2A or IgG2C plates), and the reaction was stopped by adding an equal volume of 1 M phosphoric acid; the plate was read on a DTX 880 multimode plate reader (Beckman Coulter, Brea, CA) with Multimode Analysis Software (v. 3.3.0.9). Relative antibody titers were determined by the reference serum method [17]. Briefly, reference serum was generated by pooling rF1-V-specific high-titer sera. One antibody unit was defined as the reciprocal of the reference serum dilution that gave an OD450 value of 1. Sample antibody units were interpolated from the standard curve, and were defined as the reciprocal of the dilution needed for an OD450 of 1, relative to the standard dilution.
Statistics
All analyses were performed on Prism 6 for Mac OS X, version 6.0h (GraphPad Software, Inc., La Jolla, CA), and were non-parametric as the normality of the data distribution could not be determined. Multiple comparisons were corrected for using the Bonferri method unless otherwise noted. ELISA antibody titers were interpolated from baseline-subtracted, weighted (1/y2), 4-parameter logistic regression of the standard curve. Antibody titers were log10 transformed and compared to the antigen-alone control group by Kruskal-Wallis test and adjusted for multiple comparisons by Dunn’s test. Survival curves were compared to the antigen-only control group using the log-rank test. The mean lethal dose (LD50) was calculated by the method of Reed and Muench [18]. The Spearman test was used to assess the correlation between survival and IgG subtype titer.
Results
BECC-derived novel lipid A structures
To create a TLR4L capable of activating an adjuvant level innate-immune response, BECC438 (ΔmsbB pagPYpRep) lacking the secondary lauryl acyl-transferase enzyme, MsbB, but expressing the palmitate transferase, PagP, was engineered in the avirulent Yp KIM6+ strain (Supp. Table 1). Lipooligosaccharide (LOS–LPS without O-antigen repeats) was purified from the mutant strain BECC438, the parental control strain, Yp KIM6+ (TBE358), and E. coli W3110A using a modified hot phenol and water extraction method. Hydrolyzed lipid A samples were analyzed by mass by matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) mass spectrometry. As predicted, when compared to the parent TBE358, the mass spectrum of the BECC438 construct lacked the C12 addition (Δ182 m/z) and had one or two additional saturated palmitic acids (C16) due to the presence of a functional pagP gene (Δ238 m/z). Additionally, the tetra-acylated lipid A backbone, m/z 1404, is modified by the addition of aminoarabinose (Δ131 m/z) (Figure 1, Supplemental Figures 1 and 2).
Figure 1. Control and BECC-generated lipid A structures.
Unique lipid A structures and negative ion masses are shown for the highly proinflammatory E. coli lipid A (A), the synthetic monophosphoryl lipid A PHAD (B), the lipid A of the parent Yp cultured at 26°C (C), and BECC438 from the ΔmsbB pagPYpRep Yp strain cultured at 26°C (D).
To determine the fatty acid content of lipid A, LOS was extracted from TBE358 and BECC438 after growth at 26°C, converted to fatty acid methyl esters, and analyzed by gas chromatography–flame ionization detection (GC-FID) (Supplemental Table 2) [19]. The GC data confirmed the lack of C12 in the ΔmsbB mutant and an approximately three-fold increase in C16 due to repair of the Yp pagP gene.
BECC-derived LOS elicits adjuvant-like cytokine profiles in vitro
The inflammatory profile of BECC438 was screened by in vitro cell culture stimulation initially in immortalized cell lines and subsequently in primary human cells. Initially, HEK cells expressing mouse or human TLR4 (HEK-Blue cells) and the human monocytic cell line, THP-1, were screened to determine their ability to elicit an adjuvant-like cytokine response. LOS from E. coli W3110 and PHAD, a synthetic TLR4L adjuvant, were used as controls in all assays [20, 21]. Cells were stimulated with a 5-log concentration range of BECC438 (0.1–1000 ng/mL) for 18 h. The level of production of embryonic alkaline phosphatase under NF-κB promoter (HEK-Blue; Figure 2A and B) was measured colorimetrically, and those of NF-κB–driven cytokines (THP-1; TNF-α, and IL-8) (Figure 2C and D) were measured using a multiplex Luminex assay. The proinflammatory responses elicited by the BECC438 structure in all cell lines used was consistently lower than those stimulated by the proinflammatory E. coli LOS and higher than PHAD at lower concentrations, but similar at higher concentrations (>100 ng/mL).
Figure 2. Induction of NF-κB and proinflammatory cytokines by BECC438.
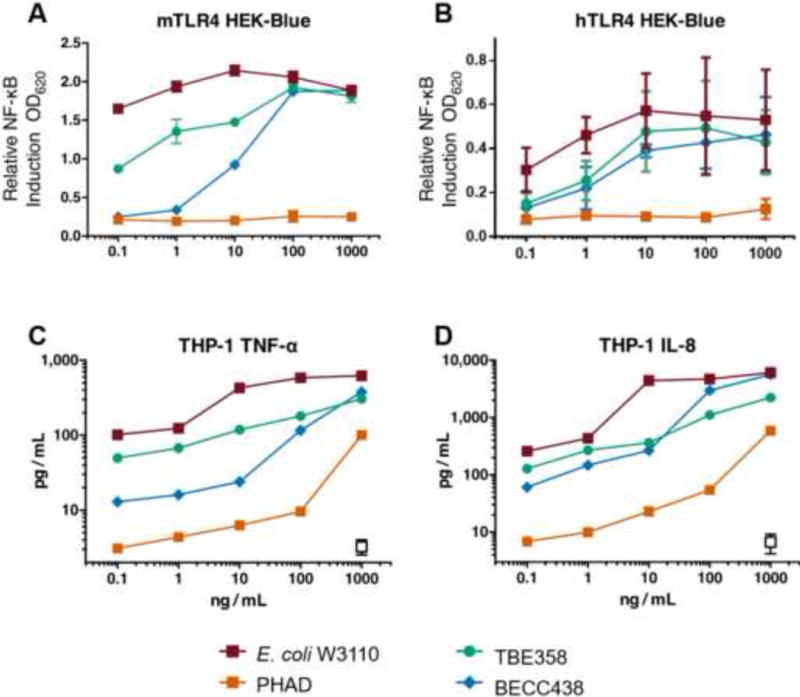
HEK-Blue NF-κB reporter cells expressing human (A) or mouse (B) CD14, MD2, and TLR4 were stimulated with E. coli LOS (red), PHAD (orange), TBE358 (green), or BECC438 (blue), at 0.1–1,000 ng/mL. After 16 hours, NF-κB-driven SEAP secretion was measured; the data are shown as means ± SD of biological duplicates. The same molecules and doses were used to stimulate THP-1 cells for 18 hours, and supernatant concentrations of TNF-α (C) and IL-8 (D) (pg/mL) were measured with the Millipore Bio-Plex Luminex assay.
To further investigate the stimulatory abilities of these molecules, primary human PBMCs were stimulated as described above and their production of an expanded cytokine panel was evaluated by multiplex cytokine analysis (Figure 3 and Supplemental Figure 3). As expected, the cytokine responses varied. After stimulation with BECC438, all three human PBMC donors showed cytokine responses markedly lower than that of E. coli LOS. Not only was a pathogenic proinflammatory response dampened (unlike LOS TBE358), the stimulatory capacity of BECC438 was titrated down and exhibited dose kinetics similar to that of PHAD. Interestingly, donors 2 and 3 showed increased production of the following TH1-associated cytokines: CCL2/macrophage inflammatory protein 1β (MIP-1β), monocyte chemotactis protein-1 (MCP-1), IFN-γ-inducible protein 10 (IP-10/CCXL10), and IFN-γ (Figure 3, Supplemental Figure 3). Despite the differences in magnitude and initial TH subtype bias among the donors’ responses, the trends between the ligands were similar.
Figure 3. BECC438 stimulates cytokine secretion by primary human PBMCs.
Primary human PBMCs from three separate donors were incubated for 36 hours with E. coli LOS (red), PHAD (orange), TBE358 (green), or BECC438 (blue) at 0.1–1,000 ng/mL. Supernatant concentrations of TNF-α (A), IL-10 (B), and MDC/CCL22 (C) (pg/mL) were measured with the Millipore Bio-Plex Luminex assay.
Finally, BECC438 was tested for its in vitro ability to induce production of costimulatory and maturation markers by primary human monocyte-derived DCs. Following differentiation, DCs were stimulated for 36 hours with 10 ng/mL BECC438 and their expression of surface markers was evaluated by staining and flow cytometry. The mean fluorescence intensity (MFI) of the costimulatory markers CD40, CD80, and CD83 induced by a low concentration of BECC438 was comparable to that by PHAD, and the MFI resulting from application of the highest concentration of BECC438 was equal or superior to that elicited by PHAD. The activity of BECC438 at all concentrations was comparable to or superior to that of TBE358. Two exceptions to this trend were CD83 (an activation marker) and CD40 in donor 1, for which BECC438 met and exceeded, respectively, the MFIs of these markers of E. coli LOS (Figure 4). The MFI values for MHC class II and CD86 show the percentage of DCs positive for each marker (Supplemental Figure 4 and 5). Taken together, the in vitro data suggest that BECC438 should provide an adjuvant potency similar to that of PHAD in vivo.
Figure 4. BECC438 induces expression of activation markers by human dendritic cells.
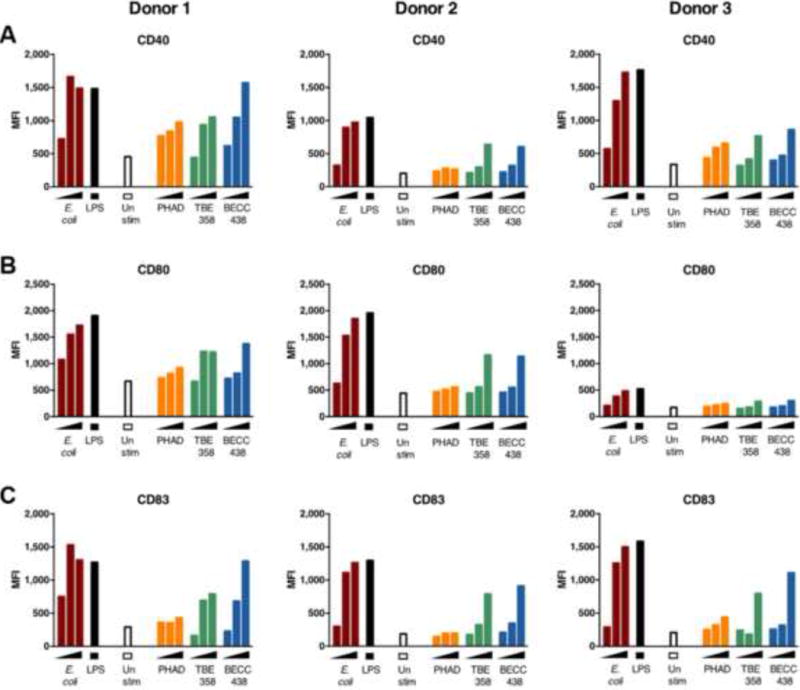
Primary human monocytes from three separate donors were differentiated into dendritic cells and incubated for 36 hours with a series of ten-fold dilutions (0.1–10 ng/mL) of E. coli LOS (red), PHAD (orange), TBE358 (green), or BECC438 (blue). The mean fluorescence intensity (MFI) of CD40 (A), CD80 (B), and CD83 (C) is graphed.
BECC438 increases antigen-specific total IgG and IgG2c antibody titers in vaccinated mice
We employed a murine immunization and challenge model using the Yp protective antigen, recombinant F1-V (rF1-V), to evaluate the in vivo adjuvant potential of BECC438. Following a prime-boost immunization regimen, C57BL/6J mice were immunized intramuscularly on days 0 (prime) and 14 (boost). The PBS and adjuvant-alone treatment groups did not mount an antigen-specific IgG antibody response, whereas the adjuvanted-rF1-V treatment groups showed higher antigen specific total IgG, IgG1, and IgG2c titers on days 14 and 28. BECC438, similarly to PHAD, induced a greater IgG2c response than alum, which is indicative of a TH1 immune response (Figure 5). This balanced TH1/TH2 response was evident after the first vaccine administration. When the BECC438 parent molecule, BECC358, was utilized as an adjuvant, a similar significant antibody induction was observed. Finally, mice immunized with rF1-V adjuvanted with Alhydrogel (n = 8) did not produce significantly higher titers of antigen specific IgG2c than the antigen-alone group. However, rF1-V adjuvanted with BECC438 showed significantly higher rF1-V-specific IgG2c immune titers compared to the rF1-V–alone group.
Figure 5. BECC438 enhances antigen-specific IgG subtype titers in C57BL/6J mice.
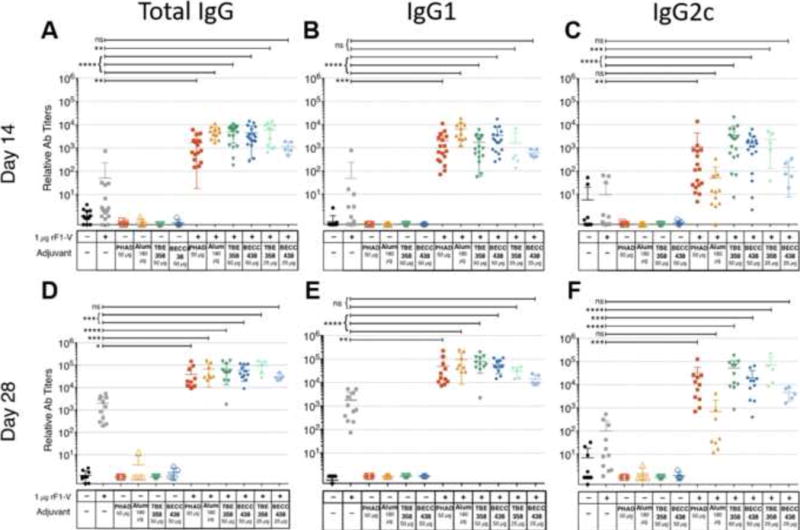
C57BL/6J mice were immunized intramuscularly on day 0 (prime) and day 14 (boost). Immune serum was harvested from the saphenous vein prior to vaccination on day 14, and on day 28. rF1-V–specific total IgG, IgG1, and IgG2c levels were measured by ELISA and are reported as relative antibody titers: (A) Total IgG day-14 titers, (B) IgG1 day-14 titers, (C) IgG2c day-14 titers, (D) Total IgG day-28 titers, (E) IgG1 day-28 titers, and (F) IgG2c day-28 titers. The adjuvanted-rF1-V–immunized treatment groups were compared to the rF1-V–alone treatment group within each IgG class using a non-parametric Kruskal-Wallis test with a post hoc Dunn’s multiple comparison test (α = 0.05, K = 6). Means ± SD are shown; *p < 0.05, **p < 0.005, ***p < 0.0005, ****p < 0.00005, ns not significant. Titers are combined from three independent vaccination experiments.
BECC438 as an rF1-V adjuvant protects mice from a lethal Y. pestis challenge
A BSL-2 Yp CO92− challenge model was used to evaluate the ability of BECC438 to strengthen the protective immune response against low levels (1 μg) of the known protective antigen, rF1-V [22]. Yp CO92− is an attenuated, but lethal to mice, strain that produces both F1 and V antigens. It is derived from the fully virulent strain, BSL-3 Yp CO92 [23, 24]. In this model, C57BL/6J mice were intramuscularly immunized using a prime and boost strategy on days 0 and 14. Mice were challenged intraperitoneally (IP) with 20 ×LD50 of Yp CO92− and 1 μg of ferrous iron (to facilitate the pathogenicity of the challenge organism), on day 36, 3 weeks after their final immunization. Mice were monitored twice daily for 14 days; the survival curves are shown in Figure 6A-D. Adjuvantation of rF1-V with alhydrogel (n = 8, p = 0.046), 50 μg TBE358 (n = 8, p = 0.015), and 50 μg BECC438 (n= 8, p = 0.015) significantly improved survival compared to the rF1-V antigen-alone group. Mice immunized with PHAD-adjuvanted rF1-V (n = 8, p = 0.055) also exhibited significantly improved survival. Furthermore, protection was positively correlated with rF1-V–specific immune titers (Figure 6E). Total IgG and IgG1 levels correlated more significantly with survival (ρ = 0.71 and 0.70, respectively) than did the IgG2c level (ρ = 0.64), indicating that a TH1-skewed, IgG2c antibody response does not predict protection better than total IgG or a TH2-skewed, IgG1 antibody response. These data demonstrate that the novel adjuvant BECC438 has an antigen-sparing function in rF1-V immunization and protects C57BL/6J mice from a lethal Yp CO92− challenge.
Figure 6. BECC adjuvanted rF1-V vaccine protects mice from lethal Yp challenge.
C57BL/6J mice were immunized intramuscularly on day 0 and day 14, and were challenged I.P. on day 36 with Yp CO92− (98 ± 16 or 101 ± 9 CFUs) and Fe(II) (1 μg). (A) PBS-immunized mice (n = 8, p = 0.267); (B) alhydrogel- (n = 8, p = 0.046) and PHAD-adjuvanted (n = 8, p = 0.055) treatment groups; (C) BECC438 50 μg (n= 8, p = 0.015) and 25 μg (n = 5, p = 0.089); and (D) TBE358 50 μg (n = 8, p = 0.015) and 25 μg TBE358 (n = 5, p = 0.089). Survival curves are pooled from two independent experiments. The log-rank (a.k.a. Mantel-Cox) pairwise test was used to compare the survival of the PBS and adjuvanted-rF1-V treatment groups to the antigen-alone control group. Bonferri-adjusted p-values were used to correct for multiple comparisons (K = 7) (N = 78, n = 5 or 8) (* = p < 0.05). (E) Survival was correlated with the rF1-V-specific IgG-subtype titer (n = 102, each) on day 28 by Spearman’s correlation analysis. The Spearman’s coefficient, ρ, 95% confidence interval, and Bonferroni-adjusted p-values (α = 0.05, K = 3) are presented. All rF1-V-specific IgG-subtype titers showed a significant, positive, and moderate correlation with survival.
Discussion
In this paper, we describe the activity of a novel BECC-derived TLR4L adjuvant, BECC438. BECC438 is a bisphosphorylated lipid A molecule derived from a Yp strain that lacks the C12:0 acyltransferase, MsbB, but expresses a functional C16:0 acyltransferase, PagP. The lipid A of BECC438 has one or two additional C16:0 groups, generating a penta-acylated (m/z 1642) or hexa-acylated (m/z 1880) structure. The structural differences in BECC438-derived lipid A result in intermediate TLR4 signaling with minimal acute murine toxicity observed after injection, which is similar to observed minimal toxicity of PHAD and in contrast to the pronounced toxicity routinely observed with E. coli LOS. Additionally, BECC438 induces a proinflammatory response that effectively adjuvants rF1-V and induces DC maturation. Similar to PHAD, this response includes both the IRF3-dependent cytokine:, IFN-γ, which promotes a TH1-type response, and the NF-κB-driven cytokine, IL-6, which promotes the development of cytotoxic memory CD8+ T cells[25, 26]. BECC438 is capable is inducing similar or superior immunity when tested using in vitro screening systems and also the Yp in vivo vaccine/challenge model presented here. Increased production speed, lowered cost, and wide accessibility make BECC438 an alternative to PHAD.
When formulated with aluminum salts in a preparation known as AS04, PHAD activates local NF-κB-driven inflammatory TNFα and IL-6 cytokine responses at the site of injection, which enhances the TH1 type immune response and prolongs the PHAD-driven cytokine response [27]. In our assays, without any formal formulation, BECC438 exhibited partial TLR4 agonist activity, suggesting that it possesses similar adjuvant-like properties to PHAD. Future studies will include vaccination efforts using the optimal BECC438 formulation, in which the adjuvant capacity of BECC438 is expected to exceed that of PHAD.
The in vitro expression levels of cytokines, chemokines, and activation markers elicited by BECC438 stimulation were consistently lower than those of the positive control E. coli LOS, but similar to the levels elicited by PHAD. In THP-1 monocytes, the stimulatory effect of E. coli LOS was approximately ten-fold that of BECC438, which was itself approximately ten-fold more stimulatory than PHAD; the effect was particularly pronounced at 10–100 ng/mL BECC438. Structural differences among these TLR4Ls could account for the observed differences in potency. PHAD is monophosphorylated, whereas BECC438 is bisphosphorylated; the presence of the two phosphate groups, as well as its hexa-acylated structure, should markedly enhance the signaling ability of BECC438 [8, 28, 29]. Additionally, the symmetry of the hexa-acylated C16:0 structure results in a more cylindrical shape, which may provide a signaling ability greater than that of PHAD but weaker than the signaling ability of the pathogenic asymmetrical hexa-acylated lipid A of E. coli.
LOS isolated from BECC438 and its parent TBE358 strain elicited IgG1 and total IgG antigen-specific immune titers similar or superior to the responses induced by the adjuvant controls, PHAD and Alum. Moreover, the mean titers of TH1-associated IgG2c induced by BECC438 were higher than those generated by PHAD. This demonstrates the usefulness of BECC for generating TH1-skewing TLR4L adjuvants. When effective T-cell antigens are identified, BECC438, a TH1-1 skewing adjuvant, should confer a high level of protection by generating a balanced cytokine response.
Our data demonstrate that use of BECC438 as an adjuvant induces strong TH1 and TH2 immune responses, similar to PHAD, and suggest that it may be beneficial as a component of next-generation vaccine formulations against intracellular pathogens and cancer, and that comprise purified protein, peptide [30], and DNA [31] antigens. In addition to enhancing the magnitude of the antibody responses in our vaccine study, BECC438 effected immunoglobulin class switching to TH1-associated subtypes. This would be beneficial for allergen immunotherapy vaccines, which aim to suppress IgE production and downstream histamine release by inducing immunoglobulin class switching to IgG1 (human) or IgG2c (C57BL/6 mice) [32, 33]. Further studies are needed to confirm the promising adjuvant potential of BECC-derived TLR4Ls.
Supplementary Material
Acknowledgments
We thank M. S. Donnenberg and J. B. Kaper at the University of Maryland, Baltimore for the allelic exchange suicide plasmid, pCVD442. We also thank B. J. Hinnebusch at the Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health for providing mutant Y. pestis strains; J. B. Bliska at the Center for Infectious Diseases, Stony Brook University for providing the BSL-2 Y. pestis CO92− challenge strain; and C. R. Raetz for providing the E. coli control strain.
Funding Information
This project was funded by NIAID/NIH R21 grant number R21AI101685 (RKE), the NIH training grant number T32AI95190 (KAG), and a MedImmune/University of Maryland joint research grant (RKE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
R.K.E. and M.R.P. hold a pending patent for the development and use of the TLR4 ligands described in the manuscript (PCT/US2014/022121).
References
- 1.Harris JB, Gacic-Dobo M, Eggers R, Brown DW, Sodha SV, Centers for Disease C et al. Global routine vaccination coverage, 2013. MMWR Morbidity and mortality weekly report. 2014;63:1055–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Mortality GBD, Causes of Death C Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bungener L, Geeraedts F, Ter Veer W, Medema J, Wilschut J, Huckriede A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine. 2008;26:2350–9. doi: 10.1016/j.vaccine.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 4.Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Frontiers in Immunology. 2012;3:406. doi: 10.3389/fimmu.2012.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–32. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 6.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. Journal of Immunological methods. 1998;212:187–92. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 7.Poltorak A, Smirnova I, He X, Liu MY, Van Huffel C, McNally O, et al. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells, Molecules & Diseases. 1998;24:340–55. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 8.Scott AJ, Oyler BL, Goodlett DR, Ernst RK. Lipid A structural modifications in extreme conditions and identification of unique modifying enzymes to define the Toll-like receptor 4 structure-activity relationship. Biochimica et Biophysica Acta. 2017;1862:1439–50. doi: 10.1016/j.bbalip.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins DJ, Vogel SN. Space and time: New considerations about the relationship between Toll-like receptors (TLRs) and type I interferons (IFNs) Cytokine. 2015;74:171–4. doi: 10.1016/j.cyto.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajaiah R, Perkins DJ, Ireland DD, Vogel SN. CD14 dependence of TLR4 endocytosis and TRIF signaling displays ligand specificity and is dissociable in endotoxin tolerance. PNAS. 2015;112:8391–6. doi: 10.1073/pnas.1424980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Didierlaurent AM, Laupeze B, Di Pasquale A, Hergli N, Collignon C, Garcon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Review of Vaccines. 2017;16:55–63. doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 12.Gregg KA, Harberts E, Gardner FM, Pelletier MR, Cayatte C, Yu L, et al. Rationally Designed TLR4 Ligands for Vaccine Adjuvant Discovery. mBio. 2017:8. doi: 10.1128/mBio.00492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler T. Plague history: Yersin's discovery of the causative bacterium in 1894 enabled, in the subsequent century, scientific progress in understanding the disease and the development of treatments and vaccines. Clinical Microbiology and Infection. 2014;20:202–9. doi: 10.1111/1469-0691.12540. [DOI] [PubMed] [Google Scholar]
- 14.Rebeil R, Ernst RK, Jarrett CO, Adams KN, Miller SI, Hinnebusch BJ. Characterization of late acyltransferase genes of Yersinia pestis and their role in temperature-dependent lipid A variation. Journal of Bacteriology. 2006;188:1381–8. doi: 10.1128/JB.188.4.1381-1388.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez K, Ditamo Y, Rodriguez L, Picking WL, van Roosmalen ML, Leenhouts K, et al. Neonatal mucosal immunization with a non-living, non-genetically modified Lactococcus lactis vaccine carrier induces systemic and local Th1-type immunity and protects against lethal bacterial infection. Mucosal Immunology. 2010;3:159–71. doi: 10.1038/mi.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramirez K, Capozzo AV, Lloyd SA, Sztein MB, Nataro JP, Pasetti MF. Mucosally delivered Salmonella typhi expressing the Yersinia pestis F1 antigen elicits mucosal and systemic immunity early in life and primes the neonatal immune system for a vigorous anamnestic response to parenteral F1 boost. Journal of Immunology. 2009;182:1211–22. doi: 10.4049/jimmunol.182.2.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muench LJRH. A Simple Method of Estimating Fifty Percent Endpoints. American Journal of Epidemiology. 1938;27:493–7. [Google Scholar]
- 19.Kawahara K, Tsukano H, Watanabe H, Lindner B, Matsuura M. Modification of the structure and activity of lipid A in Yersinia pestis lipopolysaccharide by growth temperature. Infection and Immunity. 2002;70:4092–8. doi: 10.1128/IAI.70.8.4092-4098.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantel A, Cheong C, Dandamudi D, Shrestha E, Mehandru S, Brane L, et al. A new synthetic TLR4 agonist, GLA, allows dendritic cells targeted with antigen to elicit Th1 T-cell immunity in vivo. European Journal of Immunology. 2012;42:101–9. doi: 10.1002/eji.201141855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santini-Oliveira M, Coler RN, Parra J, Veloso V, Jayashankar L, Pinto PM, et al. Schistosomiasis vaccine candidate Sm14/GLA-SE: Phase 1 safety and immunogenicity clinical trial in healthy, male adults. Vaccine. 2016;34:586–94. doi: 10.1016/j.vaccine.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Dinc G, Pennington JM, Yolcu ES, Lawrenz MB, Shirwan H. Improving the Th1 cellular efficacy of the lead Yersinia pestis rF1-V subunit vaccine using SA-4-1BBL as a novel adjuvant. Vaccine. 2014;32:5035–40. doi: 10.1016/j.vaccine.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Kummer LW, Szaba FM, Parent MA, Adamovicz JJ, Hill J, Johnson LL, et al. Antibodies and cytokines independently protect against pneumonic plague. Vaccine. 2008;26:6901–7. doi: 10.1016/j.vaccine.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin JS, Park S, Adamovicz JJ, Hill J, Bliska JB, Cote CK, et al. TNFalpha and IFNgamma contribute to F1/LcrV-targeted immune defense in mouse models of fully virulent pneumonic plague. Vaccine. 2010;29:357–62. doi: 10.1016/j.vaccine.2010.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLeod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, et al. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. PNAS. 2011;108:7914–9. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui W, Joshi NS, Liu Y, Meng H, Kleinstein SH, Kaech SM. TLR4 ligands lipopolysaccharide and monophosphoryl lipid a differentially regulate effector and memory CD8+ T Cell differentiation. Journal of Immunology. 2014;192:4221–32. doi: 10.4049/jimmunol.1302569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. Journal of Immunology. 2009;183:6186–97. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 28.Paramo T, Tomasio SM, Irvine KL, Bryant CE, Bond PJ. Energetics of Endotoxin Recognition in the Toll-Like Receptor 4 Innate Immune Response. Scientific Reports. 2015;5:17997. doi: 10.1038/srep17997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han JE, Wui SR, Kim KS, Cho YJ, Cho WJ, Lee NG. Characterization of the structure and immunostimulatory activity of a vaccine adjuvant, de-O-acylated lipooligosaccharide. PLoS One. 2014;9:e85838. doi: 10.1371/journal.pone.0085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman KD, Sosnowski DL, Kwon GS, Samuel J. Delivery of MUC1 mucin peptide by Poly(d,l-lactic-co-glycolic acid) microspheres induces type 1 T helper immune responses. Journal of Pharmaceutical Sciences. 1998;87:1421–7. doi: 10.1021/js980070s. [DOI] [PubMed] [Google Scholar]
- 31.Song P, He S, Zhou A, Lv G, Guo J, Zhou J, et al. Vaccination with toxofilin DNA in combination with an alum-monophosphoryl lipid A mixed adjuvant induces significant protective immunity against Toxoplasma gondii. BMC Infectious Diseases. 2017;17:19. doi: 10.1186/s12879-016-2147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaar O, Barth C, Jaschke C, Hormann K, Klimek L. Sublingual allergen-specific immunotherapy adjuvanted with monophosphoryl lipid A: a phase I/IIa study. International Archives of Allergy and Immunology. 2011;154:336–44. doi: 10.1159/000321826. [DOI] [PubMed] [Google Scholar]
- 33.Mothes N, Heinzkill M, Drachenberg KJ, Sperr WR, Krauth MT, Majlesi Y, et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clinical and Experimental Allergy. 2003;33:1198–208. doi: 10.1046/j.1365-2222.2003.01699.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


