Abstract
Aim:
To study the clinical outcome of shunt surgeries in children with hydrocephalus and evaluate the risk factors for ventriculoperitoneal (VP) shunt failure.
Materials and Methods:
Patients who underwent VP shunt surgery for hydrocephalus were included. Medical charts, operative reports, imaging studies, and clinical follow-up evaluations were reviewed and analyzed retrospectively.
Results:
A total of 137 patients with the average age of 20.7 months, range from 1.5 months to 8.5 years at the time of VP shunt surgery were included. The incidence of overall shunt complications was 35.76%; incidence of shunt revision was 27%, shunt blockade 45.94%, shunt infection 16.21%, shunt migration 10.81%, and shunt malfunction due to abdominal pseudocyst 10.81%. The mortality rate was 5.10%. The shunt revisions in the first 6 months after shunt placement was observed in n = 9 (24%). Hydrocephalus was associated with post-tubercular meningitis and intraventricular hemorrhage (IVH) in shunt placement was associated with multiple shunt revisions (n = 13, 35.13%) (n = 5, 45.4%), respectively.
Conclusion:
The findings of this study indicate that etiology of hydrocephalus, were associated with the shunt survival. Further prospective controlled studies are required to address the observed associations
KEYWORDS: Complications, hydrocephalus, ventriculoperitoneal shunt
INTRODUCTION
Hydrocephalus is one of the most common clinical conditions affecting the central nervous system with an incidence of 3–4 per 1000 births for congenital hydrocephalus.[1] Ventriculoperitoneal (VP) shunt is the most frequently utilized diversion procedure because of the capability of the peritoneum to absorb fluid. Initial and subsequent peritoneal catheter placements can be performed with relative ease. They are associated with complications and may require multiple surgical procedures during a patient’s life span.
Complications of VP shunt surgery may be broadly divided into (1) mechanical and (2) infective complications. Mechanical complications include obstruction, disconnection or migration of any components of a shunt system either at the ventricular or peritoneal end. Infective complications include ventriculitis, shunt tract abscess, and skin necrosis overlying the shunt device. Other complications are the subdural collection, inguinal hernia, hydrocele, ascites, pseudocyst formation, perforation of a viscus, or extrusion of the shunt.[2] We studied 137 patients with hydrocephalus and analyzed the predisposing risk factors and range of complications.
MATERIALS AND METHODS
We performed a retrospective review of children with hydrocephalus that required VP shunt placement between 2004 and 2014. Patient diagnoses were divided into the following etiologies: congenital anomaly, post-tubercular meningitis (TBM), hydrocephalus associated with spinal dysraphism, and post-intraventricular hemorrhage (IVH) of newborn. A detailed record was kept with regard to name, age, sex, etiology, and clinical features. Investigations included ultrasonography and computed tomography (CT) scan of head to assess ventricular dilatation and ventricular–parenchyma thickness ratio.
Causes of revision were divided into obstruction, infection, malposition, catheter fracture, and abdominal problems. The causes of shunt revision were analyzed with respect to the age of the patients and according to the time interval of original shunt surgery and revision.
All patients underwent VP shunt in a standard protocol. Chhabra medium pressure slit and spring value shunt system (Gurgiwear Ltd, Shahjahanpur, India) was used. Early and late shunt complication was defined according to the duration between the initial shunt placement and appearance of the first shunt problem. Those occurring within 6 months were early and later than 6 months were late complications.
RESULTS
The study included 137 patients who underwent primary VP shunt. There were 78 male and 59 female patients. Age distribution: 103 patients were below 1 year and 34 above 2 years. The median age of the patients at the time of VP shunt placement was 20.7 months (range 1.5 months to 8.5 years). The etiologies of hydrocephalus in the patient sample are shown in Table 1. Nine patients (6.5%) were operated on within 6 months after shunt placement. Twenty-three patients required 1 shunt revision, 2 revisions in 11 patients, and 3 revisions in 3 patients. Distribution of revision causes and time intervals between shunt placement and revision according to each complication are shown in Tables 2–4. Obstruction and infection were the most common causes of revision, occurring in 17 (45.9%) and 6 (16.2%) cases, respectively. Surgical procedures performed during shunt revisions in the order of frequency were the revision of peritoneal part of the shunt (n = 12, 32.43%), revision of ventricular part of the shunt (n = 11, 29.72%), revision of the entire shunt system (n = 7, 18.91%), cysts excision and revision of peritoneal catheter (n = 4, 10.81%), extra ventricular drainage and delayed re-shunt (n = 4, 10.21%), shunt removal and delayed re-shunt (n = 2, 5.4%), and opposite side shunting (n = 2, 5.40%). The mortality was 7 (5.10%).
Table 1.
Demographic distribution of patients

Table 2.
Relationship between the cause of revision surgery and the time interval after initial shunt placement (months)
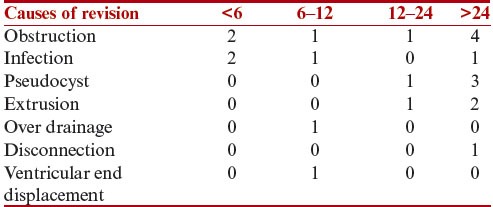
Table 4.
Relationship between the cause of revision surgery and the time interval after third revision (months)

Table 3.
Relationship between the cause of revision surgery and the time interval after second revision (months)
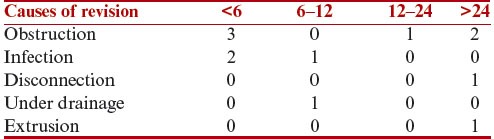
Other complications that did not require shunt revisions are shown in Table 5.
Table 5.
Complications not requiring shunt revision

DISCUSSION
Hydrocephalus is a common neurosurgical disease that develops through a variety of etiologies, including congenital anomaly, intracranial hemorrhage, infection, and tumor. Hydrocephalus has been customarily classified into obstructive and communicating variety.
Increase in head circumference was the most common sign (74%) followed by tense anterior fontanel and splayed cranial sutures (67%). A headache was present in 12% patients, vomiting in 11% and a refusal of feed in 9%, behavioral changes in 3%, and lethargy in 7%. Papilledema and sunset sign were seen in 6.5% and 5%, respectively.
The incidence of shunt failure is highest in the first 6 months following the VP shunt,[3] however, may occur at all times. The incidence of complications following VP shunt placement is reported to be around 20%–40%.[3] However, Stone et al.[4] reported 84.5%.
In this study, the most common complication was blockage of the shunt that causes it to function intermittently or not at all. We encountered shunt blockage in 15 patients (10.94%). Among the nine patients who had primary VP shunt, five were post-TBM, three were post-IVH and one was congenital hydrocephalus. The reasons for this were the presence of higher protein and cellular content in the cerebrospinal fluid (CSF), leading to early shunt obstruction. Ventricular end blockage was seen in nine [Figure 1] and peritoneal end blockage was in six. Distal obstruction was caused by collection of the tissue in the slit valve and wrapping of omentum. To improve the proper placement of the shunt, intraoperative ultrasound[5] and neuro-navigation guidance are being used particularly in patients with a small ventricle.[6] Laparoscopic insertion of a peritoneal catheter was also suggested for distal catheter insertion or revision.[7]
Figure 1.
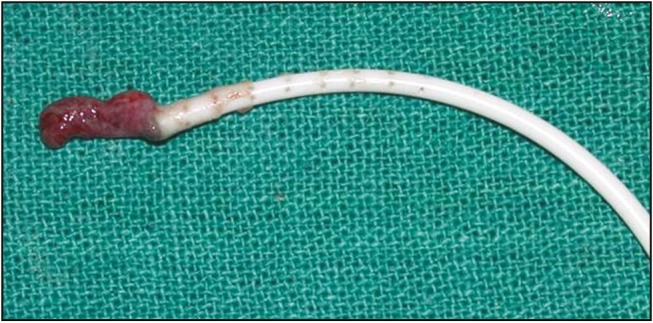
Blockage of the ventricular end
The incidence of shunt blockage complications has been reported from 5% to 47%.[8] In this study, 24.32% patients had proximal catheter blockage and 16.21% patients had distal catheter blockage.
In this study, infection was the second most common complication following shunt surgery responsible for significant morbidity and mortality. An infection rate of 4.37% was found in this study. Reported series has shown the incidence ranging from 5% to 15%. Although many factors seem to contribute to shunt infection, it is likely that the contamination of the shunt system at the time of surgery is the primary cause but also exposure during cleaning of the surgical wound is the risk factor. Approximately 70% of shunt infections present within 3 months and the remaining by 6 months of the surgical procedure. In our study, n = 4 (66.6%) developed infection within 6 months. The most common organism was coagulase negative staphylococci (n = 4). Staphylococcus epidermidis (n = 1) and Staphylococcus aureus (n = 1) were also seen.[9]
Infection rates are much higher in those patients in whom a longer period of hospitalization is required. Presence of sepsis markedly increases the probability of VP shunt infections.[10] This emphasizes the need to reduce the duration of hospitalization and to maintain special surgical wound care. Some authors[11] have reported an increased frequency of infection in children less than 6 months of ages as compared to older than 1 year. Other studies did not find differences in the infection rate between different children’s ages.[12] In this study, we did not find any correlation with age. Kulkarni et al.[11] suggested the simultaneous use of doubles gloves during surgical procedures; we followed the same to reduce the infection. Shunt infection was treated by intermittent CSF tapping and intravenous antibiotics based on culture and sensitivity of CSF and exteriorization of shunt. Revision shunt was performed only when CSF samples confirmed the absence of infection. Recently, antibiotic-impregnated shunt catheters have been shown to reduce the infection rate significantly.[13] Others have not found any reduction in the rates of complication.[14]
Disconnections occur at any section of the shunt, but they are especially frequent at the sites of mobility and with patient growth. Shunt disconnection was seen in 2 patients aged 13 and 14.6 years [Figure 2]. Both had disconnection between chamber and upper end. It is related to fibrosis and tight adhesion of the catheter to the surrounding soft tissue, which prevented the sliding of the catheter necessary to adjust to the growth of children.[15]
Figure 2.
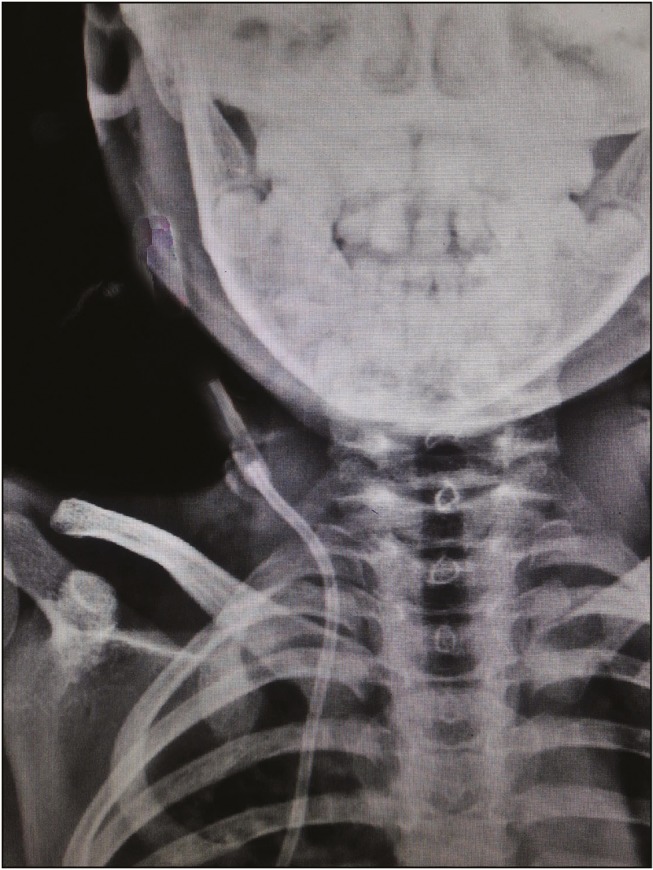
X-ray showing disconnection of chamber and ventricular end
Overdrainage was observed in one leading to slit-like ventricles [Figure 3]. To restore a balanced flow of CSF, it was necessary to change with a more accurate pressure valve.
Figure 3.
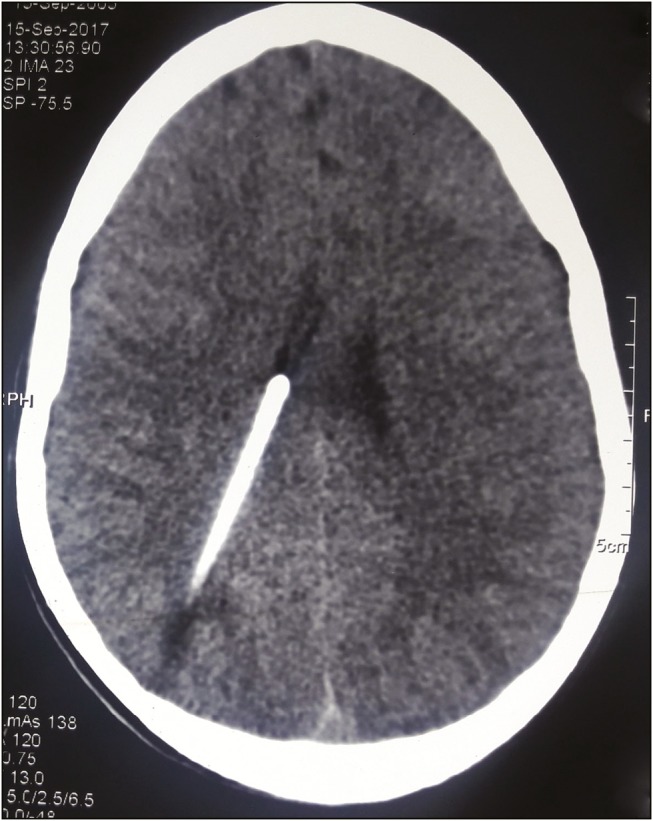
CT scan of head showing over drainage with ventricular collapse
Underdrainage was seen in one not being able to relieve the symptoms of hydrocephalus.
Subdural hematoma was observed in two [Figure 4] and craniosynostosis in one [Figure 5]. Both subdural hematoma formation and the occurrence of postshunt craniosynostosis are due to craniocephalic disproportion. The skull is not in a position to decrease in size to the same extent that the brain does after placement of the shunt, and this creates a space between the skull and the brain. Postshunt craniosynostosis is the result of apposition and overlapping of the cranial sutures in an infant following treatment of hydrocephalus.
Figure 4.
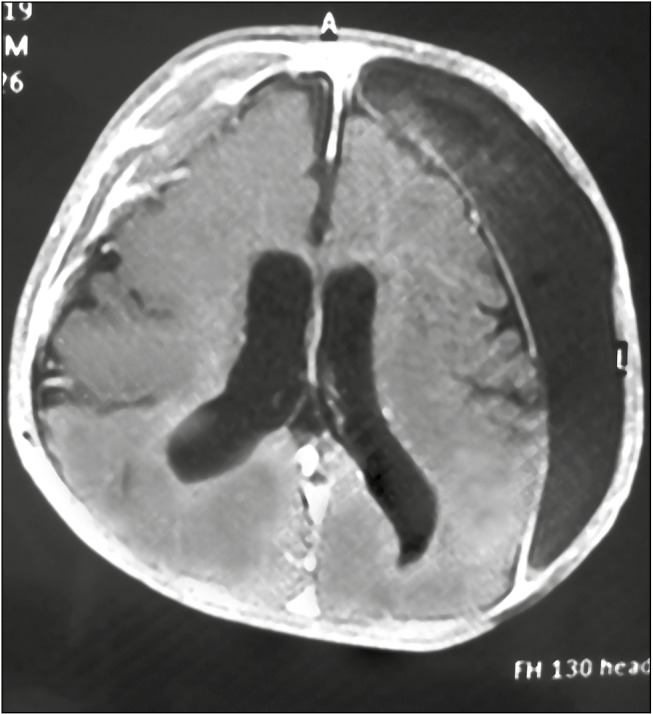
MRI of head showing subdural collection
Figure 5.
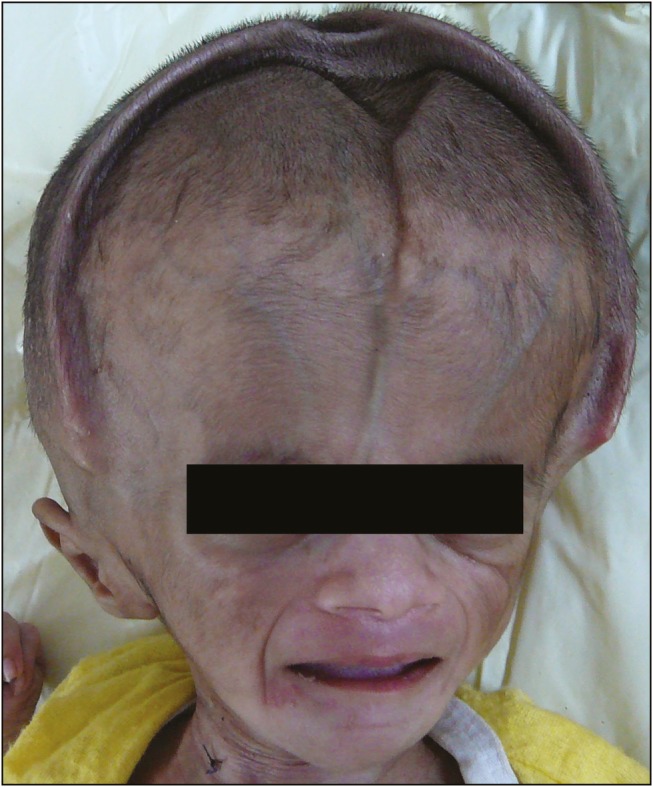
Craniosynostosis
Seizures occurred in 13 patients. Epilepsy has been involved in shunt-treated hydrocephalus. However, it is not believed to be directly related to the placement of the shunt. The reported percentage of children with VP shunts and seizures amounts to 48%, but there is a high association of seizures with underlying neurologic disorders.[16] There is no relationship between the number of shunt revisions or other complications. There is insufficient direct confirmation of an association between seizures and shunt surgery to necessitate routine prophylaxis with antiepileptic drugs. With the onset of further seizures in a child with a shunt, an infection and/or obstruction should be excluded, and then antiepileptic drugs should be considered.
Formation of CSF abdominal pseudocyst is a well-recognized but not very frequent complication (1%–4%).[17] We encountered pseudocyst in five patients presented with increased abdominal girth and shunt malfunction. All required excision of the pseudocyst and revision of the peritoneal end of the shunt [Figure 6].
Figure 6.
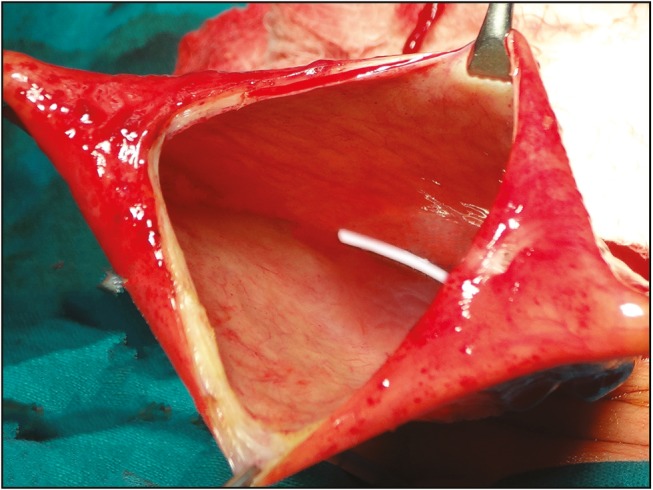
Operative photo showing shunt tube in the abdominal pseudocyst
Migration of shunt was observed in 3.64% (n = 5) of cases. The distal catheter had migrated and was extruded perrectally in two [Figure 7] and through the abdominal wound in two cases. Displacement of the ventricular end was observed in one.
Figure 7.
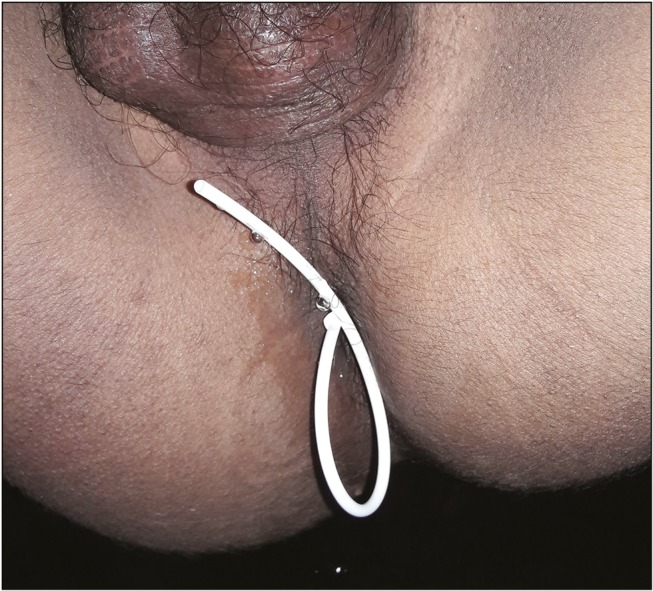
Rectal extrusion of the peritoneal end
Developmental milestones were delayed in 11 patients (20%); of them, 7 were of post-TBM, 3 were of IVH, and 1 was of spinal dysraphism.
The role of, age at the time of shunt insertion has been analyzed previously in several studies.[18] Increased shunt failure was seen in patients who had undergone shunt placement at age less than 1 year.[19] On the other hand, there are studies showing no effect of age on the incidence of shunt infection.[20] This study did not support the age at the time of shunt placement to be an important predictor of shunt function. In our study, only six patients had shunt failure who were shunted during initial 1 year of age.
In our series, 67.56% were mechanical, and 16.2% were infective complications. Similar findings were seen in other study.[21] In our study, 32.43% of patients with shunt-related complications presented within 6 months of shunt placement. On literature review, event-free survival at 1 year ranged from 62% to 80%[22] and at 10 years from 35% to 48%.[22] In this study, we found 56.12% and 37.7% event free at 1 year and 5 years, respectively. The shunt-related mortality reported being 8.6%–13.7%.[23] In our series, it was n = 7 (5.10%).
CONCLUSION
Hydrocephalus is a common neurological disorder. VP shunt placement has been the main treatment modality for hydrocephalus. It has amazingly changed the neurological outcome in these patients with enhanced chances of leading a normal life. VP shunts procedures are still associated with complications and morbidity. The development of shunt complications was found to be significantly associated with the etiology of the hydrocephalus. Other characteristics, such as age and gender, did not affect the overall shunt function. Prospective controlled studies are needed to address the observed associations between the risk factors and incidence of shunt revisions in these patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Laurence KM, Coates S. The natural history of hydrocephalus. Detailed analysis of 182 unoperated cases. Arch Dis Child. 1962;37:345–62. doi: 10.1136/adc.37.194.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sgouros S, Malluci C, Walsh AR, Hockley AD. Long-term complications of hydrocephalus. Pediatr Neurosurg. 1995;23:127–32. doi: 10.1159/000120949. [DOI] [PubMed] [Google Scholar]
- 3.Reddy GK, Bollam P, Caldito G. Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg. 2014;81:404–10. doi: 10.1016/j.wneu.2013.01.096. [DOI] [PubMed] [Google Scholar]
- 4.Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ. Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J Neurosurg Pediatr. 2013;11:15–9. doi: 10.3171/2012.9.PEDS1298. [DOI] [PubMed] [Google Scholar]
- 5.Crowley RW, Dumont AS, Asthagiri AR, Torner JC, Medel R, Jane JA, Jr, et al. Intraoperative ultrasound guidance for the placement of permanent ventricular cerebrospinal fluid shunt catheters: a single-center historical cohort study. World Neurosurg. 2014;81:397–403. doi: 10.1016/j.wneu.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Levitt MR, O’Neill BR, Ishak GE, Khanna PC, Temkin NR, Ellenbogen RG, et al. Image-guided cerebrospinal fluid shunting in children: catheter accuracy and shunt survival. J Neurosurg Pediatr. 2012;10:112–7. doi: 10.3171/2012.3.PEDS122. [DOI] [PubMed] [Google Scholar]
- 7.Martin K, Baird R, Farmer JP, Emil S, Laberge JM, Shaw K, et al. The use of laparoscopy in ventriculoperitoneal shunt revisions. J Pediatr Surg. 2011;46:2146–50. doi: 10.1016/j.jpedsurg.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi DK, Balasubramaniam S, Jayaswal SA, Savant HV, Gandhi AS. Outcome analysis of ventriculoperitoneal shunt procedures in hydrocephalus due to tubercular meningitis and non-infective cases. Int J Contemp Pediatr. 2016;3:1210–5. [Google Scholar]
- 9.Braga MH, Carvalho GTC, Brando RACS, Lima FBF, Costa BS. Early shunt complications in 46 children with hydrocephalus. Arq Neuro-Psiquiatr. 2009;67:145–8. doi: 10.1590/s0004-282x2009000200019. [DOI] [PubMed] [Google Scholar]
- 10.Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg. 1992;77:29–36. doi: 10.3171/jns.1992.77.1.0029. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni AV, Drake JM, Lamberti-Pasculli M. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg. 2001;94:195–201. doi: 10.3171/jns.2001.94.2.0195. [DOI] [PubMed] [Google Scholar]
- 12.Faillace WJ. Shunt infection. J Neurosurg. 2001;94:1019–20. doi: 10.3171/jns.2001.94.6.1019. [DOI] [PubMed] [Google Scholar]
- 13.Kandasamy J, Dwan K, Hartley JC, Jenkinson MD, Hayhurst C, Gatscher S, et al. Antibiotic-impregnated ventriculoperitoneal shunts–a multi-centre British paediatric neurosurgery group (BPNG) study using historical controls. Childs Nerv Syst. 2011;27:575–81. doi: 10.1007/s00381-010-1290-z. [DOI] [PubMed] [Google Scholar]
- 14.Costa BS, Maitrot TD. Prise en charge de l’hidrocéphalie de l’enfant au Brésil. Congres Table Ronde de La SNCLF, Paris. 2001 [Google Scholar]
- 15.Park MK, Kim M, Park KS, Park SH, Hwang JH, Hwang SK. A retrospective analysis of ventriculoperitoneal shunt revision cases of a single institute. J Korean Neurosurg Soc. 2015;57:359–63. doi: 10.3340/jkns.2015.57.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klepper J, Büsse M, Strassburg HM, Sörensen N. Epilepsy in shunt-treated hydrocephalus. Dev Med Child Neurol. 1998;40:731–6. doi: 10.1111/j.1469-8749.1998.tb12340.x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma AK, Pandey AK, Diyora BD, Mamidanna R, Sayal PP. Abdominal CSF pseudocyst in a patient with ventriculo-peritoneal shunt. Indian J Surg. 2004;66:360–3. [Google Scholar]
- 18.Agarwal N, Shukla RM, Agarwal D, Gupta K, Luthra R, Gupta J, et al. Pediatric ventriculoperitoneal shunts and their complications: an analysis. J Indian Assoc Pediatr Surg. 2017;22:155–7. doi: 10.4103/0971-9261.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liptak GS, McDonald JV. Ventriculoperitoneal shunts in children: factors affecting shunt survival. Pediatr Neurosci. 1985;12:289–93. doi: 10.1159/000120268. [DOI] [PubMed] [Google Scholar]
- 20.Kinasha AD, Kahamba JF, Semali IT. Complications of ventriculoperitoneal shunts in children in Dar es Salaam. East Cent Afr J Surg. 2005;10:55–9. [Google Scholar]
- 21.Lee JY, Wang KC, Cho BK. Functioning periods and complications of 246 cerebrospinal fluid shunting procedures in 208 children. J Korean Med Sci. 1995;10:275–80. doi: 10.3346/jkms.1995.10.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, et al. Long-term follow-up data from the shunt design trial. Pediatr Neurosurg. 2000;33:230–6. doi: 10.1159/000055960. [DOI] [PubMed] [Google Scholar]
- 23.Tuli S, Tuli J, Drake J, Spears J. Predictors of death in pediatric patients requiring cerebrospinal fluid shunts. J Neurosurg. 2004;100:442–6. doi: 10.3171/ped.2004.100.5.0442. [DOI] [PubMed] [Google Scholar]


