Abstract
Introduction:
Childhood epilepsy is a generalized epilepsy syndrome with a favorable response to antiepileptic drugs; however, a small percentage of typical absence seizures remain refractory to drugs. We studied the safety and efficacy of amantadine in children with refractory absence seizures.
Materials and Methods:
Of 48 children with typical absence seizures attending the outpatient department of a tertiary care neurological center over a period of 3 years from July 2013 to June 2016, 4 children who were refractory to standard treatment for at least 1 year were selected and were started on amantadine 4–6 mg/kg/day, after obtaining informed consent.
Observations:
The children, aged between 7 and 14 years, had more than 10 episodes of seizures per day in spite of polytherapy with valproate, lamotrigine, clonazepam, levetiracetam, and topiramate in various combinations. Electrographically, all showed the typical generalized 3 Hz spike wave discharges activated by hyperventilation. All the children became seizure free within 1 week after starting amantadine, and there was improvement in their school performance. The children continue to remain seizure free for 6–30 months now. No significant adverse effects were observed on addition of amantadine.
Discussion:
Amantadine can be tried as a safe add-on drug for children with absence epilepsy refractory to multiple drugs. Further multicenter trials may be needed to prove its effectiveness, as the numbers are small.
KEYWORDS: Amantadine, childhood absence epilepsy, refractory absences
INTRODUCTION
Childhood absence epilepsy (CAE) accounts for 10%–15% of all childhood epilepsies. Valproic acid (VPA) and ethosuximide are often considered to be the drug of choice for the treatment of CAE. These drugs can control absences in about 75% of children with CAE.[1] Approximately 10%–15% of children remain refractory to various drug combinations. Younger age at onset, longer duration before starting antiepileptic drugs (AEDs), and more number of episodes per day are associated with refractoriness.[2] The children with refractory absences become progressively inattentive and hyperactive, with a decline in their scholastic achievement.[3] Various drugs such as lamotrigine, benzodiazepines, topiramate, and levetiracetam (LEV) are used as second-line drugs.[4]
Different modalities of treatment such as drugs (steroids, acetazolamide, sulthiame, amantadine), vagal nerve stimulation, and ketogenic diet were tried in these subset of children and were found to be beneficial to some extent, as per a few small, open-label studies.[5,6,7,8,9] Ethosuximide, the drug of choice in typical absence epilepsy, is not yet available in India. Interestingly, carbamazepine and phenytoin were known to aggravate absences.[10]
The antiviral drug amantadine was found to be useful in refractory generalized seizures from as early as 1985.[7] Shahar and Brand[8] used amantadine in four children with refractory absence epilepsy, and demonstrated rapid achievement of freedom from seizures, which was sustained for 24–36 months. Amantadine exerts its action by increasing the release and inhibiting the reuptake of dopamine in the brain. The drug also has an antagonistic action on the thalamocortical excitatory N-methyl D-aspartate (NMDA) receptors.
In this small series, we sought to review our experience with amantadine in four children with refractory absence epilepsy. A short review of available evidence showing the efficacy and safety of the drug in absence seizures is also included.
MATERIALS AND METHODS
All the children with typical absence seizures, fulfilling the international league against epilepsy diagnostic criteria, and attending the Paediatric Neurology Clinic of Government Medical College Thiruvananthapuram, Kerala, India from July 2013 to June 2016 were reviewed. The children who were refractory to at least two AEDs in maximum tolerated doses for a minimum period of 1 year were included in the study group. Amantadine hydrochloride (4–6 mg/kg/day) was started after obtaining informed consent from both the parents. The children were followed up for a period of 6–30 months after starting amantadine. The seizure freedom was assessed from parental and teachers’ reports, and follow-up video electroencephalograms (EEGs), looking for activation of interictal epileptiform discharges (IEDs) and/or clinical absences on hyperventilation (HV). Adverse effects of amantadine, if any, were also monitored. The blood counts and hepatic and renal function tests were repeated every month for the first 3 months.
OBSERVATIONS
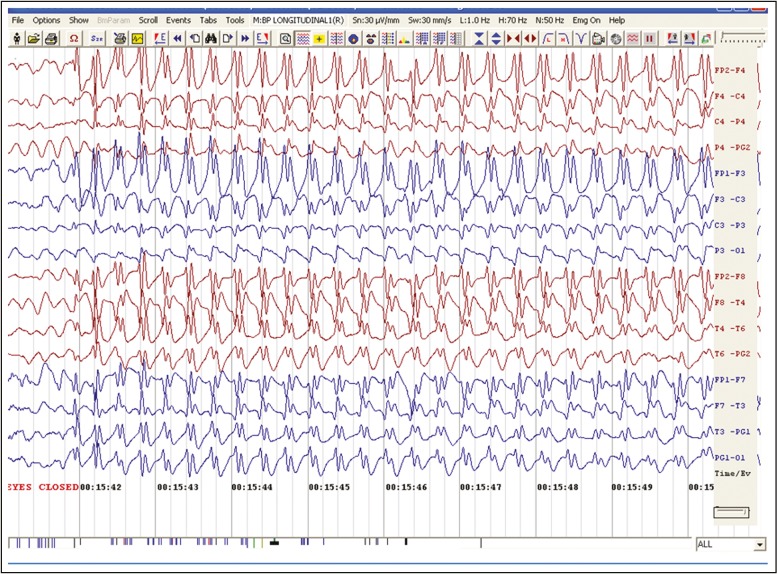
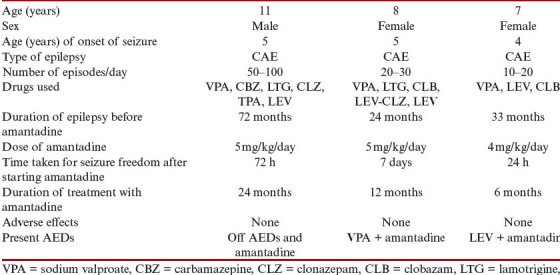
The four children, three girls and a boy, were aged between 7 and 14 years at the time of starting amantadine. All had a normal perinatal history and normal milestones of development before the onset of absence seizures. All of them had more than 10 episodes of typical absences lasting 5–30 s per day, characterized by arrest of activity, uprolling of eyes, simple automatisms such as lip smacking, and precipitation of attacks during HV. Electrographically, all showed the typical 3–3.5 Hz generalized frontally dominant spike wave (SW) discharges, lasting 8–25 s with an abrupt onset and offset, associated with clinical absences, and marked activation on HV [Figure 1]. There was no evidence of photosensitivity. The children were on polytherapy with sodium valproate, clonazepam, LEV, topiramate, and zonisamide in various combinations for an average period of 31 months, before starting amantadine. Seizure control was never achieved in two of them, one girl had improvement with a combination of valproate and lamotrigine for 9 months, another girl became seizure free on a combination of valproate, clonazepam, and topiramate for a year, but became refractory later. Within a week of starting amantadine, all became free of clinical absences, and the EEGs were also normalized. The children were found to be seizure free for 6–30 months after starting amantadine. We could taper other anticonvulsants slowly, and the three girls were maintained on a single drug along with amantadine. For the boy, we could taper and stop all the antiepileptics including amantadine after 2.5 years of seizure freedom. No serious adverse effects were observed on adding amantadine. The parents observed a significant improvement in school grades. Table 1 shows the patient characteristics and the outcome.
Figure 1.
Typical 3/sec spike wave discharges in EEG (Child 1)
Table 1.
Patient characteristics and outcome
Case 1
Our index case was an 11-year-old boy with refractory absences for 6 years. He was born to nonconsanguineous parents, and started developing multiple daily episodes of seizures (>50 absences/day) from the age of 5 years. The seizures were brief episodes of behavioral arrest lasting for less than a minute. He had no aura or postictal confusion or drowsiness. There were no falls or generalized convulsions. He had no perinatal risk factors. His growth and development were normal. His academic skills had declined after the onset of seizures. There was no family history of epilepsy. He was put on AEDs after 6 months of onset of seizures. He initially received sodium valproate 40 mg/kg, and his seizures continued. Later on, carbamazepine (25 mg/kg) and phenytoin (5 mg/kg) were added by his family physician. He reported to us at the age of 8 years with complaints of increasing frequency of daily seizures. Bedside HV test precipitated typical absences. He underwent short-term video EEG, which showed normal background rhythm and multiple absence seizures characterized by abrupt onset of 3 Hz generalized SW discharges lasting for 10–30s. No focal slowing, focal IEDs, or postictal slowing was noted. Sleep tracing showed normal sleep organization and a few frontally dominant fragmentary generalized spikes of 200–400ms duration. Hyperventilation precipitated absences. Intermittent photic stimulation (IPS) did not produce any activation. Carbamazepine and phenytoin were discontinued, and valproate dose was hiked to 60 mg/kg/day without any benefit. Subsequently, he was put on polytherapy with valproate (60 mg/kg), LEV (60 mg/kg/day), followed by clobazam (1 mg/kg/day), lamotrigine (5 mg/kg), and topiramate (5 mg/kg) without any benefit. Ethosuximide could not be tried, as it was not available in the country. Because of very frequent absences and declining academic skills, he was given a course of intravenous methyl prednisolone (30 mg/kg/day) for 5 days. He remained refractory to all the AEDs. At the age of 9 years also he continued to get multiple seizures. At this time, he underwent a repeat short-term video EEG. Several brief seizures of varying duration from 10 to 30 s were recorded. The semiology of seizures consisted of complete loss of awareness of surroundings. He had repeated eye blinks but no myoclonia or eye or head deviation. His torso was found to be slightly bent forward. He exhibited mild oral automatisms in the form of licking of lips and used to drop objects held in his hands. He recovered consciousness fully immediately after the ictus. The ictal EEG consisted of abrupt onset of bilateral frontally dominant SWs of 3.5–3 Hz, which toward the end slowed to slightly less than 3 Hz. No leading frontal spikes were recorded. The duration of ictal EEG record correlated with clinical events. Normal background rhythm was noted immediately after the end of ictal discharges. Interictal awake record showed normal alpha rhythm and no focal spikes or slow waves. Sleep architecture was normal, and a few fragmentary generalized spikes were recorded. No clinical events were recorded during sleep.
He was investigated for structural malformations/gliotic changes by magnetic resonance imaging (1.5 tesla), which was normal. A cerebrospinal fluid (CSF) study was carried out to look for Glut 1 deficiency, and no CSF hypoglycorrhachia was reported. He was started on 5 mg/kg of once daily amantadine along with his ongoing AED therapy (VPA, LEV, and clobazam). Within 72 h, all the clinical seizures stopped. A repeat video EEG at this time showed normal awake and sleep EEG. No activation was noted during HV or IPS. Clobazam and LEV were tapered and stopped in 6 months. Sodium valproate dose was also brought down to 20 mg/kg without any seizure recurrence. His scholastic performance improved significantly. As he remained seizure free for 2 years, he is off all the drugs now, and the 3-h video EEG remains absolutely normal. He continues to remain seizure free on follow-up.
Case 2
An 8-year-old girl presented with a history of 20–30 episodes of staring spells and unresponsiveness with chewing movements, which was noticed since the age of 4.5 years. The episodes lasted for a few seconds only. The events were precipitated by HV. There was no postictal confusion. The interictal EEG showed normal, symmetrical background alpha activity. During the ictus, there was an abrupt onset of 3-3.5 Hz, generalized frontally dominant SW discharges lasting for 5-12 seconds. The absences were more prolonged after HV that lasted for 10-25 s. She was started on sodium valproate, and the number of episodes decreased, but the attacks persisted in spite of increasing the dose of valproate to 50 mg/kg and adding clobazam 0.5 mg/kg. Lamotrigine was added and the dose was titrated slowly to 2 mg/kg without any significant improvement. The child was showing decline in her school performance, and was sleepy at school. After 36 months of starting AEDs in maximum tolerated doses, amantadine was tried at 5 mg/kg/day, after obtaining informed consent. There was a significant reduction in the number of episodes within 72 h, and she became free of clinical absences within a week. A short-term video EEG carried out 2 weeks after starting the treatment was found to be completely normal. The anticonvulsants were slowly tapered, and now 12 months after starting amantadine, she is seizure free on amantadine 5 mg/kg and sodium valproate 20 mg/kg. No adverse events were reported after starting amantadine. Her academic performance improved after the control of seizures, as reported by her parents.
Case 3
A 7-year-old girl, with a history of 10–20 daily typical absence seizures from the age of 5 years, was started on sodium valproate, and she became seizure free for 9 months with a dose of 40 mg/kg; however, the absences recurred. Levetiracetam was added and the dose was increased to 50 mg/kg, and as there was no reduction in the number of episodes, clonazepam was also added. The child became very sleepy and continued to have absences. On starting amantadine at 4 mg/kg/day, she became free of attacks within 24 h, and remains seizure free after 6 months of treatment. The short-term video EEG for 3h did not show any clinical or electrographic seizure activity. Sodium valproate and clobazam were tapered and stopped, and the dose of LEV was reduced to 25 mg/kg/day. The girl became active, less sleepy, and her academic performance also improved.
Case 4
A 14-year-old girl with a history of repeated episodes of absence seizures from 7 years of age was presented. The absences were very frequent at 50–100 times/day, associated with staring spells, and a few automatisms involving the upper limb. There was no family history of seizures. EEG was suggestive of typical absence seizures with generalized 3Hz frontally dominant generalized SW discharges with an abrupt onset and offset, which were also activated by HV. She was started on sodium valproate and clobazam. On increasing the dose of sodium valproate to 40 mg/kg/day, she started gaining weight rapidly. Thus, the dose of valproate was reduced and lamotrigine was added, because of which the number of attacks decreased, but she never became seizure free. Later on, topiramate was added, and there was a significant improvement with 2 mg/kg of topiramate. She reduced topiramate on her own and the episodes recurred, which could not be controlled by restarting topiramate at 5 mg/kg. She also became free of clinical absences within 72 h after starting amantadine at 5 mg/kg/day. The short-term video EEG carried out after 2 weeks of starting amantadine was found to be completely normal. Now she is on amantadine and topiramate combination while remaining seizure free for 6 months.
DISCUSSION
Amantadine hydrochloride was approved by the Food and Drug Administration for the treatment and prophylaxis of influenza A in 1976 in children 1 year and older. It acts on the transmembrane domain of the viral M2 protein preventing the release of viral RNA into the host cell. Later, it was found that the drug has significant effects on Parkinson’s disease, probably by its effect on dopaminergic neurons. The drug also acts as an NMDA receptor antagonist. Recently, the drug was found to be effective in various behavioral disorders such as attention deficit hyperactivity disorder, autism, and unipolar depression, and it was found to augment recovery from traumatic brain injury probably by its NMDA receptor–blocking action.[11]
The effectiveness of amantadine in generalized seizure disorder was identified serendipitously, when children with epilepsy treated with amantadine for influenza experienced seizure freedom. Shields et al.[7] demonstrated effectiveness of amantadine as an add-on therapy in 10 children with refractory generalized epilepsy and found that it is maximally effective in absence and myoclonic seizures. Shahar and Brand[8] used amantadine in four children with refractory absence epilepsy and noted rapid achievement of seizure freedom in all, which was sustained for 24–36 months. Perry et al.[9] noted more than 90% reduction in seizure freedom in 6 of 12 children with refractory absence, myoclonic, or continuous spike wave discharges of sleep, and more than 50% reduction of attacks in another two within 3 months of starting amantadine. The response was better in typical absence seizures.[9]
All the four children in our series had clinical and electrographic features of typical CAE, who were resistant to at least three standard medications, and all of them had complete freedom from clinical absences on starting amantadine, with normalization of the EEG recording, even after HV. Interestingly, there was no recurrence of events even after tapering and stopping other antiepileptics. The girls are now seizure free on amantadine and low-dose sodium valproate (20 mg/kg) or LEV (25 mg/kg) or topiramate (2 mg/kg). The boy is off AEDs and amantadine, and remains seizure free for more than 2 years now.
Typical absence seizures result from the abnormal cyclic activation of a bilateral network that comprises three structures: cortex, thalamic relay nuclei, and thalamic reticular nuclei. The regular oscillatory activation of these corticothalamocortical loops is responsible for the generation of SW discharges, the spike corresponding to synchronous excitatory neuronal activity, and the slow wave to neuronal inactivation. This cycle involves reciprocal excitatory glutamatergic connections between the thalamus and cortex, and inhibitory GABAergic influence of the thalamic reticular nuclei on the thalamic relay nuclei. Low-threshold (T-type) voltage-dependent calcium qchannels in the thalamocortical neurons are activated in an oscillatory way by this GABA-induced hyperpolarization. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and dopaminergic influence from the basal nuclei also regulate oscillatory thalamocortical activity.[4]
The exact mechanism of action of amantadine still remains unknown. The drug may exert its action by its dopamine agonistic action or by antagonizing the excitatory NMDA receptors in the thalamocortical circuits.[8] The inhibitory effect of apomorphine, a dopamine agonist, was found to be effective in reducing epileptic photosensitivity.[12]
The drug was tolerated very well by all the four children, and no side effects were reported. All the earlier studies also noted the drug to be very safe in children. The common adverse effects reported in adults with Parkinson’s disease include vomiting, dry mouth, hallucinations, transient elevation of liver enzymes, and rarely neuroleptic malignant syndrome and angle closure glaucoma.
CONCLUSION
Our initial experience suggests that amantadine may be a safe and effective add-on drug for the treatment of refractory absence epilepsy. It is to be emphasized that one of the two key drugs for absence epilepsy, ethosuximide, is not available in India till now. A trial of amantadine may be worthwhile, after failure of sodium valproate, LEV, clonazepam, and/or lamotrigine. As all the studies were open label and included very few children, randomized, double-blind multicenter trials are needed to prove its safety and efficacy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Duncan JS. Idiopathic generalized epilepsies with typical absences. J Neurol. 1997;244:403–11. doi: 10.1007/s004150050113. [DOI] [PubMed] [Google Scholar]
- 2.Ollivier ML, Dubois MF, Krajinovic M, Cossette P, Carmant L. Risk factors for valproic acid resistance in childhood absence epilepsy. Seizure. 2009;18:690–4. doi: 10.1016/j.seizure.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Wirrell EC, Camfield CS, Camfield PR, Dooley JM, Gordon KE, Smith B. Long-term psychosocial outcome in typical absence epilepsy. Sometimes a wolf in sheep’s clothing. Arch Pediatr Adolesc Med. 1997;151:152–8. doi: 10.1001/archpedi.1997.02170390042008. [DOI] [PubMed] [Google Scholar]
- 4.Vrielynck P. Current and emerging treatments for absence seizures in young patients. Neuropsychiatr Dis Treat. 2013;9:963–75. doi: 10.2147/NDT.S30991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorman KM, Shahwan A. Sultiame revisited: treatment of refractory absence seizures. Epileptic Disord. 2016;18:329–33. doi: 10.1684/epd.2016.0850. [DOI] [PubMed] [Google Scholar]
- 6.Arya R, Greiner HM, Lewis A, Mangano FT, Gonsalves C, Holland KD, et al. Vagus nerve stimulation for medically refractory absence epilepsy. Seizure. 2013;22:267–70. doi: 10.1016/j.seizure.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Shields WD, Lake JL, Chugani HT. Amantadine in the treatment of refractory epilepsy in childhood: an open trial in 10 patients. Neurology. 1985;35:579–81. doi: 10.1212/wnl.35.4.579. [DOI] [PubMed] [Google Scholar]
- 8.Shahar EM, Brand N. Effect of add-on amantadine therapy for refractory absence epilepsy. J Pediatr. 1992;121:819–21. doi: 10.1016/s0022-3476(05)81922-5. [DOI] [PubMed] [Google Scholar]
- 9.Perry MS, Bailey LJ, Kotecha AC, Malik SI, Hernandez AW. Amantadine for the treatment of refractory absence seizures in children. Pediatr Neurol. 2012;46:243–5. doi: 10.1016/j.pediatrneurol.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Kochen S, Giagante B, Oddo S. Spike-and-wave complexes and seizure exacerbation caused by carbamazepine. Eur J Neurol. 2002;9:41–7. doi: 10.1046/j.1468-1331.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- 11.Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55–60. [PMC free article] [PubMed] [Google Scholar]
- 12.Quesney LF, Andermann F, Lal S, Prelevic S. Transient abolition of generalized photosensitive epileptic discharge in humans by apomorphine, a dopamine-receptor agonist. Neurology. 1980;30:1169–74. doi: 10.1212/wnl.30.11.1169. [DOI] [PubMed] [Google Scholar]