Abstract
Extent of resection is a very important prognostic marker in adult and pediatric brain tumors. Therefore, radical resections confer an oncological benefit. Resection of intra-axial tumors in proximity to eloquent regions requires intraoperative mapping and monitoring. Continuous subcortical mapping using a suction monopolar device has been recently described for adult tumors. This allows a real-time dynamic mapping of the advancing resection cavity walls, synchronized with the surgeon’s actions during resection. We describe the application of this technique in a child who presented with a rapidly increasing right parietal mass. It was resected using this dynamic mapping technique. This is the first such report of its use in a pediatric brain tumor. We also review the relevant literature briefly.
KEYWORDS: Dynamic mapping, intraoperative monitoring, pediatric brain tumors, subcortical mapping
INTRODUCTION
Extent of resection is an important prognostic factor in most pediatric brain tumors.[1,2] The benefits of radical resection have to be balanced against the risks of imparting new neurological deficits. This is particularly important in tumors close to eloquent regions. Intraoperative neurophysiological mapping and monitoring techniques have evolved in the last decade and provide a valuable means of assessing the functional status and thereby guide the resection even in pediatric cases.[3] Motor mapping (both cortical and subcortical) is one such very reliable technique. Recently, a new technique for continuous and dynamic subcortical mapping was described in adults using a suction monopolar stimulator device.[4] We describe the application of this technique in a pediatric brain tumor for the first time.
CASE AND TECHNICAL DESCRIPTION
A 3-year-old girl presented with multiple episodes of seizures for 6 months. When evaluated at another center at that time, magnetic resonance imaging (MRI) (noncontrast) of the brain revealed a small intra-axial right parietal lesion, which was initially observed. Serial imaging showed significant increase in the size of the mass when the girl was referred to our center. At presentation, she was active, cheerful with no focal neurological deficits. MRI of the brain [Figure 1] revealed a well-circumscribed intra-axial mass measuring 7 × 7 × 6 cm in the right parietal lobe. It was hypointense on T1 images, iso- to hypo-intense on T2 images with patchy enhancement on contrast. The tumor abutted the descending motor fibers anteriorly, reaching up to the posterior limb of the internal capsule (white arrow, Figure 1). The child underwent a right parietal craniotomy and excision of the tumor with intraoperative neurophysiological stimulation and recordings. We used the NIM Eclipse 4.2 (Medtronics, Minneapolis, MN) system. Anesthesia was induced with oxygen and sevoflurane, and after an intravenous (IV) access was secured, injections propofol 2 mg/kg, fentanyl 2 μg/kg, and atracurium 0.5 mg/kg were given IV to facilitate endotracheal intubation. Following this, anesthetic depth was maintained with total IV anesthesia with propofol infusion 100–150 μg/kg/min and fentanyl infusion at 1 μg/kg/h. Transcranial electrical stimulation (TES) using a multipulse paradigm (train of 5-, 0.5-ms pulse duration, at 333 Hz) was done by delivering the stimulus at C1–C2 montage (EEG 10–20 international system) using corkscrew electrodes. Threshold current strengths were achieved at 250 V by starting with 100 V and increasing in steps of 10 V till all the monitored muscles were recruited. Muscle motor-evoked potentials (MEPs) were recorded using subdermal needle electrodes inserted on the contralateral side: upper limbs—flexor carpi radialis and abductor pollicis brevis; lower limbs—tibialis anterior and extensor digitorum brevis. One control muscle was monitored on the ipsilateral side. Muscle MEPs were recorded with a band-pass filter of 30–1500 Hz and a sweep length of 100 ms. We also performed subdural direct cortical stimulation (DCS) using a 4-point contact-strip electrode (train of 5, pulse duration of 500 µs with an interstimulus interval of 4 ms). We started at a very minimal stimulus intensity at 3 mA and gradually increased it in steps of 1 mA till we got the MEP responses from the target muscles (minimum amplitude 20 µV). At 9 mA, we achieved consistent reproducible responses from upper limb and maintained this motor threshold (MT) for continuous cortical strip MEP (at 1 Hz) till the end of tumor resection. The tumor was delineated using intraoperative ultrasound. It seemed to occupy the entire superior parietal lobule. After confirming the location of the motor cortex anteriorly (using the strip electrode), corticectomy was performed and tumor rapidly debulked with a suction aspirator. As the tumor was debulked, we switched to the suction monopolar device for dynamic subcortical mapping. We used the monopolar probe with suction capabilities [Figure 2] as described by Raabe et al.[4] Stimulation was activated by connecting it to our standard monopolar finger-stick probe connection in the stimulator box (NIM Eclipse 4.2 [Medtronics]), with the reference electrode on the exposed muscle near the surgical field. Cathodal stimulation with a monophasic current (stimulation parameters same as DCS) was used. To avoid concurrent seizure with continuous stimulation, we used a 2-Hz low repetition rate of the trains. A high-pitch sound was delivered with every single train of stimulation as a feedback signifying adequate current delivery. For determining the MT, we set our amplitude criteria of 20 µV for an MEP response in the recorded muscles. The responses were observed in both the free-running and triggered electromyography screens and instantly reported to the surgeon in a real-time manner. As tumor debulking proceeded, we started getting responses at 15 mA near the anteromedial border of the tumor. The current was then reduced in steps of 2 mA till the tumor resection was completed. We stopped at 8 mA MT because we achieved the expected resection of the tumor. DCS and TES MEPs remained stable throughout the resection [Figures 3 and 4]. The child recovered uneventfully. Complete excision was documented on the immediate postoperative computed tomography (CT) scan [Figure 5], and the child was discharged on the fifth day. Histopathology revealed it to be an embryonal tumor with multilayered rosettes (WHO grade IV) and the child was planned for adjuvant radiotherapy.
Figure 1.
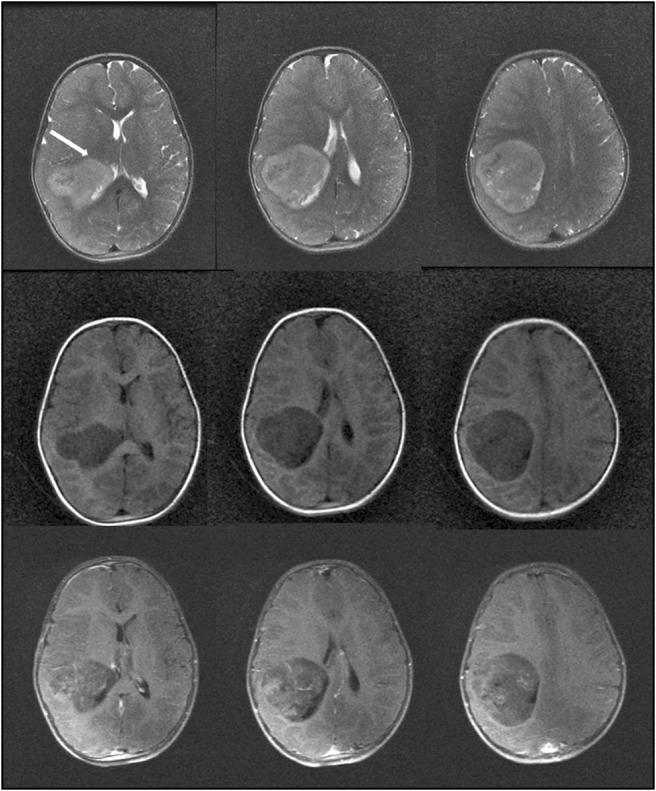
Preoperative MRI of the child showing the T2 images (top row), T1 images (middle row), and postcontrast T1 images (bottom row). The tumor abuts the corticospinal tract anteriorly (white arrow, top row)
Figure 2.

Picture showing the suction monopolar device used
Figure 3.
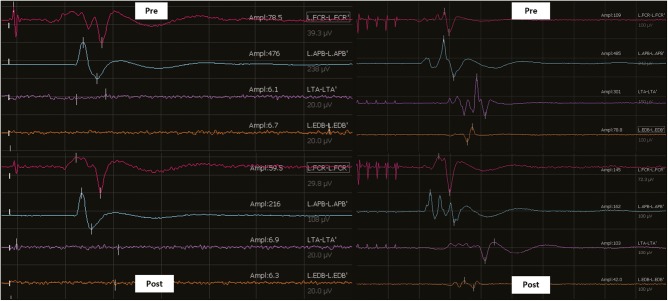
Snapshot of the screen depicting the direct cortical strip MEP (left panel) and transcranial MEP responses (right panel) before (pre) and after (post) resection. Both remained stable throughout
Figure 4.
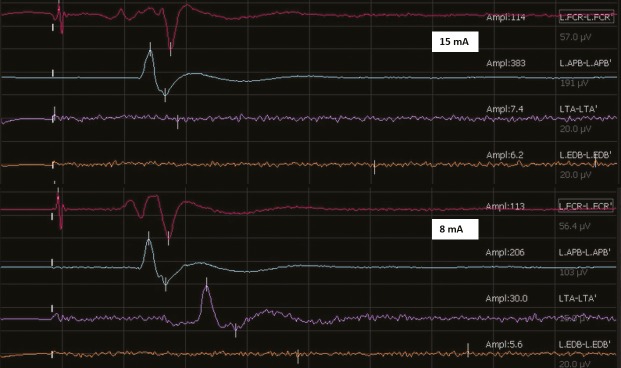
Snapshot of the monitoring screen showing the dynamic mapping responses obtained during subcortical mapping with the suction probe at an MT of 15 mA (upper panel) and at 8 mA (lower panel) at the end of the resection
Figure 5.
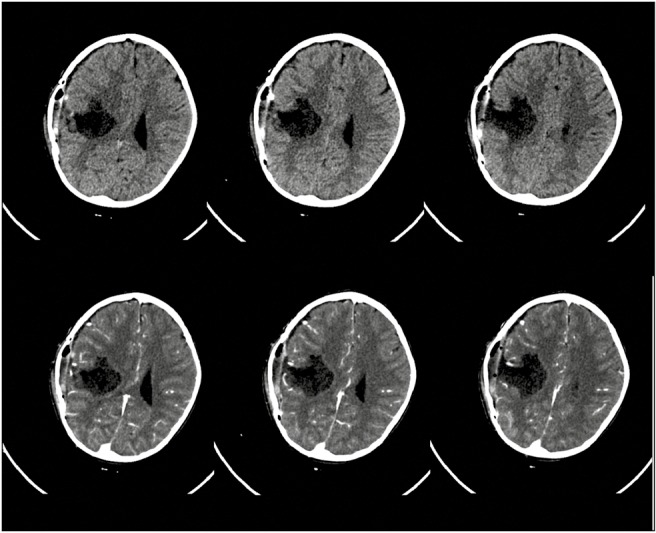
Postoperative CT scan (plain, top row; contrast enhanced, bottom) showing complete tumor excision
DISCUSSION
Technical adjunct such as intraoperative imaging has improved radicality of resection in pediatric brain tumors.[5,6] However, preservation of function is of utmost importance. Intraoperative neurophysiological techniques have emerged as very reliable intraoperative real-time assessment tools for functional characterization and assessment of integrity of eloquent areas. This is particularly so for motor cortex and motor tracts where cortical mapping and subcortical mapping, respectively, have been found to be very effective. The low-frequency bipolar stimulation protocol has long been used for this purpose.[1] With the introduction of monopolar high-frequency stimulation, it has become widespread for cortical and subcortical stimulation allowing even continuous monitoring of motor tracts and permits objective evaluation of the elicited response by way of recorded MEPs.[7] This may actually be a better alternative to the low-frequency technique in pediatric population where because of immature myelination of central tracts, responses may be unpredictable. Though limited, experience with high-frequency monopolar stimulation in pediatric cases has shown that it is safe and feasible and can be reliably used when coupled with other complementary techniques such as phase reversal.[3] During resection of tumors around the central motor areas, besides mapping the cortex, it is extremely important to preserve the descending fibers of the corticospinal tract. Subcortical mapping is therefore very crucial. A combination of mapping and continuous monitoring is usually preferred. Typically, this involves intermittent stimulation of the advancing resection cavity edge, alternating with debulking of the tumor bulk in a repetitive manner. This intermittent stimulation technique (even with continuous MEP monitoring) assumes that in between two consecutive trials of subcortical stimulation, the surgical “act” will not impart any structural damage.[8] If such damage does occur, it may not be reversible. Thus, it is desirable that this mapping of the “approaching” subcortical white matter tracts be done almost continuously. However, this is very tedious and entails very frequent interruption of the surgical resection that is not surgeon friendly. To overcome this limitation, Raabe et al.[4] described a novel technique using a modified suction tip that doubles up as a monopolar stimulator, providing continuous stimulation (at a rate of almost 0.5–1 Hz) as the surgery proceeds. Because the suction tip is almost always at the advancing edge of the resection cavity, it ensures near-continuous stimulation without interrupting the surgical procedure. This technique relies on the concept of MTs whereby an almost linear relationship is postulated to exist between the strength of the stimulating current and the distance from the motor fibers (1 mA = 1 mm). Simply put, positive responses obtained at 10 mA current (MT) imply a distance of 10 mm from the motor fibers, 9 mA imply 9 mm, 8 mA imply 8 mm, and so on. Therefore, by progressively reducing the strength of the current (MT), it is possible to gauge the distance from the motor fibers and tailor the resection safely. Whereas this is also possible to judge using navigated tractography images, they may be inaccurate in the event of brain shift, which is often the case. The experience with this suction monopolar device is reported only in adult cases. Ours is the first case in a child. We have additionally used this technique in 20 other adult cases (unpublished data). A similar experience in pediatric tumors using an electrified suction aspirator has been recently described by Roth et al.[9] They used a modified aspirator tip as a stimulator. In our experience, a suction monopolar device is more favored as the suction tip is usually a constant instrument in one hand whereas the other hand may use many other instruments (including the aspirator) interchangeably. We used the same suction monopolar device as described by Raabe et al. but connected to another equipment (NIM Eclipse [Medtronics]). The device was compatible and worked well. Modifications of the software to incorporate a dual auditory feedback for recorded MEPs (as described originally by Raabe et al.) would improve the surgeon experience and make the technique more effective.
CONCLUSION
We report the first case of using a suction monopolar device for continuous dynamic subcortical mapping of motor tracts in a child. The technique is easy to use and feasible and provides a reliable real-time guide for the proximity to eloquent motor fibers.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this article.
REFERENCES
- 1.Berger MS. The impact of technical adjuncts in the surgical management of cerebral hemispheric low-grade gliomas of childhood. J Neurooncol. 1996;28:129–5. doi: 10.1007/BF00250195. [DOI] [PubMed] [Google Scholar]
- 2.Hervey-Jumper SL, Berger MS. Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol. 2014;16:284. doi: 10.1007/s11940-014-0284-7. [DOI] [PubMed] [Google Scholar]
- 3.Coppola A, Tramontano V, Basaldella F, Arcaro C, Squintani G, Sala F. Intra-operative neurophysiological mapping and monitoring during brain tumour surgery in children: An update. Childs Nerv Syst. 2016;32:1849–59. doi: 10.1007/s00381-016-3180-5. [DOI] [PubMed] [Google Scholar]
- 4.Raabe A, Beck J, Schucht P, Seidel K. Continuous dynamic mapping of the corticospinal tract during surgery of motor eloquent brain tumors: Evaluation of a new method. J Neurosurgery. 2014;120:1015–24. doi: 10.3171/2014.1.JNS13909. [DOI] [PubMed] [Google Scholar]
- 5.Giordano M, Arraez C, Samii A, Samii M, Di Rocco C. Neurosurgical tools to extend tumor resection in pediatric hemispheric low-grade gliomas: iMRI. Childs Nerv Syst. 2016;32:1915–22. doi: 10.1007/s00381-016-3177-0. [DOI] [PubMed] [Google Scholar]
- 6.Moiyadi AV, Shetty P, Degaonkar A. Resection of pediatric brain tumors: Intraoperative ultrasound revisited. J Ped Neuroscs. 2017;12:19–23. doi: 10.4103/jpn.JPN_141_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taniguchi M, Cedzich C, Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: Technical description. Neurosurgery. 1993;32:219–26. doi: 10.1227/00006123-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Seidel K, Beck J, Stieglitz L, Schucht P, Raabe A. The warning-sign hierarchy between quantitative subcortical motor mapping and continuous motor evoked potential monitoring during resection of supratentorial brain tumors. J Neurosurgery. 2013;118:287–96. doi: 10.3171/2012.10.JNS12895. [DOI] [PubMed] [Google Scholar]
- 9.Roth J, Korn A, Bitan-Talmor Y, Kaufman R, Ekstein M, Constantini S. Subcortical mapping using an electrified Cavitron Ultrasonic Aspirator in pediatric supratentorial surgery. World Neurosurg. 2017;101:357–64. doi: 10.1016/j.wneu.2017.02.023. [DOI] [PubMed] [Google Scholar]