Abstract
Objective
Otogenic brain abscesses are one of the most significant life‐threatening complications of otologic infections. Given their low prevalence, otogenic brain abscesses require a high index of suspicion for diagnosis. In this systematic review, we aim to provide an analysis of otogenic brain abscesses and describe common clinical signs and symptoms, bacteriology, location, treatment options, morbidity, and mortality.
Data Sources
PubMed, Cochrane CENTRAL database, Google Scholar, and Scopus.
Methods
A systematic review of literature was performed using the Preferred Reporting Items for Systematic Reviews and Meta‐analyses recommendations. Variables assessed included clinical signs and symptoms, bacteriology, location, treatment, morbidity, and mortality.
Results
Twenty‐nine studies met inclusion and exclusion criteria, corresponding to a total of 1307 otogenic abscess cases for review. Fifty‐five percent of abscesses were found in the temporal lobe and 28% in the cerebellum. Most patients (88.3%) had a history of suppurative chronic otitis media. The most common symptoms were headache, altered mental status, papilledema, and meningeal irritation. Fever, nausea, and vomiting affected about 40% of patients. The most commonly cultured bacterial species was Proteus mirabilis. In addition to antibiotics, most otogenic brain abscesses were treated by burr hole aspiration. Average mortality following advent of computed tomography was 8.11%.
Conclusion
Although rare, otogenic brain abscesses may occur as a complication of suppurative otitis media and require a high index of suspicion. Appropriate imaging studies and multidisciplinary expertise are crucial in the diagnosis and management.
Level of Evidence
4.
Keywords: Brain abscess, otologic infection, magnetic resonance imaging, computed tomography
INTRODUCTION
Otitis media (OM) is a common otologic condition in pediatric and adult populations. OM is typically classified into acute otitis media (AOM) chronic otitis media (COM) and otitis media with effusion (OME).1 Chronic suppurative otitis media (CSOM) is a subtype of COM characterized by persistent drainage from the middle ear associated with a perforated ear drum, with or without cholesteatoma.2, 3 CSOM affects 65–330 million people worldwide, mainly in developing countries and has been estimated that there are 31 million new cases of CSOM per year, with 22.6 % in children less than 5 years old.4 Similar to AOM and OME, CSOM has a profound impact on society in terms of hearing, but also has increased morbidity and mortality due potential for life‐threatening complications.5
Although rare, complications of OM are commonly encountered given its high prevalence. Complications of OM are classified as extracranial or intracranial. Brain abscess are commonly considered the second most common intracranial complication of OM after meningitis (Fig. 1).6 Historically, it has been reported that 25% of brain abscesses in children were otogenic, whereas in adults it is thought that more than 50% of brain abscesses were otogenic.7, 8 The development of antibiotics and the availability of advanced imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI) have decreased the incidence and mortality of otogenic brain abscesses over the past two decades, particularly in developed nations.6
Figure 1.
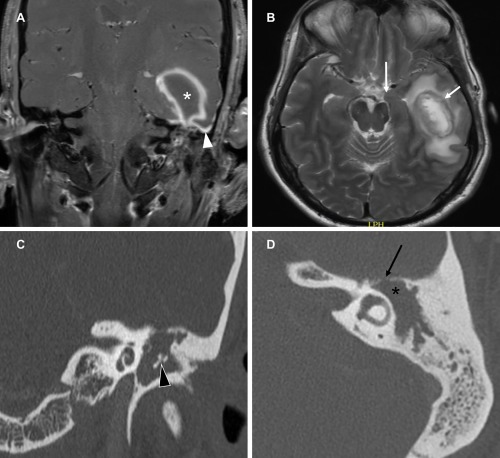
Coronal T1 fat‐suppressed post gadolinium (A) and axial T2 (B) MR images demonstrate an intra‐axial left temporal lobe peripherally enhancing lesion (white asterisk), adjacent dural enhancement (white arrowhead) and a peripheral rim of T2 hypointense signal (short white arrow). There is surrounding edema resulting in uncal herniation (long white arrow). On coronal (C) and axial (D) CT, there is a soft tissue mass in the left middle ear and mastoid with erosion of the middle ear ossicles (black arrowhead), expansion of the aditus ad antrum (black asterisk) and erosion of the tegmen (black arrow).
Despite its rarity, otogenic brain abscesses are still encountered in the developed world. Few large‐scale reports have investigated common features of otogenic abscesses. Most reports on otogenic brain abscess derive from single‐center case series. In this systematic review, we aim to provide an analysis of otogenic brain abscesses and describe common clinical signs and symptoms, bacteriology, location, treatment, morbidity, and mortality. A better understanding of clinical features of otogenic brain abscess may assist in improved diagnosis and management.
METHODS
Search Strategy
A review of the literature was conducted to comprehensively identify articles related to otogenic brain abscesses. We used the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) checklist and statement recommendations as a guide to this qualitative systematic review. In October 2016, we searched PubMed, Cochrane CENTRAL database, Google Scholar, and Scopus for relevant publications for all available dates. A principal electronic search strategy was designed for use in PubMed and then tailored for the other electronic databases. The initial search combined key terms related to complications of otitis media, brain abscess, and the following Medical Subject Headings (MeSH terms): “Abscess, Brain” (MeSH Terms), “Ear, Middle” (MeSH Terms), “otitis media, suppurative” (MeSH Terms), and “Brain abscess, pyogenic” (MeSH Terms). Additional publications were identified by reviewing the reference lists of articles in the search described above.
Inclusion and Exclusion Criteria
We sought to examine studies that specifically addressed otogenic brain abscesses to determine clinical features, treatment, and outcomes. The patient population addressed included adult and pediatric individuals with an otogenic brain abscess. As outlined below, we attempted to include all forms of study design. Papers must have been available in the English language with a title, abstract, and full manuscript available. All study types, including case reports and case series, were included. Articles were then reviewed to confirm a focus on otogenic brain abscess. Full publications were obtained following initial selection of titles and abstracts. Two investigators (E.D.K., M.D.) independently reviewed articles for inclusion and exclusion criteria.
Study Extraction, Categorization, and Analysis
Articles were assessed for variables including study size, location, setting, main outcome measures (as described above), and conclusions. Data was subsequently extracted and compiled in an electronic data extraction form. In the event of uncertainty, two independent investigators (E.D.K., M.J.D.) discussed the relevant finding and determined an outcome based on consensus.
Level and Quality of Evidence
To assess the level of evidence, studies were categorized based on the 2011 Oxford Centre for Evidence‐based Medicine‐Levels of Evidence.9, 10 Level 1 was defined as a systematic review of randomized trials; Level 2 was defined as a randomized trial or observational study with dramatic effect; Level 3 was defined as a nonrandomized controlled cohort/follow‐up study; Level 4 was defined as a case‐series, case‐control, or historically controlled study.
To assess the quality of each study, a 14‐item checklist was generated a priori, assessing research design, patient selection, and presentation of outcome data (Table 1). Criteria were chosen based on previous systematic reviews. Studies were assigned one point based on each listed item. Studies with a total score of 10 to 14 points were considered “high quality”; studies with a score of 6 to 9 were considered “moderate quality”; and studies with a score of 0 to 5 were considered “low quality.”
Table 1.
Fourteen‐Item Checklist to Assess Methodological Quality of Studies.
|
1. Clear description of research design, eg, observational, prospective, or retrospective 2. Well‐defined description of consecutive samples 3. Well‐formulated inclusion and exclusion criteria 4. Demographics of population described 5. Location and extent of disease described, when appropriate 6. Chronicity of symptoms included 7. Otologic comorbidities included 8. Prior treatments noted 9. Diagnostic criteria described 10. Microbiology included 11. Follow‐up rate and length described 12. Treatment described 13. Complications addressed 14. Mortality included |
Statistical Analysis
Data on overlapping patients from different studies published by the same authors were determined. Descriptive analysis was performed. Statistical analysis was performed using SPSS (version 22.0; Chicago, IL, U.S.A.).
RESULTS
Study Selection
The primary search strategy initially identified 105 articles. The study selection process is illustrated in Figure 2. Studies were excluded for several reasons: 35 were duplicates, 32 had titles or abstract indicating irrelevance to study, and 9 were not available in English, had overlapping patients, and/or had discussion of brain abscess without clear description of etiology. After the above screening, the total number of articles that met inclusion and exclusion criteria was 29.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 Year of publication ranged from 1960 to 2016. The country of origin of papers included Brazil, China, England, France, India, Israel, Scotland, South Africa, Turkey, Thailand, Taiwan, and the United States. Of the papers reviewed, 45% (13 of 29) of papers discussed only otogenic abscesses, corresponding to 601 abscesses. Fifty one percent (15 of 29) of papers described brain abscesses of multiple origins and included a total of 701 otogenic abscesses. Most papers reviewed (23 of 29) were of high quality based on previously described criteria. A complete list of reviewed articles is available from the authors upon request.
Figure 2.
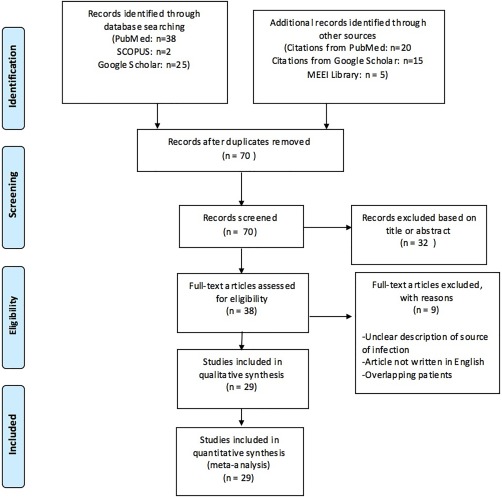
Flowchart demonstrating the study selection process, following the established Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) recommended guidelines.
Patient Demographics
The mean number of abscess cases reviewed per paper was 134 (range: 4–973). Of papers that evaluated all types of brain abscess (n = 15), 31% (701 of 2245) were otogenic. Specific ages were not given in most studies on otogenic brain abscess. Where ages were available, patients ranged from 2 months to 76 years of age. A qualitative review of data indicates most patients with otogenic brain abscess were pediatric patients. Gender was not routinely quantified. In papers where gender was available, gender ranged from 60% to 78% male. (Table 2)
Table 2.
Studies Meeting Inclusion and Exclusion Criteria.
| Publication | Date Range | Design, Evidence Level, Quality | n (abscesses) | n (otogenic abscesses) | Age | Gender |
|---|---|---|---|---|---|---|
| Yang, 1981 | 1952–1972 | RCR, III, mod | 400 | 263 | 85% under 65 y | 71% M |
| Bradley et al., 1984 | 1950–1979 | RCR, III, high | 139 | 139 | 4.5–76 y | 2.5:1 M:F |
| Keet et al., 1990 | 1952–1986 | RCR, III, mod | 641 | 233 | Not specified | Not specified |
| Samuel et al., 1986 | 1978–1983 | RCR, III, mod | 53 | 53 | 75% under age 15 y | 59% M |
| Nahoo et al., 2011 | 1983–2002 | RCR, III, high | 973 | otorhinogenic: 369 | Not specified | 74.2% M, (for all abscesses) |
| Rupa and Raman,1991 | 1981–1989 | RCR, III, mod | 27 | 27 | 83.6% under 25 y | 2:1 M to F |
| Beller et al., 1973 | 1941–1971 | RCR, III, high | 89 | 27 | 2 mo–70 y; 43% under 15 y | 60 M |
| Sichiza et al., 2005 | 1993–2003 | RCR, III, high | 121 | 25 | Not specified | 101 M |
| Carey et al., 1972 | 1946–1965 | RCR, III, mod | 86 | Sinus or mastoid: 25 | Not specified | Not specified |
| Kangsanarak et al, 1995 | 1978–1990 | RCR, III, high | 29 | 29 | 60% of otogenic abscess patients were between 11–20 y | Not specified |
| Lakshmi et al. 1993 | 1987–1991 | RCR, III, mod | 50 | 25 | 1–55 y | 41 M |
| Pennybacker et al., 1961 | 1960 | RCR, III, high | 85 | 85 | Not specified | Not specified |
| Dawes et al., 1960 | 1944–1960 | RCR, III, mod | 30 | 30 | 0–80; greatest amount 10–20 y | Not specified |
| Morgan et al., 1973 | 1946–1971 | RCR, III, high | 88 | 21 | 2 mo–69 y; most between 10–20 y | 61 M |
| Lavin et al., 2016 | 2009 | RCR, III, low | 37 | 37 | Not specified | Not specified |
| Penido et al., 2005 | 1987–2002 | RCR, III, high | 26 | 26 | 6 mo–79 y; 66% of patients younger than 25 y | |
| Prasad et al., 2006 | 1997–2004 | Prospective cultures/RCR, III, high | 118 | 37 | 3 mo–63 y (for all abscesses) | 95 M (for all abscesses) |
| Nunez and Browning, 1990 | 1976–1986 | RCR, III, mod | 517 | 44 | 0–80; equally distributed | 31 M |
| Newlands, 1965 | 1953–1962 | RCR, III, high | 80 | 80 | 7–80 y | 63 M |
| Sennaroglu and Sozeri, 2000 | 1968–1999 | RCR, III, high | 41 | 41 | 65% between 5–15 | 27 M |
| Brand et al., 1984 | 1962–1982 | RCR, III, high | 17 | 15 | 8–65y | 12 M |
| Osma et al., 2000 | 1990–1999 | RCR, III, high | 10 | 10 | 7–49 y, 58% under age 20 | 67% M |
| Yen et al., 1995 | 1981–1994 | RCR, III, high | 93 | 19 | 1 mo–79 y (for all abscesses) | 3.1:1 M to F |
| Chun et al., 1986 | 1970–1983 | RCR, III, high | 45 | 7 | 5–79 y (for all abscesses) | 33 M (for all abscesses) |
| Carpenter and Holliman, 2007 | 2000–2004 | RCR, III, high | 49 | 4 | 8–77 (for all abscesses) | 1.7:1 M to F (for all abscesses) |
| Kao et al., 2003 | 1991–2001 | RCR, III, high | 53 | 8 | 19–70 y | 5 M |
| Barry et al., 1999 | 1977–1995 | RCR, III, high | 4 | 3 | Not specified | Not specified |
| Murthy et al., 1991 | 1984–1990 | RCR, III, high | 10 | 10 | 6–15 y | 2 M |
| Sun and Sun, 2013 | 1996–2012 | RCR, III, high | 9 | 9 | For all patients with complicated OM: 12–62 y | For all patients with complicated OM: 12 M 5 F |
Most studies focused on brain abscesses over a certain time period, of which a subset were otogenic in origin. There were some that focused on only otogenic abscesses and some that focused on complications of ear disease. All study designs were Retrospective Cohort Studies (RCR). Corresponding levels of evidence are based on the Centre for Evidence‐Based Medicine, Oxford, where III‐IV represents cohort studies. A level of evidence of IV is assigned to poor quality cohort studies, based on available data and design. The quality of evidence is based on a 14‐item checklist was generated a priori, taking into account research design, patient selection, and presentation of outcome data (Table 1). Criteria were chosen based on previous systematic reviews. Studies were assigned one point based on each listed criteria. Studies with a total score of 10 to 14 points were considered “high quality”; studies with a score of 6 to 9 were considered “moderate quality”; and studies with a score of 0 to 5 were considered “low quality.” M, Male; F, Female; y, years.
Location and Symptoms of Otogenic Brain Abscess
Of papers identifying otogenic comorbidities in patients presenting with brain abscess (n = 21; 1046 otogenic abscesses), all stated that patients most commonly also suffered from suppurative chronic otitis media, with a prevalence of 43% to 100% (mean 88.3% ± 15.9). Seven studies also explicitly mentioned the prevalence of cholesteatoma, ranging from 21–100% (mean 75% ± 30.2). Two studies, Chun et al. and Fernandes et al., also identified mastoiditis as prevalent in 71% and 62%, respectively, of patients with otogenic brain abscess.
Most papers specifically addressed the intracranial location of abscesses of otogenic infection. Out of 1302 total otogenic abscesses, 55% (n = 722) were found in the temporal lobe and 28% (n = 369) were in the cerebellum. Other locations mentioned included the frontal and parietal lobes, which were implicated in 5% (n = 66). A total of 156 out of 1302 abscesses did not have an identified location. Across papers, the range of otogenic abscesses in the temporal lobe comprised 11% to 90% (median 57.5%), while otogenic abscesses in the cerebellum ranged from 4% to 80% (median 21.5%) (Fig. 3, Table 3).
Figure 3.
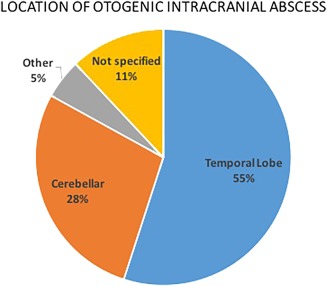
Location of otogenic intracranial abscesses across all studies. The location of 905 out of 1302 total otogenic abscesses was specified. Most were located in the temporal lobe (n = 722, 55% of total) or cerebellum (n = 369, 28% of total). “Other” includes frontal lobe, parietal lobe and subdural locations (n = 66, 5% of total). The location of 145 otogenic abscesses was not specified (11%).
Table 3.
Location of Otogenic Abscesses.
| Publication | n (otogenic) | Temporal lobe n (%) | Cerebellar lobe n (%) | Other n (%) |
|---|---|---|---|---|
| Yang, 1981 | 263 | 168 (42%) | 112 (28%) | 50 (12.5%) |
| Keet et al., 1990 | 233 | 140 (60%) | 70 (30%) | |
| Bradley et al., 1984 | 139 | 76 (54%) | 39 (25%) | |
| Pennybacker et al., 1961 | 85 | 55 (65%) | 30 (35%) | |
| Newlands, 1965 | 80 | 52 (65%) | 25 (31%) | 3 (3.75%) |
| Fernandes et al., 1986 | 53 | Not specified | 10 (18%) | |
| Sennaroglu and Sozeri, 2000 | 41 | 44 (22%) | 8 (18%) | |
| Nunez and Browning, 1990 | 41 | 29 (70%) | 19 (46%) | |
| Prasad et al., 2006 | 37 | Not specified | Not specified | |
| Lavin et al., 2016 | 37 | Not specified | Not specified | |
| Dawes et al., 1960 | 30 | Not specified | Not specified | |
| Kangsanarak et al., 1995 | 29 | 20 (84%) | 9 (31%) | |
| Sichiza et al., 2005 | 27 | 23 (84%) | 4 (16%) | |
| Rupa and Raman,1991 | 27 | 12 (44%) | 15 (55%) | |
| Beller et al., 1973 | 27 | 14 (52%) | 4 (16%) | |
| Penido et al., 2005 | 26 | Not specified | Not specified | |
| Lakshmi et al., 1993 | 24 | 6 (24%) | 4 (16%) | 12 (49%) |
| Morgan et al., 1973 | 21 | 19 (90%) | Not specified | |
| Yen et al., 1995 | 19 | 31 (11%) | 1 (6%) | |
| Brand et al., 1984 | 15 | 10 (66%) | Not specified | |
| Osma et al., 2000 | 10 | 9 (90%) | 1 (10%) | |
| Murthy et al., 1991 | 10 | 2 (20%) | 8 (80%) | |
| Sun and Sun, 2013 | 9 | 5 (55%) | 5 (44%) | |
| Kao et al., 2003 | 8 | 4 (50%) | 1 (12.5%) | 1 (12.5%) |
| Chun et al., 1986 | 7 | 3 (43%) | 4 (57%) | |
| Carpenter and Holliman, 2007 | 4 | Not specified | Not specified | |
| Barry et al., 1999 | 3 | Not specified | Not specified | |
| Total otogenic: 1302 | Total Temporal lobe: 722 (55%) | Total Cerebellar: 369 (28.3%) | Total Other: 66 (5%) |
Throughout all studies, the most common symptoms of patients with intracranial abscess secondary to ear disease were headache (82.5% of pooled patients) and altered mental status (75%). Meningeal irritation was present in 52.9% of patients, and papilledema was seen in 66.9% of those examined. Fever was identified in 35.5% of patients, and nausea/vomiting identified in 42.3%. Purulent otorrhea was mentioned in four studies, ranging from 5.7% to 92% of patients.19, 30, 34, 39 Seizure was present in 24.2% of patients, and hemiparesis affected 42.3%. Focal neurological signs and seizures were associated with larger and more advanced abscesses and were reported most often in older studies that predated the use of CT scan (1977) for diagnosis (Fig. 4).
Figure 4.
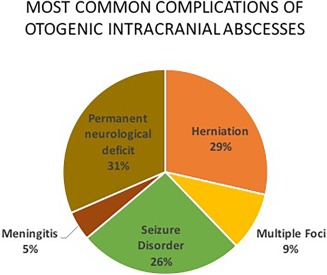
Complications: 12 of 18 studies specified complications in patients with otogenic abscesses. Out of the 681 otogenic abscesses covered in those studies, 238 (35%) were cases with complications. The figure demonstrates the most common complications patients suffered.
Imaging and Bacteriology
In the CT era (commonly defined as after 1977)21, all reported abscesses were diagnosed with CT. Prior to CT, angiography was used in most cases. There were few reports of MRI diagnosis.15 Of 29 studies, 14 described the specific bacteriology of otogenic abscesses (48%). The most commonly isolated bacteria was Proteus mirabilis (79%, 11 of 14) (Fig. 5, Table 4). There was one report of undefined Streptococci species, and two reports of multiple isolates: one with P. mirabilis and Streptococcus species and one with Streptococcus and Staphylococcus species. In addition, there were other reports of Staphylococcus aureus, and Streptococci including milleri and viridans species; however, these reports did not note specific abscess location.
Figure 5.
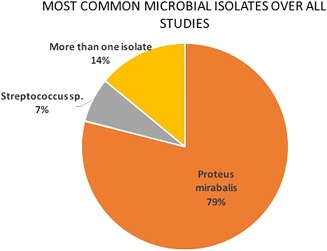
Microbiology. Microbiology data was available from 14 studies that specified a total of 16 common isolates from otogenic intracranial abscesses. Eleven of 14 (78.5%) of studies had P. mirabilis as the most common isolate while 1 of 14 (7.1%) specified Streptococcus species as the most common isolate. Two studies had more than one common isolate: one with P. mirabilis + Streptococcus species and one with Streptococcus and Staphylococcus species.
Table 4.
Microbiological Isolates in Intracranial Otogenic Abscesses Across All Studies.
| Publication | Primary isolate |
|---|---|
| Yang, 1981 | Proteus spp. and Streptococcus spp. |
| Newlands, 1965 | Proteus spp |
| Sennaroglu and Sozeri, 2000 | Proteus mirabilis |
| Kangsanarak et al., 1995 | Proteus mirabilis |
| Rupa and Raman,1991 | Proteus mirabilis |
| Sichiza et al., 2005 | Proteus mirabilis |
| Morgan et al., 1973 | Streptococci and Staphylococi |
| Yen et al., 1995 | Proteus mirabilis |
| Sun and Sun, 2013 | Proteus mirabilis |
| Kao et al., 2003 | Proteus mirabilis |
| Nahoo et al., 2011 | Proteus mirabilis |
| Dawes et al., 1960 | Streptococcus pyogenes |
| Penido et al., 2005 | Proteus mirabilis |
| Barry et al., 1999 | Proteus mirabilis |
Surgical Management of Otogenic Brain Abscess
All the studies included in this review examined cases of brain abscess of otogenic origin, but many also included cases of brain abscess from other sources. A subset of papers (16 of 29) addressed treatment on otogenic abscesses specifically, and five of these provided detailed information on the number of cases undergoing each modality of treatment.13, 14, 25, 34, 38 Of the 16 studies that specifically examined otogenic brain abscess treatment, 13 addressed the treatment of the abscess itself. Eleven of these 13 papers (85%) mentioned burr hole aspiration as a treatment modality for temporal otogenic abscess, and of these 11 studies, 10 considered it first‐line treatment, with a range of 50% to 100% of patients undergoing burr hole aspiration. Burr hole aspiration was often coupled with antibiotic irrigation of the abscess cavity. In one study, Brand et al. (U.S.A., 1984), patients underwent craniotomy and burr hole aspiration at roughly equal proportions.14 Treatment for otogenic abscesses in the cerebellum was less clearly defined, but Bradley et al. (U.K., 1984) specified that excision was performed for cases of cerebellar abscess.13
Excision was also the most common second line treatment for temporal lobe abscesses.12, 13, 18, 38 All studies that addressed the topic described excision of the abscess cavity if repeated aspirations failed to control the abscess. Few papers quantified the number of patients undergoing excision as a second line treatment, but Yang et al. mentioned that 30% of cases initially treated with aspiration ultimately required excision, and Bradley et al. stated that 11% of aspiration patients required excision.13, 38 Other studies specified a rate of excision from 15% to 20%.16, 25 Several papers made a mention of excision without quantification and some stated that the treatment modality was per the neurosurgeon's choice. A final treatment mentioned in some studies was a “drainage procedure,” which could refer to burr hole aspiration, but was not further specified.22, 23, 33
The other important component of treating an otogenic brain abscess lies in controlling the source of infection, eg, middle ear. Sixteen of 29 papers mentioned control of the otogenic infection as part of the treatment of patients, and all described a mastoidectomy as the first line treatment. Eleven of 16 papers described the surgical approach in more detail. Radical mastoidectomy was the most common approach, described in 10 of 11 papers. One paper used a canal wall up or down mastoidectomy/tympanoplasty approach in most patients.14 A modified radical approach was used in a subset of patients (6.5%) in two studies.13, 29
While radical mastoidectomy was generally the treatment of choice for source control, the timing of the surgery differed between papers. Bradley et al. stated that in most cases, neurosurgical drainage was performed first and then mastoidectomy was performed at a second operation.13 Four patients in this cohort did receive concurrent surgeries due to severity of infection. Yen et al. (Taiwan, 1995) followed a similar approach, in which all patients that underwent mastoidectomy had it performed after an initial neurosurgical procedure.39 Sun et al. (China, 2013) reported that abscess drainage and mastoidectomy were performed simultaneously, and that all patients underwent these procedures within 24 hours of admission.36 Similarly, Nathoo et al. (South Africa, 2011) reported that in all cases, mastoidectomy was performed at the same setting as the neurosurgical intervention.26 Other studies reported sequential surgeries versus simultaneous surgeries based on the patient presentation.13, 38
Complications and Mortality
Major complications of otogenic brain abscesses included meningitis, herniation, need for repeat surgical drainage, and death. Long‐term complications included epilepsy (across all studies, a range of 11–48%) and permanent neurological deficits (5–33%), including aphasia, visual disturbances, ataxia, hemiparesis, and facial nerve damage. Several reports documented mortality from otogenic brain abscesses. There was a wide range from 0% mortality to 53% mortality, with a sharp decrease in mortality after the advent of the CT scan for diagnosis in 1977. Studies that explicitly recorded mortality before 1977 (n = 12) had an average mortality of 34.4%, while studies that recorded mortality after 1977 (n = 8) had an average mortality of 8.11% (Table 5).
Table 5.
Complications and Mortality from Brain Abscesses Across All Studies
| Publication | Complications | Mortality | Other Complications |
|---|---|---|---|
| Yang, 1981 | Herniation in 25.8% of cases | 21% | None specified |
| Keet et al., 1990 | Not specified | 37% | None specified |
| Bradley et al., 1984 | 44% of patients undergoing radical mastoidectomy required further aural surgery after abscess decompressed due to persistent disease | 47.2% | Epilepsy (23%), ataxia (13.8%), hemiplegia (12%), visual disturbance (7%), dysphagia (7.7%) |
| Pennybacker et al., 1961 | 23% | Not specified | |
| Newlands, 1965 | 10% with concurrent meningitis | 275% | Not specified |
| Samuel et al., 1986 | Not specified | 36% | None specified |
| Nunez and Browning, 1990 | Not specified | 20% | Not specified |
| Sennaroglu and Sozeri, 2000 | 32% with concurrent meningitis | 29% overall; 45% before CT and 10% after CT | 31%of cases required revision surgery |
| Lavin et al., 2016 | Not specified | Not specified | Not specified |
| Prasad et al., 2006 | Not specified | Not specified | Not specified |
| Dawes et al., 1960 | 73% of abscesses with multiple foci | 50% | Not specified |
| Kangsanarak et al., 1995 | Not specified | 31% | Not specified |
| Rupaand Raman,1991 | Not specified | Not specified | None specified |
| Beller et al., 1973 | Not specified | 40% | Epilepsy (15%), Permanent neurologial deficit (33%), |
| Sichiza et al., 2005 | Not specified | 13% | Epilepsy (11%), hemiparesis (5%) |
| Penido et al., 2005 | 55% of cases developed more than one intracranial complication | Not specified | Not specified |
| Lakshmi et al., 1993 | Notspecified | Not specified | Not specified |
| Morgan et al., 1973 | Not specified | 36.4% | Seizures (48%), Severe neurological deficit (17%) |
| Yen et al., 1995 | Not specified | 5% | 5% permanent deficit |
| Brand et al., 1984 | Not specified | 26.6% (all before CT scan) | 6.6% incomplete aphasia, 6.6% facial nerve paralysis; 6.6% other permanent neurological deficit |
| Osma et al., 2000 | Not specified | 20% | Not specified |
| Murthy et al., 1991 | 30% with mastoiditis | 0% | 0 |
| Sun and Sun, 2013 | Not specified | 0% | 0 |
| Kao et al., 2003 | Not specified | 12.5% | |
| Chun et al., 1986 | Not specified | Not specified | Not specified |
| Carpenter and Holliman, 2007 | Not specified | 0% | 0 |
| Barry et al., 1999 | 100% concurrent meningitis | Not specified | Not specified |
| Nahoo et al., 2011 | Otic hydrocephalus developed in 6 patients and 13 patients required repeat surgery. | 29.4% | None specified |
| Carey et al., 1972 | Excessive bleeding (30%) | 53% | Epilepsy (26%) |
Table 6.
Treatment
| Publication | n | Treatment |
|---|---|---|
| Yang, 1981 | 263 | Repeated aspiration (44.2%), excision (32%), aspiration then excision (19%), drainage (2.5%) + antibiotics |
| Keet et al., 1990 | 233 | Burr hole aspiration (100%) + antibiotics |
| Bradley et al., 1984 | 139 | Aspiration (69%), late excision (8%), primary excision (6.4%), no surgical procedure (5%) + antibiotics |
| Pennybacker et al., 1961 | 85 | Burr hole aspiration + antibiotics |
| Newlands, 1965 | 80 | Burr hole aspiration + antibiotics |
| Fernandes et al., 1986 | 53 | Mastoidectomy; aspiration (100%) + antibiotics |
| Nunez and Browning, 1990 | 41 | Neurosurgery + antibiotics |
| Sennaroglu and Sozeri, 2000 | 41 | Mastoidectomy and abscess drainage (61%), burr hole aspiration (20%) craniotomy (15%) |
| Lavin et al., 2016 | 37 | Not specified |
| Prasad et al., 2006 | 37 | Burr hole aspiration + antibiotics |
| Dawes et al., 1960 | 30 | Burr hole aspiration + antibiotics |
| Kangsanarak et al., 1995 | 29 | Drainage, aspiration + antibiotics |
| Rupa and Raman,1991 | 27 | Surgical aspiration + antibiotics |
| Beller et al., 1973 | 27 | Drainage (7%), aspiration (40%), primary resection (32%), aspiration and resection (20%) + antibiotics |
| Sichiza et al., 2005 | 27 | Aspiration (87%), excision (13%) + antibiotics |
| Penido et al., 2005 | 26 | Surgical aspiration + antibiotics |
| Lakshmi et al., 1993 | 24 | Burr hole aspiration + antibiotics |
| Morgan et al., 1973 | 21 | For all brain abscesses: excision (31%), aspiration (66%) using thorostat + abx |
| Yen et al., 1995 | 19 | Neurosurgical management + antibiotics; radical mastoidectomy (63%) |
| Brand et al., 1984 | 15 | Neurosurgical management + antibiotics |
DISCUSSION
In this systematic review, we present an analysis of otogenic brain abscesses and describe common clinical signs and symptoms, bacteriology, location, treatment, morbidity, and mortality. Few large‐scale reports have investigated common features of otogenic brain abscesses. Our study has several notable findings. The most common presenting symptoms of otogenic brain abscess in the post‐CT era are fever, headache, and nausea. Of reported otogenic abscesses, 67% were found in the temporal lobe and 27% were in the cerebellum. Second, most identified patients suffered from suppurative chronic otitis media, with a prevalence of 43% to 100% amongst all papers. Eight studies also explicitly mentioned the prevalence of cholesteatoma, ranging from 21% to 100%.11, 19, 25, 29, 30, 33, 36, 39
The most common symptoms across all studies were headache, altered mental status, papilledema, and meningeal irritation. Patients presenting with otogenic abscess before the advent of CT imaging for diagnosis complained overwhelmingly of headache (97.5%).27 A majority had altered mental status (78%) and fever (54%), and 37% displayed signs of increased intracranial pressure such as vomiting.27 After CT imaging became a first‐line diagnostic tool, fewer patients presented with headache (35%), altered mental status (5%), and vomiting (4%).20, 25, 31, 39 This is likely due to earlier diagnoses and smaller abscesses. Thus, the most common presenting symptoms in the post‐CT era were fever (with a similar proportion to the pre‐CT era) at 44%, headache at 33%, and nausea at 18%.20, 25, 31, 39 A temporal bone and brain CT scan or MRI (Fig. 1) should therefore be considered in any patient with a history of chronic ear disease presenting with new‐onset fever, headache, and nausea, although it should be emphasized that only a minority of patients with otogenic brain abscess will present with any one of these symptoms. A condition with a potentially subtle presentation and a mortality rate of almost 10% requires a very high index of suspicion, and clinicians treating otologic infections should be alert to symptoms and the importance of imaging in making an early diagnosis.
Major complications of otogenic brain abscess included concurrent meningitis, brain herniation, and death. Long‐term complications included epilepsy, aphasia, visual disturbances, ataxia, hemiparesis, and facial nerve damage. The rate of complications in the pre‐CT era were much higher than the post‐CT era. Of studies that quantified long‐term complications in the pre‐CT era (n = 73 otogenic abscesses), 29% of patients (n = 21) experienced the development of a seizure disorder, and 18% (n = 13) experienced a permanent neurological deficit.12, 16, 17, 24 Studies that quantified complications after the advent of CT (n = 42 otogenic abscesses) recorded no patients with seizure disorder and only one patient with a long‐term neurological deficit.15, 25, 36, 39 Studies that explicitly recorded mortality after 1977 had an average mortality of 8.11%, compared to an average mortality of 34.4% prior to 1977.
Treatment of otogenic brain abscess was heterogeneous, but mostly consisted of intravenous antibiotics and surgical drainage via burr hole aspiration, craniotomy, or mastoidectomy (Table VI). In many of the papers, the specific approach was not well‐delineated. While some papers focused on the removal of the abscess and decompression of the intracranial cavity, others also emphasized the importance of source control and described approaches to mastoidectomy. Overall, treatment focused on surgery. While all patients across all studies received intravenous antibiotic therapy, very few of them (5% in Bradley et al.) underwent only medical treatment.13
While specific antibiotics were rarely mentioned in the included studies, antibiotics to empirically treat brain abscesses commonly target potential pathogens, including streptococci, anaerobic bacteria, staphylococci, and gram‐negative rods. Modern guidelines call for a third‐generation cephalosporin plus metronidazole, with consideration of adding another drug to provide coverage for methicillin‐resistant Staphylococcus aureus (MRSA). The duration of antibiotic treatment for brain abscess is usually at least 4 weeks.40
Interestingly, Proteus mirabilis was the most commonly isolated bacterial pathogen from brain abscesses of otogenic origin, although Proteus is not the most common middle ear pathogen. One possible reason for this is that in all papers the patients most commonly suffered from CSOM, in which bacteriology is known to differ from that of AOM.41, 42 Moreover, the gram‐positive organisms are usually more often sensitive to the most commonly prescribed antibiotics for OM, leading to an increase of gram‐negative organisms. Some older studies have also posited that more virulent flora, such as Streptococcus pneumoniae, lead to a more fulminant suppurative meningitis, while species such as Proteus and Pseudomonas are more likely to be associated with a more indolent infection with bone erosion, chronic otitis media with or without cholesteatoma, and, rarely, intracranial abscess formation.43 Finally, anaerobic bacteria likely play a role in these infections, but are difficult to isolate due to challenges in culturing. Indeed, studies that include specific techniques to culture anaerobes, such as Hafidh et al., demonstrated that anaerobes were the most commonly cultured organism from otogenic abscesses.44 Unfortunately, little to no information is available on the antibiotic exposure patients had prior to collection of cultures, which may skew microbiological findings at the time of culture collection.
At our institution, otogenic brain abscess cases are treated via a team approach, following the algorithm described in Figure 6. In patients presenting with an otogenic infection and other “red flag” systemic symptoms such as fever, headache, altered mental status, nausea/vomiting, or any localizing neurologic signs, initial work‐up consists of labs, imaging, and cultures. If an abscess is identified on imaging, neurosurgical and otolaryngology consults are called for determination of urgent operative intervention. Empiric intravenous antibiotic therapy, such as Metronidazole and Ceftriaxone, is initiated, and a tympanostomy tube may be placed in the case of a nondraining ear.
Figure 6.
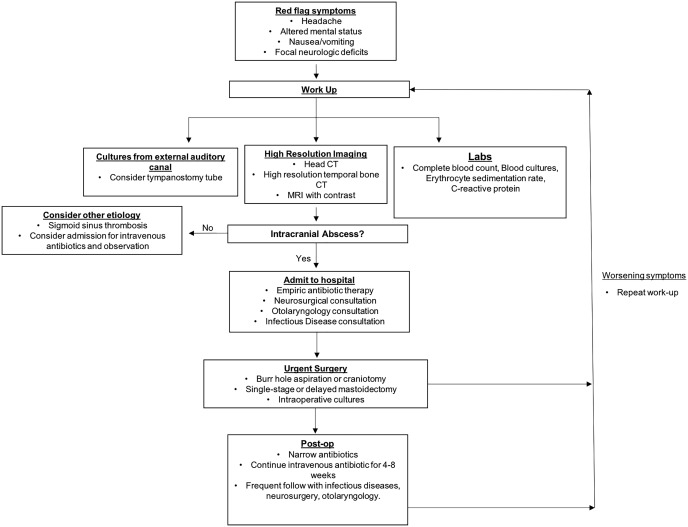
Treatment algorithm of otogenic brain abscesses at our institution.
Neurosurgical treatment of the abscess is first‐line for lesions 2.5 cm or larger. The specific method of abscess treatment (burr hole, stereotactic drainage, or open craniotomy) has been found to be less important than infection control and radiographic character of the lesion.44, 45 It has been proposed that abscesses less than 2.5 cm can be managed conservatively with antibiotics, though this is controversial.46 Removal of the abscess via excision is the first‐line surgical technique for superficial brain abscesses (as is often the case with an otogenic origin),8 encapsulated abscesses, or abscesses of the cerebellum. Otherwise, the abscess is stereotactically aspirated under image guidance (generally CT) via burrhole. If needle aspiration fails to decrease the size of the abscess, excision is performed. Cultures are taken at the time of aspiration. Coordination between otology and neurosurgical teams is key in the treatment of otogenic abscesses where source control must be obtained via mastoidectomy, in a single staged or delayed fashion, depending on availability and patient stability. In cases of clinical deterioration or neurological alteration, the brain abscess should be evacuated urgently by the neurosurgical team, and the mastoidectomy can follow as operative planning allows for source control. However, a combined surgical approach between the two teams when allowed by the clinical scenario is optimal. Intravenous antibiotic coverage is narrowed based on culture data and continued for four to eight weeks and follow‐up CT scans are used to ensure that the abscess has resolved. Prophylactic anticonvulsants can be used throughout and several weeks after hospitalization to prevent seizures.
There are several weaknesses of the present study. First, our search criteria may have missed articles that describe otogenic brain abscess. Our search criteria lead to the examination of papers over a 50‐year period, during which time the evolution in (MeSH terms) made identification of all studies involving otogenic abscess challenging. Second, there may have been bias introduced into our systematic review due to our inclusion and exclusion criteria. Non‐English articles may have yielded additional studies deserving of review, especially on the topic of operative otogenic brain abscesses. Negative findings may not have been published. Finally, many of the papers described herein have nonstandardized approaches to describing outcomes data. As part of the data analysis, best attempts were made to: 1) describe the study design, and 2) fit disparate data into comparable and interpretable datasets that may serve as a reference to the reader. We acknowledge that data reorganization is subject to author interpretation. To address this limitation, two authors independently reviewed papers for accuracy.
CONCLUSION
Although rare, otogenic brain abscess may occur as a complication of acute and chronic suppurative otitis media. Otolaryngologists should have a high index of suspicion for otogenic abscesses in patients with a history of chronic ear disease and new symptoms of fever, headache, and nausea. Where warranted, prompt imaging may aid in more rapid diagnosis and treatment of otogenic brain abscess.
ACKNOWLEDGMENTS
We would like to thank Louise Collins of The LeRoy A. Schall Library of Otolaryngology at the Massachusetts Eye and Ear Infirmary, Boston, MA.
Presentation: Study presented at the 2018 Triological Society in Scottsdale, Arizona (January 2018).
BIBLIOGRAPHY
- 1. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics 2016. doi: 10.1542/peds.2015-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wintermeyer SM, Nahata MC. Chronic suppurative otitis media. Ann Pharmacother 1994;28:1089–1099. [DOI] [PubMed] [Google Scholar]
- 3. Harkness P, Topham J. Classification of otitis media. Laryngoscope 1998;108:1539–1543. [DOI] [PubMed] [Google Scholar]
- 4. Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One 2012;7:e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yorgancilar E, Akkus Z, Gun R, et al. Temporal bone erosion in patients with chronic suppurative otitis media. B‐ENT 2013;9:17–22. [PubMed] [Google Scholar]
- 6. Kangsanarak J, Fooanant S, Ruckphaopunt K, Navacharoen N, Teotrakul S. Extracranial and intracranial complications of suppurative otitis media. report of 102 cases. J Laryngol Otol 1993;107:999–1004. [DOI] [PubMed] [Google Scholar]
- 7. Nicolosi A, Hauser WA, Musicco M, Kurland LT. Incidence and prognosis of brain abscess in a defined population: Olmsted County, Minnesota, 1935–1981. Neuroepidemiology 1991;10:122–131. [DOI] [PubMed] [Google Scholar]
- 8. Lu C, Chang W, Lui C. Strategies for the management of bacterial brain abscess. J Clin Neurosci 2006;13:979–985. [DOI] [PubMed] [Google Scholar]
- 9.Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM Levels of Evidency (Introductory Document). Oxford Centre for Evidence‐Based Medicine. http://www.cebm.net/index.aspx?o=5653.
- 10.Howick J, Chalmers I, Glasziou P, et al. The 2015 Oxford CEBM Levels of Evidency (Introductory Document). Oxford Centre for Evidence‐Based Medicine. http://www.cebm.net/index.aspx?o=5653.
- 11. Barry B, Delattre J, Vié F, Bedos J‐P, Géhanno P. Otogenic intracranial infections in adults. Laryngoscope 1999;109:483–487. [DOI] [PubMed] [Google Scholar]
- 12. Beller AJ, Sahar A, Praiss I. Brain abscess Review of 89 cases over a period of 30 years. J Neurol Neurosurg Psychiatry 1973;36:757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bradley PJ, Manning KP, Shaw MDM. Brain abscess secondary to otitis media. J Laryngol Otol 1984;98:1185–1192. [DOI] [PubMed] [Google Scholar]
- 14. Brand B, Caparosa RJ, Lubic LG. Otorhinological brain abscess therapy—past and present. Laryngoscope 1984;94:483–487. [DOI] [PubMed] [Google Scholar]
- 15. Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis 2006;26:1–11. [DOI] [PubMed] [Google Scholar]
- 16. Carey M, Chou S, French L. Experience with Brain Abscesses. J Neurosurg 1972;36:1–9. [DOI] [PubMed] [Google Scholar]
- 17. Chun CH, Johnson JD, Hofstetter M, Raff MJ. Brain abscess: a study of 45 consecutive cases. Medicine (Baltimore) 1986;65:415–431. [PubMed] [Google Scholar]
- 18. Pennybacker J, Dixon JW, Fulton Christie J, Dawes JD. Discussion on intracranial complications of otogenic origin. Proc Royal Soc Med 1961;54:309–320. [PMC free article] [PubMed] [Google Scholar]
- 19. Kangsanarak J, Navacharoen N, Fooanant S, Ruckphaopunt K. Intracranial complications of supparative otitis media: 13 years' experience. Am J Otol 1995;16:104–109. [PubMed] [Google Scholar]
- 20. Kao PT, Tseng HK, Liu CP, Su SC, Lee CM. Brain abscess: clinical analysis of 53 cases. J Microbiol Immunol Infect 2003;36:129–136. [PubMed] [Google Scholar]
- 21. Keet PC. Cranial intradural abscess management of 641 patients during the 35 years from 1952 to 1986. Br J Neurosurg 1990;4:273–278. [DOI] [PubMed] [Google Scholar]
- 22. Lakshmi V, Rao RR, Dinakar I. Bacteriology of brain abscess—observations on 50 cases. J Med Microbiol 1993;38:187–190. [DOI] [PubMed] [Google Scholar]
- 23. Lavin JM, Rusher T, Shah RK. Complications of pediatric otitis media. Otolaryngol Head Neck Surg 2016;154:366–370. [DOI] [PubMed] [Google Scholar]
- 24. Morgan H, Wood MW, Murphey F. Experience with 88 consecutive cases of brain abscess. J Neurosurg 1973;38:698–704. [DOI] [PubMed] [Google Scholar]
- 25. Murthy PSN, Sukumar R, Hazarika P, Rao AD, Mukulchand, Raja A. Otogenic brain abscess in childhood. Int J Pediatr Otorhinolaryngol 1991;22:9–17. [DOI] [PubMed] [Google Scholar]
- 26. Nathoo N, Nadvi SS, Narotam PK, van Dellen JR. Brain abscess: management and outcome analysis of a computed tomography era experience with 973 patients. World Neurosurg 2011;75:716–726. [DOI] [PubMed] [Google Scholar]
- 27. Newlands WJ. Otogenic brain abscess: a study of eighty cases. J Laryngol Otol 1965;79–130‐140. [DOI] [PubMed] [Google Scholar]
- 28. Nunez DA, Browning GG. Risks of developing an otogenic intracranial abscess. J Laryngol Otol 1990;104:468–472. [DOI] [PubMed] [Google Scholar]
- 29. Osma U, Cureoglu S, Hosoglu S. The complications of chronic otitis media: report of 93 cases. J Laryngol Otol 2000;114:97–100. [DOI] [PubMed] [Google Scholar]
- 30. Penido NDO, Borin A, Iha LCN, et al. Intracranial complications of otitis media: 15 years of experience in 33 patients. Otolaryngol Head Neck Surg 2005;132:37–42. [DOI] [PubMed] [Google Scholar]
- 31. Prasad KN, Mishra AM, Gupta D, Husain N, Husain M, Gupta RK. Analysis of microbial etiology and mortality in patients with brain abscess. J Infect 2006;53:221–227. [DOI] [PubMed] [Google Scholar]
- 32. Rupa V, Raman R. Chronic suppurative otitis media: complicated versus uncomplicated disease. Acta Otolaryngol 1991;111:530–535. [DOI] [PubMed] [Google Scholar]
- 33. Samuel J, Fernandes CMC, Steinberg JL. Intracranial otogenic complications: a persisting problem. Laryngoscope 1986;96:272–278. [DOI] [PubMed] [Google Scholar]
- 34. Sennaroglu L, Sozeri B. Otogenic brain abscess: review of 41 cases. Otolaryngol Head Neck Surg 2000;123:751–755. [DOI] [PubMed] [Google Scholar]
- 35. Sichizya K, Fieggen G, Taylor A, Peter J. Brain abscesses–the Groote Schuur experience, 1993–2003. S Afr J Surg 2005;43:79–82. [PubMed] [Google Scholar]
- 36. Sun J, Sun J. Intracranial complications of chronic otitis media. Eur Arch Otorhinolaryngol 2013;271:2923–2926. [DOI] [PubMed] [Google Scholar]
- 37. Loh S, Loh WS. Malignant otitis externa: an Asian perspective on treatment outcomes and prognostic factors. Otolaryngol Head Neck Surg 2013;148:991–996. [DOI] [PubMed] [Google Scholar]
- 38. Yang SY. Brain abscess: a review of 400 cases. J Neurosurg 1981;55:794–799. [DOI] [PubMed] [Google Scholar]
- 39. Yen PT, Chan ST, Huang TS. Brain abscess: with special reference to otolaryngologic sources of infection. Otolaryngol Head Neck Surg 1995;113:15–22. [DOI] [PubMed] [Google Scholar]
- 40. Arlotti M, Grossi P, Pea F, et al. Consensus document on controversial issues for the treatment of infections of the central nervous system: bacterial brain abscesses. Int J Infect Dis 2010;14:S79–S92. [DOI] [PubMed] [Google Scholar]
- 41. Orji FT, Ukaegbe O, Alex‐Okoro J, Ofoegbu VC, Okorafor IJ. The changing epidemiological and complications profile of chronic suppurative otitis media in a developing country after two decades. Eur Arch Otorhinolaryngol 2016;273:2461–2466. [DOI] [PubMed] [Google Scholar]
- 42. Brook I. The role of anaerobic bacteria in chronic suppurative otitis media in children: implications for medical therapy. Anaerobe 2008;14:297–300. [DOI] [PubMed] [Google Scholar]
- 43. McGovern F, Khuri A. Chronic otitis media and mastoiditis due to proteus vulgaris (Bacillus Proteus). AMA Arch Otolaryngol 1958;67:403–409. [DOI] [PubMed] [Google Scholar]
- 44. Hafidh M, Keogh I, Walsh R, Walsh M, Rawluk D. Otogenic intracranial complications. a 7‐year retrospective review. Am J Otolaryngol 2006;27:390–395. [DOI] [PubMed] [Google Scholar]
- 45. Sharma B, Gupta S, Khosla V. Current concepts in the management of pyogenic brain abscess. Neurol India 2000;48:105–111. [PubMed] [Google Scholar]
- 46. Harbaugh R, Shaffrey CI, Couldwell WT, Berger MS. Neurosurgery Knowledge Update: A Comprehensive Review. New York: Thieme; 2015. [Google Scholar]


