Abstract
Background
Virtual surgical planning (VSP), intraoperative cutting guides and stereolithographic models, provides the head and neck reconstructive surgeon with powerful tools for complex reconstruction planning. Despite its use in fibular osteocutaneous reconstruction, application to the scapular tip has not been as widely reported.
Methods
From 2013 to 2014, four cases of either mandibular or maxillary reconstruction were completed with the scapular tip osseous free flap. All four cases underwent preoperative VSP with patient‐specific guide design.
Results
Patient‐specific guides were generated for scapular tip harvest. Guide placement was improved using a stabilizing flange and bracket design. With minimal disruption of the overlying periosteum a wedge osteotomy was successfully implemented in one case.
Conclusions
Unlike the fibula and iliac crest donor sites, the scapular tip has overlying muscle attachments that make intraoperative osteotomies challenging. Attention to key aspects of scapular anatomy, including the fibrous tip and extensive overlying muscle, permits effective guide design.
Level of evidence
4
Keywords: Scapula, VSP, virtual, surgical, planning, guide
INTRODUCTION
Management of extensive head and neck defects that include both bone and soft tissue is a challenging reconstructive problem. Successful reconstruction aims to achieve restoration of both function as well as a normal appearance. The development of microvascular techniques, using osseous free‐tissue transfer, can provide these functional and aesthetic outcomes. The harvest techniques of anatomic sites that contain adequate bone stock have evolved since the first report of mandibular reconstruction.1 Historically, the iliac crest was first used for mandibular reconstruction; however, the introduction of the fibular free flap resulted in this osseous flap being adopted as the primary donor site for mandibular reconstruction due to excellent bone quantity and vascular reliability. The two donor sites provide excellent bone stock, but they have limitations with regard to the quantity and quality of cutaneous tissue available for reconstruction. The iliac crest skin paddle has an inconsistent perforator and therefore less reliable skin perfusion. Additionally, the iliac crest has been associated with significant donor site morbidity.2 While the fibula free flap reliably includes one or two excellent cutaneous perforators, they course through the septum, which is fixed along the lateral surface of the bone and restricts the rotation of the skin paddle. In addition, the fibula is limited in terms of the ability to harvest a larger soft tissue component. An alternative source for small segments of bone is the osteocutaneous radial forearm free flap (OCRFFF).3 Here a partial‐thickness portion of the radius is transferred with the forearm skin. This has been effective for maxillary reconstruction and short segments of the mandible, but carries the risk of soft‐tissue donor site morbidity and the potential for debilitating radius fractures.4 In addition, the volume of bone available with the OCRFFF is limited, although a double barrel technique has recently been reported.5
In order to address these issues, many reconstructive surgeons turn to the subscapular system for reconstructive alternatives. The scapular donor site is an excellent alternative when faced with complex defects requiring both bone and significant soft tissue requirements. It has many benefits, including limited donor site morbidity, large donor‐vessel diameter, and the ability to reconstruct defects that require both intraoral and external skin. The subscapular system can be designed to include multiple independent skin paddles with or without muscle, bone, and rib. This flexibility is unmatched by other donor sites. In addition, the scapular donor site has distinct benefits when reconstructing elderly patients because they can mobilize more quickly6 and the subscapular system is associated with less atherosclerotic disease when compared to peripheral extremity donor sites.7
Despite its versatility, the scapula remains under‐utilized for several reasons. Simultaneous harvest and ablation are not feasible, an unfamiliarity with the anatomy and a relatively short pedicle using the traditional harvest technique. There is also an under‐appreciation for the available bone. A comparative anatomical study, specifically addressing available bone stock as a criteria for dental implantability, demonstrated that the scapula had an average cross‐sectional area comparable to the iliac crest and could support implants in nearly 80% of the cadavers studied.8 This has been supported by clinical reports.9 The issue of pedicle length has been addressed with the technical modification which elongates the pedicle by using the angular branch off the thoracodorsal artery.10 This allows harvest of bone from the scapular tip and a significant length of lateral border.11, 12, 13 Finally, for novice surgeons, reconstructive design and execution can be challenging and time consuming due to unfamiliarity with the subscapular system.
The use of preoperative modeling to assist complex reconstruction requiring bone has gained significant momentum with greater commercial availability. Stereolithography, a rapid prototyping method available since the 1980s, generates a three‐dimensional (3D) model using data from a computed tomography (CT) scan. The accuracy of these models is well documented14 and this technology has been used in preoperative planning in a variety of craniofacial reconstructive settings.15, 16, 17, 18, 19 The use of computer‐aided planning and stereolithographic modeling in head and neck reconstruction has also increased as the technology has improved and the cost has decreased. Reconstructive surgeons now not only have the ability to virtually plan osteotomies, but also generate preoperative 3D models and cutting guides for use during the operation. In addition, patient‐specific allographic implants can also be designed to support the reconstruction.20 Long purported to save money, primarily as a result of decreased operative time, there is emerging evidence that supports this claim in experienced hands.21 There is no doubt that computer planning has offered the benefit of performing more complex reconstructions. Free‐hand osteotomies or the application of simple two‐dimensional (2D) cutting guides (tongue‐depressor, foam, rulers) are unable to anticipate the 3D nature of the reconstruction and requires both operative experience and intraoperative adjustments to achieve precise outcomes.
Here we present our initial experience demonstrating the use of VSP and donor‐specific cutting guides for reconstruction using the scapular angle osteomyocutaneous donor site. We highlight relevant learning points in guide design that stabilizes the guide placement, minimizes stripping of overlying periosteum and maximizes bone to bone contact for reconstruction.
METHODS
Four patients requiring a segmental mandibulectomy or partial maxillectomy, for which th fibular donor site was either not available or would necessitate an additional myocutaneous flap, were selected for virtual planning. The scapular angle osteomyocutaneous flap was chosen as the reconstructive option and preoperative CT scans were obtained of the head, neck, and chest.
Design Process and VSP
Preoperative CT scans were analyzed electronically with Synthes Proplan CMF (Synthes, West Chester, PA, U.S.A.), an interactive technology that can simulate skeletal osteotomies and reconstruction through 3D modeling. The DICOM axial CT images in 1‐ to 2.5‐mm slice thickness were imported into the software and a virtual 3D model of the mandible and scapula were created. Utilizing the software, the surgeon and support specialist met in a live, virtual planning session to mark out the resection margins and to decide on the number of bone segments and placement of the proposed reconstruction. The software then outlined the harvest margins of the scapula as well as the trajectory of the osteotomies. Prior to finalizing the plan a second virtual meeting took place where the proposed resection and harvest including osteotomies were reviewed and an overlay of the resection and reconstruction were approved. Surgical guides for the resection were then printed and sterilized. Each guide was custom designed with clear landmarks to ensure the preoperative planned osteotomies align with the operative osteotomies. Similarly, a surgical guide for the scapular harvest, with attention to the location of the scapular tip and anticipated muscular attachments, was printed and sterilized.
Flap Harvest
Exposure of the scapular subsystem and scapular tip has been described previously.13 Briefly, an axillary incision is made to expose the anterior border of the latissimus dorsi muscle. The dissection is then carried through subcutaneous fat and soft tissue to expose the thoracodorsal artery and serratus anterior branches of the system. These branches can then be traced proximally to determine the blood supply of the scapular tip and identify the angular branch. While the variability of the branching pattern has been previously described,10 the angular branch, supplying the scapular tip, is a relatively consistent finding regardless of the parent vessel (thoracodorsal or serratus anterior). Once the angular branch has been identified it is important to visualize the muscular attachments to the scapular tip. The teres major and infraspinatus muscles converge on the scapula and attach to the most distal portion of the scapular tip, which is somewhat cartilaginous in nature. At this point the bone is palpated and the muscles are incised along the lateral border of the scapula at the desired length for the planned osteotomy. One of the primary advantages of VSP planning is that the actual position of the highest quality bone stock is known preoperatively, via the planning session. The cutting guide positions of the osteotomy at that appropriate site despite the obscuring nature of overlying soft tissue, thereby avoiding harvesting inadequate lengths of the lateral border of the scapula. In addition, the inset of the flap is facilitated by VSP planning as harvesting a flap of excess osseous and soft tissue bulk is avoided and intraoperative adjustments to the flap are minimized.
RESULTS
Of the four cases described, three had oral cavity squamous cell carcinoma (SCCa) with mandibular invasion requiring segmental mandibulectomy. The fourth case demonstrates maxillary reconstruction following resection of an ameloblastoma. In all cases a virtual resection and reconstruction using the scapular tip donor site was completed using Proplan CMF software (Synthes, West Chester, PA, USA). Following completion of the virtual planning session cutting guides were manufactured for intraoperative use. Additionally stereolithographic models of the reconstructed defects were also used.
Case 1
A 65‐year‐old male presented with an erosive right lateral tongue SCCa, extending through the right mandible and involving the lateral cutaneous skin. The anticipated defect included internal oral cavity lining, mandibular bone, and external cheek. Reconstruction using the scapular tip was chosen to provide a single pedicle with two myocutaneous skin paddles and a scapular tip osseous component. The resection margins for the mandible were planned virtually and highlighted on the model (Fig. 1). Using this resection plan, medial and posterior measurements were determined for the scapula flap to match the resection defect. The medial measurements were 8.0 x 1.5 cm with a flap length of 7.1 cm. These margins were highlighted on the scapula model. To ensure a proper fit of the planned flap with the resection site, the software overlays the images for verification. Three guides were then created: one scapula and two mandible guides. Both guides had 2.2‐mm fixation holes suitable for a 1.5‐mm drill and 2.0‐mm screw for temporary fixation to the patient's bone. Two key adaptations were created for the scapular template (Figs. 1B and 1C). First, a cup was created to sit on the tip of the scapula. The cup was deigned to sit onto the scapular tip 1 cm off the bone to anticipate the fibrous tip of the scapula. Of note, this was later modified on subsequent plans, to 5 mm for an improved fit. Second, a thick curved flange was created to stabilize the guide on the lateral border. Intraoperative execution of the plan is depicted in Figure 2. The template fit reasonably well on the scapula and a postoperative panorex shows adaptation of the bone with union across the segments (Fig. 2D). Postoperatively, the patient had radiation and chemotherapy, exhibited excellent bony union on follow‐up imaging. The patient was unable to undergo implant restoration due to financial considerations.
Figure 1.

(A) 3D reconstruction of an erosive right mandibular SCCa with proposed single segment resection (green) and patient specific cutting guides (grey): Case 1. (B) 3D reconstruction of right scapula with proposed reconstructive segment (green) and patient specific cutting guides (grey). (C) Highlight of upper and lower cutting slots demonstrating the curved stabilizing piece (upper) and cup design with 1cm offset for fibrous tip (lower). 3D = three dimensional.
Figure 2.

Implementation of scapular tip reconstruction for Case 1. (A) Harvest of scapular tip with cutting guide in place. (B) Harvested flap with two musculocutanous paddles from the lastissimus and serratus muscles as well as the scapular tip. (C) Inset of flap. D. Panorex showing union of scapular tip with native mandible.
Case 2
A 50‐year‐old man, nine months status‐post a partial mandibulectomy and reconstruction with an iliac crest osteocutaneous free flap for an anterior floor of mouth SCCa, recurred at the anterior floor of mouth with tumor invading the osseous reconstruction. His initial reconstruction was with an iliac crest because he had suffered a motor‐vehicle accident with bilateral tibia and fibular fractures. Lower limb angiography demonstrated aberrant blood flow bilaterally, and the scapula was chosen for osseous reconstruction.
The virtual resection margins included the iliac crest flap and the defect involved what was originally the left body and symphysis (Fig. 3). In this case, two segments were planned to contour to the anterior mandibular arch. One segment was 3.9 cm and the other 3.6 cm. The scapular template was modified from the first case so that it could slide along the lateral border of the scapular tip, in case the fibrous portion was not exactly 1 cm. The stabilizing curved flange was maintained for guide stabilization (Fig. 3C). With the planned open osteotomy, free bone chips were used to fill in the gap. Postoperatively osseous healing was uneventful and the patient went on to receive adjuvant radiotherapy without incident.
Figure 3.
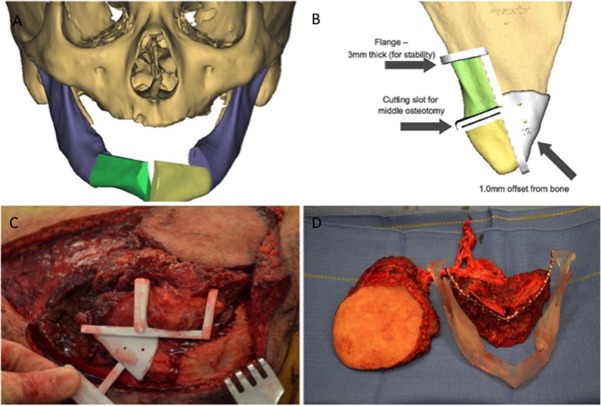
(A) 3D reconstruction of anterior mandibular defect with proposed two segments and open osteotomy. (B) Patient‐specific cutting guide with stabilizing flange and 1 mm offset from medial border. (C) Intraoperative placement of scapular tip cutting guide. (D) Comparison of harvested flap with stereolithographic model.
Case 3
A 63‐year‐old patient presented with an anterior floor of mouth SCCa eroding the mandible. He had significant peripheral vascular disease and absence of three‐vessel run‐off on CT angiography of the lower extremities. Similar to case 2, this patient required a central partial mandibulectomy with reconstruction requiring a middle osteotomy. Virtual planning was performed for the resection and reconstruction flap (Fig. 4). As opposed to using the patient's scapula for planning, as in case 1 and 2, a generic stock scapula was used for VSP. In this particular case, a wedge osteotomy was designed. Intraoperatively the recipient vessels were healthier in the contralateral neck and an adjustment was made to the proposed plan. The sliding scapular guide was used to compensate for the change in pedicle position. By trimming the tip it allowed for the pedicle to be reversed and the lower segment to plate to the Left mandibular body (Figs. 4B, 4C).
Figure 4.

(A) Virtual plan for Case 3 involving an anterior mandibular segment with wedge osteotomy. (B) Intraoperative inset of scapular tip. (C) Postoperative reconstruction of CT showing submental view. CT = computed tomography.
Case 4
A 41‐year‐old man presented with a left maxillary ameloblastoma posterior to the first pre‐molar. Resection margins extended from the infraorbital foramen superior medially, laterally to the zygoma and inferiorly through the alveolar ridge. The posterior hard palate and pterygoid plates were resected as well (Fig. 5). An isolated scapular tip was designed to reconstruct the maxillary defect. Two cutting guides were designed, one to guide the midface resection and the other to facilitate the left scapular harvest. In this case, there was no mucosal resection, so a bone‐only flap was used. The tip was secured with the lateral border attached to the maxilla. Two‐year post‐operative results demonstrate preservation of occlusion as well as midface projection (Fig.5B).
Figure 5.

(A) Proposed resection of left maxillary ameloblastoma (red) with proposed scapular reconstruction (blue). (B) Preoperative (upper left) and Postoperative images following reconstruction. (C) Planned guide design with cup overlying scapular tip.
DISCUSSION
The subscapular free flap system, originally described by dos Santos,22 provides unprecedented versatility to harvest cutaneous, muscular, and osseous components based on a single vascular pedicle. While the bony stock may be more limited then the iliac or fibula counterparts, the ability to reconstruct complex multidimensional defects with a single flap is unique to the scapular system. The growing experience with the angular branch as the osseous perforator has significantly improved pedicle length compared to the traditional parascapular flap. There is also evidence of less atherosclerotic disease in the scapular system compared to the lower limbs.7 Nevertheless, compared to the fibula and iliac crest, there is less experience in the number and length of osteotomies that can maintain a vascularized bone. The bulky muscular insertions to the scapula and the variable fibrous tissue at the tip can make calculating intraoperative osteotomies a time‐consuming and challenging process that may compromise the accuracy of the reconstruction. Virtual surgical planning as the potential to improve these results.
Traditionally, an intraoperative mandibular reconstruction plate is contoured and pre‐adapted prior to ablation. When the neoplasm prevents contouring, alternative methods are implemented including relying on occlusion of the contralateral dentition with mandibulo‐maxillary fixation, free hand proximal segment positioning, or a variety of external fixation systems. Virtual planning allows for contouring a mandibular reconstruction plate preoperatively based on the virtual model. There is accumulating evidence that this may be more efficient then traditional techniques, especially in complicated reconstructions.23, 24 In mandibular reconstruction, using the fibula, VSP can be accurate to the millimeter with less than 10 degrees of difference in segment positioning.25 Applying VSP for scapular reconstruction may have additional benefits, particularly when a surgeon has less familiarity with the available bone stock. Preoperative planning allows for identification of the thickest portion of bone, which is particularly important for osteointegrated dental implant placement.
In this initial experience, a number of learning points were appreciated (Table 1). First, a curved flange proved very helpful in stabilizing the guide to the scapula. This was used for all three cases of mandibular reconstruction. Second, a cup shaped guide can accommodate the overlying muscle, hug the scapular tip and allow for accurate osteotomies. Of note, the cup design was adjusted after the first case. In Case 1, the cup covered the entire tip and it was estimated that the fibrous component would be 1 cm. The design was modified to one that slides along the scapular tip with approximately a 5‐mm clearance. In doing so, the exact position of the osteotomy could be decided intraoperatively. This flexibility provides more precise osteotomies, despite the variable fibrous tip, and allows for the use of a standardized stock scapula when a chest CT of adequate resolution is unavailable (Case 3).
Table 1.
Case Characteristics With Defect, Reconstruction, and Learning Points.
| Case | Defect | Reconstruction | Learning points in guide design |
|---|---|---|---|
| 1 |
Right mandibular body, intraoral and external skin |
Right Scapular border (single bone segment) with two skin paddles |
• Anchoring guide with 1 cm offset to allow for fibrous portion of scapular tip (cup design later shortened to 0.5 mm for better adaptation) • Stabilizing flange |
| 2 |
Anterior Mandible, floor of mouth |
Left Scapular tip and lateral border (two segments with open osteotomy and packed bone chips |
• 0.5 mm offset from lateral border to account for overlying teres muscle • Stabilizing flange |
| 3 |
Left body and symphysis, floor of mouth |
Right Scapular tip and lateral border (two segments with wedge osteotomy) |
• Wedge osteotomy implemented • Generic stock scapula used with sliding guide design |
| 4 | Left maxilla (bone only) | Left Scapular tip | • Preoperative identification of optimal bone stock for future dental implants |
CONCLUSION
Virtual surgical planning can be performed for mandibular and maxillary reconstruction using the scapular tip. Patient‐specific guides can be created that assist with intraoperative osteotomies during scapular reconstruction. With attention to particular aspects of the scapular anatomy (variable fibrous tip, location of highest quality bone stock, and overlying musculature), predetermined osteotomies can be accomplished with minimal disruption of the overlying periosteum. This technology allows for a variety of configurations, including wedge osteotomies. The accuracy of virtually planned scapular reconstruction and the cost‐benefit analysis of this technology remain to be evaluated.
BIBLIOGRAPHY
- 1. Hayden RE, Mullin DP, Patel AK. Reconstruction of the segmental mandibular defect. Curr Opin Otolaryngol Head Neck Surg 2012;20(4):231–236. [DOI] [PubMed] [Google Scholar]
- 2. Valentini V, Gennaro P, Aboh IV, Longo G, Mitro V, Ialongo C. Iliac crest flap: donor site morbidity. J Craniofac Surg 2009;20(4):1052–1055. [DOI] [PubMed] [Google Scholar]
- 3. Davidson J, Boyd B, Gullane P, et al. A comparison of the results following oromandibular reconstruction using a radial forearm flap with either radial bone or a reconstruction plate. Plast Reconstr Surg 1991;88(2):201–208. [DOI] [PubMed] [Google Scholar]
- 4. Clark S, Greenwood M, Banks RJ, Parker R. Fracture of the radial donor site after composite free flap harvest: a ten‐year review. Surgeon 2004;2(5):281–286. [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez‐Castro J, Petrisor D, Ballard D, Wax MK. The double‐barreled radial forearm osteocutaneous free flap. Laryngoscope 2016;126(2):340–344. [DOI] [PubMed] [Google Scholar]
- 6. Dowthwaite SA, Theurer J, Belzile M, et al. Comparison of fibular and scapular osseous free flaps for oromandibular reconstruction: a patient‐centered approach to flap selection. JAMA Otolaryngol Head Neck Surg 2013;139(3):285–292. [DOI] [PubMed] [Google Scholar]
- 7. Urken ML, Bridger AG, Zur KB, Genden EM. The scapular osteofasciocutaneous flap: a 12‐year experience. Arch Otolaryngol Head Neck Surg 2001;127(7):862–869. [PubMed] [Google Scholar]
- 8. Moscoso JF, Keller J, Genden E, et al. Vascularized bone flaps in oromandibular reconstruction. A comparative anatomic study of bone stock from various donor sites to assess suitability for enosseous dental implants. Arch Otolaryngol Head Neck Surg 1994;120(1):36–43. [DOI] [PubMed] [Google Scholar]
- 9. Lanzer M, Gander T, Grätz K, Rostetter C, Zweifel D, Bredell M. Scapular free vascularised bone flaps for mandibular reconstruction: are dental implants possible? J Oral Maxillofac Res 2015;6(3):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seneviratne S, Duong C, Taylor GI. The angular branch of the thoracodorsal artery and its blood supply to the inferior angle of the scapula: an anatomical study. Plast Reconstr Surg 1999;104(1):85–88. [PubMed] [Google Scholar]
- 11. Hanasono MM, Skoracki RJ. The scapular tip osseous free flap as an alternative for anterior mandibular reconstruction. Plast Reconstr Surg 2010;125(4):164e–166e. [DOI] [PubMed] [Google Scholar]
- 12. Clark JR, Vesely M, Gilbert R, Frcs C. Scapular angle osteomyogenous flap in postmaxillectomy reconstruction: defect, reconstruction, shoulder function, and harvest technique. Head Neck 2008;30(1):10–20. [DOI] [PubMed] [Google Scholar]
- 13. Miles BA, Gilbert RW. Maxillary reconstruction with the scapular angle osteomyogenous free flap. Arch Otolaryngol Head Neck Surg 2011;137(11):1130–1135. [DOI] [PubMed] [Google Scholar]
- 14. Chow LK, Cheung LK. The usefulness of stereomodels in maxillofacial surgical management. J Oral Maxillofac Surg 2007;65(11):2260–2268. [DOI] [PubMed] [Google Scholar]
- 15. Thomas CV, McMillan KG, Jeynes P, Martin T, Parmar S. Use of a titanium cutting guide to assist raising the composite radial forearm free flap. Int J Oral Maxillofac Surg 2013;42(11):1414–1417. [DOI] [PubMed] [Google Scholar]
- 16. Cohen A, Laviv A, Berman P, Nashef R, Abu‐Tair J. Mandibular reconstruction using stereolithographic 3‐dimensional printing modeling technology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108(5):661–666. [DOI] [PubMed] [Google Scholar]
- 17. Cunningham LL, Madsen MJ, Peterson G. Stereolithographic modeling technology applied to tumor resection. J Oral Maxillofac Surg 2005;63(6):873–878. [DOI] [PubMed] [Google Scholar]
- 18. Sannomiya EK, Silva JVL, Brito AA, Saez DM, Angelieri F, da Silva Dalben G. Surgical planning for resection of an ameloblastoma and reconstruction of the mandible using a selective laser sintering 3D biomodel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008;106(1):2–3. [DOI] [PubMed] [Google Scholar]
- 19. Hanasono MM, Jacob RF, Bidaut L, Robb GL, Skoracki RJ. Midfacial reconstruction using virtual planning, rapid prototype modeling, and stereotactic navigation. Plast Reconstr Surg 2010;126(6):2002–2006. [DOI] [PubMed] [Google Scholar]
- 20. Mertens C, Lowenheim H, Hoffmann J. Image data based reconstruction of the midface using a patient‐specific implant in combination with a vascularized osteomyocutaneous scapular flap. J Craniomaxillofac Surg 2013;41(3):219–225. [DOI] [PubMed] [Google Scholar]
- 21. Toto JM, Chang EI, Agag R, Devarajan K, Patel SA, Topham NS. Improved operative efficiency of free fibula flap mandible reconstruction with patient‐specific, computer‐guided preoperative planning. Head Neck. 2015;37(11):1660–1664. [DOI] [PubMed] [Google Scholar]
- 22. dos Santos LF. The vascular anatomy and dissection of the free scapular flap. Plast Reconstr Surg 1984;73(4):599–604. [DOI] [PubMed] [Google Scholar]
- 23. Dérand P, Hirsch JM. Virtual bending of mandibular reconstruction plates using a computer‐aided design. J Oral Maxillofac Surg 2009;67(8):1640–1643. [DOI] [PubMed] [Google Scholar]
- 24. Prisman E, Haerle SK, Irish JC, Daly M, Miles B, Chan H. Value of preoperative mandibular plating in reconstruction of the mandible. Head Neck 2014;36(6):828–833. [DOI] [PubMed] [Google Scholar]
- 25. Hanken H, Schablowsky C, Smeets R, et al. Virtual planning of complex head and neck reconstruction results in satisfactory match between real outcomes and virtual models. Clin Oral Investig 2014;19(3):63–71. [DOI] [PubMed] [Google Scholar]


