Abstract
Objective
Skull base osteomyelitis (SBO) is a rare but life‐threatening disease observed in elderly diabetic patients, with high risk of recurrence and difficult therapeutic management. The diagnosis is ascertained from a set of clinical, biological, and imaging findings. CT and MRI allow initial diagnosis, but are not accurate to affirm healing at the end of therapy. 99mTc‐HMPAO‐Leucocyte Scintigraphy (LS) is highly sensitive and specific for the detection of infection. The aim of this study was to evaluate LS i) for initial diagnosis, and ii) to confirm healing at the end of antibiotherapy in SBO.
Study design
We retrospectively reviewed from November 2011 to September 2015 all patients with confirmed SBO who underwent LS twice, at diagnosis and at the end of antibiotic therapy in our nuclear medicine department (n = 27).
Methods
Clinical, biological, CT, LS, and follow‐up data were recorded in all patients. LS images (planar and tomographic performed 4 hours and 24 hours after intravenous injection of autologous Tc‐99m‐HMPAO‐leucocytes) were visually assessed and quantified.
Results
At initial diagnosis, 25 of 27 patients had a positive LS. At the end of antibiotic therapy (3 ± 1 months duration), 26 of 27 patients had a negative LS. During subsequent follow‐up (= or >6 months), the disease recurred in four patients including three with a negative postantibiotherapy LS scan.
Conclusion
In this retrospective study, LS was powerful for initial diagnostic of SBO and for healing assessment at the end of antibiotic therapy. We conclude it is a useful technique for therapeutic monitoring of SBO.
Level of Evidence
4
Keywords: Skull base osteomyelitis, scintigraphy, infection, recurrence
INTRODUCTION
Skull base osteomyelitis (SBO) is a rare but life‐threatening disease, the prevalence of which tends to increase with the growing incidence of diabetes mellitus and the increase in life expectancy. The most common form occurs after a malignant external otitis in elderly diabetic patients and affects the temporal bone. Another form is characterized by neurological symptoms and occurs in contexts of immunosuppression (eg, HIV or organ transplantation) and more specifically affects the occipital and sphenoid bones.
An early diagnosis, hence the rapid administration of an adequate therapy, is essential for the management of this potentially lethal disease. The diagnosis of SBO is suspected in patients with persistent otalgia and otorrhea despite outpatient treatment for several weeks. CT and MRI are recommended for initial diagnosis: the CT scan assesses osteolysis and the MRI assesses soft tissue and potential bone marrow involvement. Final diagnosis is established after microbiological and radiological findings that prove i) the causal agent—the most frequent being Pseudomonas aeruginosa—from bone biopsy,1, 2 and ii) osteolysis. One of the major challenges in managing SBO is the high rate of recurrence highlighted in several studies (9–27%).3, 4 Indeed, the infection can recur as long as 4 to 12 months after the end of the antibiotic therapy,5 with undertreatment being the major contributor.3, 4 These data plead for a long follow‐up of patients with SBO to ensure that adequate therapy duration is met. There is no current guideline regarding treatment duration for SBO. In our hospital, otolaryngologists empirically administrate antipseudomonal antibiotics for four to six weeks, a timeframe that covers the three‐to‐four‐week window necessary for bone revascularization,6 then follow SBO patients for six months after the end of antibiotherapy to ensure the absence of symptom recurrence, before referring the patient to his primary care provider for a longer follow‐up. The antibiotics commonly used against P. aeruginosa include aminoglycosides, penicillin, and cephalosporins.7
Various studies on SBO highlighted the need for a sensitive and specific technique that could help physician to affirm healing in order to adapt case by case the duration of antibiotherapy.6 The persistence of MRI and CT abnormalities after treatment despite resolution of the disease makes these techniques unreliable.7, 8, 9, 10, 11 Bone scintigraphy (HMDP‐Tc‐99m) has the same limitations.9 Leukocyte scintigraphy (LS) is known to specifically detect bacterial infections, but very few data are available regarding LS in SBO.12, 13 The aim of this study was to evaluate LS in SBO patients as a tool for the initial diagnosis and to confirm healing after antibiotherapy.
MATERIALS AND METHODS
Patients
We retrospectively studied the medical records and imaging studies of 27 consecutive patients treated for SBO and who underwent LS scans at the time of diagnosis and at the end of antibiotic therapy in our nuclear medicine department, from May 2011 to September 2015. The diagnosis of SBO was suspected on clinical signs, laboratory parameters, inefficiency of short‐term oral antibiotherapy (clinical symptoms continuation or exacerbation), and confirmed by the identification of the causative agent from biopsy and the presence of osteolysis on diagnostic CT scan. All patients received a combination of antipseudomonal antibiotics. All patients underwent two LS: one performed at initial diagnosis, the second performed at the end of therapy when clinicians thought that the disease was cured on the basis of clinical and biological parameters (leukocytes blood counts, CRP). Then the patients had clinical follow‐up from 6 to 12 months after the end of antibiotherapy, based on clinical examination and biological parameters. Conventional imaging (CT or MRI) is not routinely performed during follow‐up in our institution.
Leukocyte Isolation and Radiolabeling
According to routine procedure in Nuclear Medicine Departments, autologous leukocytes were isolated from peripheral blood and radiolabeled ex‐vivo, then reinjected back to the same patient for scintigraphic imaging.
Leukocytes were labeled with Tc‐99m‐hexamethylpropyleneamineoxime, (Tc‐99m HMPAO) as recommended in the consensus protocol from the International Society of Radiolabeled Blood Elements.14 Peripheral blood (80 ml) was collected into two 60‐mL syringes containing 8 ml of Acid Citrate Dextrose solution (ACD); 10 mL of ACD blood were removed from each syringe and transferred into a tube, then centrifuged at 2000 g for 10 min to obtain Cell Free Plasma (CFP). The remaining 40 mL of ACD blood in both syringes were incubated with 6.5 mL of sedimentation agent (Plasmion) for 30 to 90 minutes. The red blood cells–free supernatant was transferred into a sterile tube and centrifuged at 150 g for 5 minutes. The supernatant was discarded to obtain a leukocyte pellet.
Tc‐99m‐HMPAO was prepared by adding 1000 MBq (0,027 Ci)/2,5 mL of freshly eluted Tc‐99m‐perthechnetate in a Ceretec bottle (Ceretec, GE Healthcare, Little Chalfont, UK). 666 MBq of Tc‐99m‐HMPAO was added to the leukocyte suspension. After 15 minutes of incubation, 10 ml of CFP were added to stop the labeling reaction, and the solution was centrifuged at 150 g for 5 minutes. The supernatant that contained unbound Tc‐99m‐HMPAO was discarded, and the labeled leukocyte pellet was resuspended in 4 ml of CFP. The amounts of radioactivity in the pellet and the supernatant were measured to calculate the labeling yield. Tc‐99m‐HMPAO‐labeled leukocytes (200–500 Mbq) were then rapidly injected into the patient intravenously (IV).
Image Acquisition
Planar and SPECT/CT images centered on the head and neck were obtained 4 hours and 24 hours after IV injection of Tc‐99m‐HMPAO‐labeled leukocytes. A large field of view gamma camera (Flash 3D, Siemens SAS Medical Solutions, Saint‐Denis, France) with 2 low energy high‐resolution collimators was used. The pulse height analyzer was centered at 140 keV using a 15% to 20% window. Fixed time and fixed duration planar and SPECT/CT acquisitions were performed using a 128 x 128 matrix. Planar images duration was 600 s at 4 hours post‐IV and 900 s at 24 hours post‐IV. SPECT images consisted in 64 projections over 360°, with 30 s per projection at 4 hours post‐IV and 45 s per projection at 24 hours post‐IV. Three‐dimensional (3D) images were reconstructed using Ordered Subset Expectation Maximization (OSEM) algorithm. CT scans were performed immediately after SPECT. Scanning parameters were 110 kV, 80 mAs, and 6 x 1 mm collimation; bone‐weighted (B70, Medium Sharp) reconstruction was performed to obtain 1.25 mm thick slices.
Image Analysis
LS images were analyzed by two experienced nuclear medicine physicians. In case of discrepancy, a consensus reading was made. LS was considered positive or negative for infection on visual qualitative analysis of planar and SPECT/CT images. It was considered positive when showing a focal increased radiotracer uptake on skull base bones that could not be related to normal physiologic biodistribution, with stable or increased intensity on 24 hours post‐IV images compared to 4 hours post‐IV images (Fig. 1). Quantitative analysis was performed on planar images, according to the method recommended by Glaudemans et al15: a region of interest (ROI) was drawn on the affected area on the anterior view then copied to the contralateral non‐affected side, and the total counts in ROIs were recorded to calculate the lesion‐to‐non‐lesion (L/NL) ratio.
Figure 1.
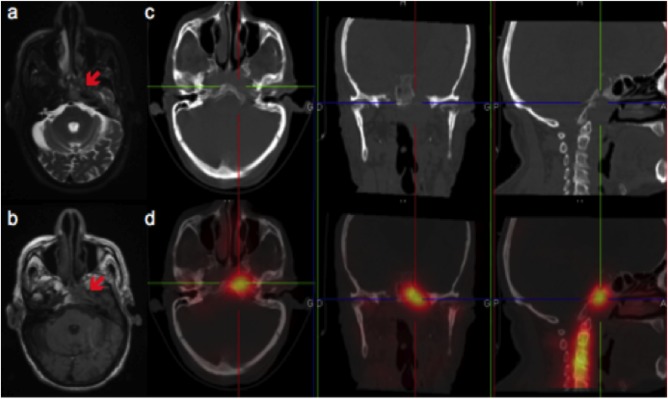
Typical imaging features of skull base osteomyelitis on MRI and Leukocyte Scintigraphy (SPECT/CT).
MRI: T2 axial weighted‐sequences (A), T1 axial weighted‐sequences (B); LS: axial, coronal, sagittal CT (C), and fused SPECT/CT images (D). MRI shows an hypersignal on the T2 weighted‐sequence and an hyposignal on the T1 weighted‐sequence of the left part of the sphenoid, left part of the clivus and of the petrous part of the left temporal bone, and soft tissue infiltration in the left retropharyngeal and parapharyngeal spaces. CT enables localization of the tracer uptake and shows bony rarefaction of the clivus. SPECT/CT shows high uptake of the radiotracer in the left part of the clivus and in the petrous part of the left temporal bone.
Statistical Analysis
Data are presented as median (interquartile range) or number (%) as appropriate. Skewed variables were compared using paired or unpaired Sum‐rank Wilcoxon test, as appropriate. A P value < .05 was considered statistically significant.
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration. For this type of study formal consent is not required.
RESULTS
Patients
The baseline characteristics of the study patients are shown in Table 1. Most patients were men (89%), mean age was 72 ± 9 years. Most patients (25 of 27, 93%) presented with typical history of SBO originating from necrotizing external otitis: persistent otorrhea for one to five months despite local or oral antibiotic therapy; two patients presented with atypical SBO (radiation‐induced infection after an epidermoid carcinoma of the floor of the mouth, cystic mass of the cavum). Eight patients had cranial nerve paralysis at diagnosis. Diabetes was the most common risk factor (81% of patients), and one patient was under immunosuppressant. All but three patients had elevated plasma CRP and six had elevated white blood cell counts. Identification of the causative organisms was obtained in all patients (biopsy in 25, culture of the ear canal in 2). P. aeruginosa alone or associated was identified in the majority of cases (n = 25). Antibiotherapy was applied as recommended, using a combination of ceftazidim (FORTUM) and ciprofloxacin (CIFLOX) for most of the patients (24 of 27). The three other patients were infected by a quinolone‐resistant germ and were treated with other antibiotics.
Table 1.
Patient Characteristics.
| SBO | ||
|---|---|---|
| n = 27 | ||
| Age | Mean ± SD | 72 ± 9 |
| Sex | Men | 24 (89%) |
| Risk factor | Diabetes | 22 (81%) |
| Other | 1 (3%) | |
| None | 4 (14%) | |
| Organism | Pseudomonas aeruginosa | 25 (89%) |
| Staphylococcus epidermidis | 2 (7%) | |
| Staphylococcus aureus | 2 (7%) |
SBO = skull base osteomyelitis.
Scintigraphic Results
Injected dose was 345 ± 130 MBq (0.09 ± 0.003 Ci). Overall, the radiolabeling yield was 61 ± 13%. WBC counts were not different in the patients with positive and negative LS scans (6635 ± 2242/ml vs. 6450 ± 150/ml, NS).
First leukocyte scintigraphy at initial diagnosis
The first LS was performed before treatment in seven patients, less than 15 days (mean: 8 ± 4 days) after the start of treatment in 19, and after 21 days of treatment in one. If began, the antibiotherapy was not stopped before LS. LS was positive in 25 patients (Fig. 1) and negative in 2 (performed 10 days before the start of treatment in 1 patient, and 7 days after the start of treatment in the other).
Second leukocyte scintigraphy at the end of antibiotherapy
The second LS was performed three days to one month after the end of antibiotherapy in 13 patients, and between 1 and 20 days before the end of antibiotherapy (under treatment) in 14 patients. It was negative for 26 patients (including 24 with positive initial LS, Fig. 2), and positive for 1 (Table 2).
Figure 2.
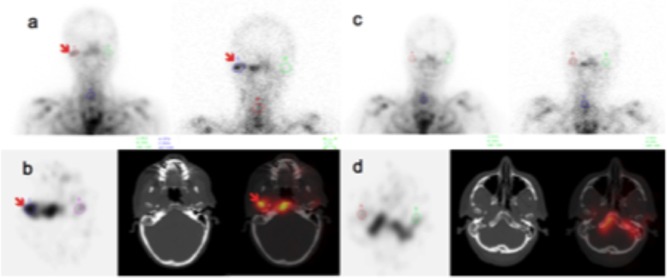
Typical Leucocyte Scintigraphy (LS) scans before and after antibiotic therapy in a patient with no recurrence.
A. Planar 4 and 24 hours anterior images before treatment; B. SPECT, CT and SPECT/CT axial 24 hours images before treatment; C. planar 4 and 24 hours anterior images after treatment; D. SPECT, CT and SPECT/CT axial 24 hours images after treatment.
In September 2014, planar and SPECT/CT images showed high uptake of radiotracer on the tympanic part of the temporal bone and mandibular condyle. After two months of systemic antibiotherapy against pseudomonas aeruginosa, LS scan was normalized.
Table 2.
Descriptive Leukocyte Scintigraphy Results.
| LS RESULTS | Recurrence | No recurrence | Total | |
|---|---|---|---|---|
| LS1 | LS2 | |||
| + | + | 1 | 0 | 1 |
| + | ‐ | 3 | 21 | 24 |
| ‐ | ‐ | 0 | 2 | 2 |
| ‐ | + | 0 | 0 | 0 |
LS1, leukocyte scintigraphy at initial diagnosis; LS2, post‐therapeutic leukocyte scintigraphy. + indicates Positive LS scan; ‐ indicates negative LS scan.
In LS scans declared positive on visual analysis, quantitative analysis showed a statistically significant increase in mean L/NL ratio between 4 hours post‐IV and 24 hours post‐IV images (1.59 ± 1.00 vs. 2.46 ± 1.14, P < .05). L/NL ratios on negative LS scans were 1.07 ± 0.20 and 1.08 ± 0.20 on 4 hours post‐IV and 24 hours post‐IV images, respectively.
Follow‐up
Four of 27 patients had recurrence of the disease two to nine months after the end of antibiotherapy (resurgence of earache, othorroea): one patient with positive post‐therapeutic LS, and three others with normalized post‐therapeutic LS (Table 2, Fig. 3). So the likelihood of being cured (no recurrence) for patients with negative post‐therapeutic LS (negative predictive value) was 23 of 26 (88%). The timings of false negative second scans were one and six days before the end of antibiotherapy in two patients and one month after the end of therapy in one. The patients with disease recurrence had higher L/NL ratios on 24 hours post‐IV images at initial diagnosis than cured patients, although this difference was not statistically significant. In cured (non‐recurrent) patients, quantitative analysis showed a significant decrease in L/NL ratios between initial and post‐therapeutic LS on both 4 hours post‐IV and 24 hours post‐IV images. In the patients with disease recurrence, a slight but not statistically significant decrease in L/NL ratios was observed between the initial and the post‐therapeutic LS (Table 3).
Figure 3.
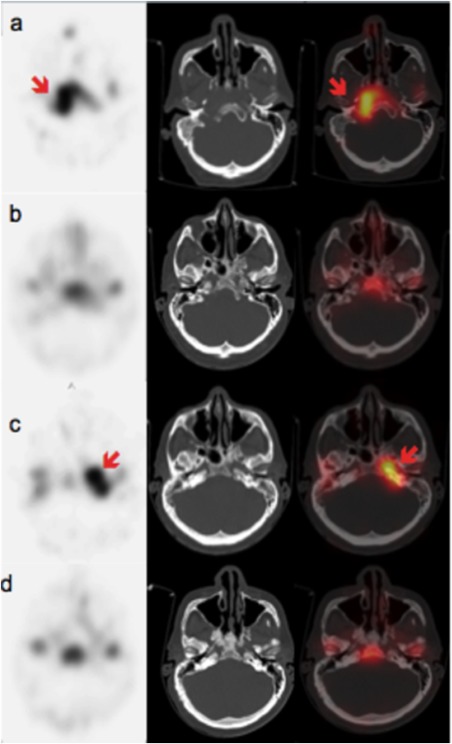
False‐negative post‐therapeutic LS: a case of recurrence 7 months after the end of the antibiotic treatment.
A. June 2014, SPECT, CT and fused SPECT/CT axial slices: high radiotracer uptake in the right part of the clivus corresponding to bone osteolysis.
B. August 2014, SPECT, CT and fused SPECT/CT axial slices: normalization 1 month after the end of adequate systemic therapy.
C. January 2015, SPECT, CT and fused SPECT/CT axial slices: radiotracer uptake in the left part of the clivus and in the petrous part of the left temporal bone23.
D. April 2015, SPECT, CT and fused SPECT/CT axial slices: normalization after 3 months of adequate systemic therapy against pseudomonas aeruginosa.
Table 3.
Lesion to Non‐Lesion 99mTc‐HMPAO‐Leukocytes's Uptake Ratios in Patients With and Without Recurrent Disease.
| Patients With Recurrent Disease (n = 4) | Cured Patients (n = 23) | |||||
|---|---|---|---|---|---|---|
| 4 h | 24 h | 4 h | 24 h | P valuea | ||
| Pre‐therapeutic LS | 1.38 [1.31–1.39] | 2.84 [2.60–3.08] | 1.36 [1.10–1.57] | 2.00 [1.63–2.59] | .33 | |
| Post‐therapeutic LS | 1.25 [1.15–1.36] | 1.18 [1.07–1.29] | 1.05 [0.88–1.18] | 1.10 [0.92–1.20] | .64 | |
| P value b | .75 | 1 | .0006 | <.0001 | ||
Comparison of lesion to non‐lesion 99mTc‐HMPAO‐leukocytes's uptake (L/NL) ratios on 4 h planar post‐IV images between recurrent and cured patients.
Comparison of L/NL ratios on 24 h planar post‐IV images between recurrent and cured patients.
Comparison of L/NL ratios between pre‐therapeutic and post‐therapeutic planar Leucocyte Scintigraphy.
We did not observe any false positive LS scan.
DISCUSSION
In this study, we retrospectively evaluated LS for initial diagnosis and therapeutic monitoring of SBO. LS detected 25 of 27 SBO lesions at initial diagnosis, and correctly affirmed healing in 23 of 26 patients at the end of antibiotherapy.
At initial diagnosis, CT and MRI are respectively required to assess bone and soft tissue involvements of the infectious process. CT shows a thickening of the external auditory canal mucosa and auricle, which are enhanced after contrast agent administration. An erasure of fat planes in the soft tissues beneath the temporal bone around the styloid foramen and infra‐temporal fossa can also be seen.7 MRI has a lower sensitivity than CT for bone erosion imaging but this technique better detects changes in soft tissues, especially dural enhancement and involvement of bone marrow.16, 17 The most consistent imaging findings on MRI are bone marrow T1 hypointensity and T2 hyperintensity. However, MRI is unable to discriminate SBO from other differential diagnoses, especially tumor processes. Also, CT and MRI abnormalities persist despite the resolution of the disease, and so are not useful for therapeutic monitoring in SBO.7, 8 Bone remineralization after disease resolution is a late phenomenon making CT not reliable for early therapeutical management.9, 11, 18 On MRI, soft tissue abnormalities also persist after the resolution of the disease, as well as bone marrow abnormal signals that persist from 6 to 12 months after successful treatment.16, 18, 19, 20
Bone scintigraphy allows earlier diagnosis of SBO than CT, with a sensitivity of almost 100%.21, 22, 23, 24 However its specificity is low and it does not differentiate osteomyelitis from simple otitis externa, traumatism, or neoplasm. Moreover, it remains positive for many years and is not a reliable marker of the response to therapy and disease resolution.21, 22, 23
Conversely 67Ga‐citrate and leukocytes scintigraphy demonstrate high specificity for infection in SBO.25, 26, 27, 28, 29, 30 In a series of eight patients with SBO, Stokkel et al. reported that 67Ga‐citrate scintigraphy was useful at initial diagnosis (8 of 8 positive scans), and could confirm healing at the end of antibiotherapy in five of eight patients.28 To our knowledge, only one study compared LS to CT, MRI and bone scintigraphy during post‐therapeutical follow‐up in SBO, showing higher accuracy of LS.12 In a series of patients suspected for SBO, mostly postoperative cases (15 of 25), the authors reported that 111Indium‐oxine‐leukocytes scintigraphy (performed once in each patient at time of diagnosis or during therapeutic follow‐up) was truly positive at diagnosis in eight of eight patients, and truly negative during follow‐up in three of four cured patients.12 Tc‐99m‐HMPAO‐leukocytes scintigraphy is nowadays more often used because of higher image quality (compared to 67Ga‐citrate) and lower radiation dose to the leukocytes and to the patients (compared to 67Ga‐citrate and 111Indium‐oxine‐leukocytes scintigraphy). Our patient series differs from that of Seabold12 et al. by higher rate of diabetic patients (22 of 27 vs. 6 of 25), higher number of patients with proven SBO (n = 27 vs. n = 12), and by the fact that all our patients with proven SBO underwent LS twice, at diagnosis (n = 27 vs. 8) and during follow‐up (n = 27 vs. 4). We obtained comparable results while diabetic status could be a disadvantage for the technique because of restrained blood flow or impaired leukocyte migratory ability.29 It is worth noting that all five false negative cases concerned diabetic patients with high levels of glycosylated Hb (HbA1c = 8.9%, 10%, 7.4%, and 8.3%). All the other parameters did not differ from those of other patients. Most patients presented with otitis evolving since several weeks before they were managed in our hospital ORL department. Causing pathogens, especially Pseudomonas aeruginosa, have two different lifestyles: cytotoxic living cells causing acute inflammatory reaction and biofilm‐forming communities which cause refractory chronic infection. Recent studies suggest that both lifestyles can coexist within the bacterial population.31, 32 Plausible hypotheses for false negative LS scans are biofil‐forming communities' escape to immune cellular response, or alteration of vasculature by necrotizing and vasoactive endotoxins. Finally low‐grade infections (reduced bacterial load) in patients under antibiotherapy could theoretically result in false negative scans: this is controversial since several studies27, 31 previously reported that antibiotherapy does not affect the sensitivity of the diagnostic LS. In our study three of five false negative results (two at initial diagnosis, three during follow‐up) concerned LS performed under antibiotherapy (>7 days duration). This raises potential interest of a therapeutic window before LS, especially for healing assessment at the end of therapy. An interesting point is that the patients who experienced disease recurrence had higher L/NL ratios than those who did not recur after the end of antibiotherapy: 2.84 (2.60–3.08) versus 2.00 (1.63–2.59) (24 hour images) at initial diagnosis, then 1.18 (1.07–1.29) versus 1.10 (0.92–1.20) (24 hour images) at the end of antibiotherapy. Despite very small effective of recurrent patients and no statistical difference, this suggests potential prognostic significance of the intensity of radiotracer uptake in the lesions; indeed it can be assumed that the amount of accumulated leukocytes in the lesion is related to bacterial load, and that SBO with higher bacterial load need longer treatment duration. Also it suggests that quantitative criteria could be considered for scans interpretation to improve LS sensitivity. However it is not possible to determine a threshold value because of the too small effective of patients with recurrent disease (n = 4).
Alternatively to SPECT, PET (positron emission tomography) may be proposed to improve spatial resolution and contrast, eg, to improve sensitivity of detection. Inflammatory cells exhibit overexpression of hexokinase and glucose transporter proteins with a high affinity for 18F‐Fluoro‐Desoxy‐Glucose (18F‐FDG), the most often used PET radiotracer. However, 18F‐FDG‐PET may be less specific for infection than LS, since 18F‐FDG is taken by all type of activated cells especially during healing processes.33, 34 Alternatively, PET using leukocytes labeled with a positron emitter may be more sensitive and as specific as Tc‐99m‐HMPAO‐leukocytes SPECT imaging in this setting.34 However, PET isotopes with adequate radioactive half‐lives are more destructive to cells regarding the nature of radiations,35 and labeling yields and stability are still challenging issues. Imaging of bacteria using radiolabeled antibiotics may be problematic in SBO with drug‐resistant pathogens. Finally 68Ga–citrate or 68Ga–radiolabeled antimicrobioal peptides, such as UBI, are emerging in the setting of infection imaging, with high potential because of high sensitivity, high specificity, fast and easy radiolabeling, and reasonable cost (68Ga generator, dramatic decrease in PET acquisitions duration on current PET cameras).36, 37, 38, 39, 40
Study Limitations
This is a retrospective study yet the largest published so far. Comparison with more powerful PET technology was not performed for technical reasons, and should be initiated because of high potential in this setting.
CONCLUSION
In this retrospective study, LS was powerful for initial diagnostic of SBO and for healing assessment at the end of antibiotic therapy. Further studies should evaluate PET technology using leukocytes labeled with a positron emitter, or 68Ga‐citrate.
All authors declare they have no specific financial interests, relationships, and affiliations relevant to the subject of the manuscript.
All authors declare they have no conflict of interest.
BIBLIOGRAPHY
- 1. Rubin J, Yu VL. Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy. Am J Med 1988;85(3):391–398. [DOI] [PubMed] [Google Scholar]
- 2. Ridder GJ, Breunig C, Kaminsky J, Pfeiffer J. Central skull base osteomyelitis: new insights and implications for diagnosis and treatment. Eur Arch Otorhinolaryngol 2015;272(5):1269–1276. [DOI] [PubMed] [Google Scholar]
- 3. Singh A, M AK, Hyder MJ. Skull base osteomyelitis: diagnostic and therapeutic challenges in atypical presentation. Otolaryngol Head Neck Surg 2005;133(1):121–125. [DOI] [PubMed] [Google Scholar]
- 4. Clerc NL, Verillaud B, Duet M, Guichard JP, Herman P, Kania R. Skull base osteomyelitis: incidence of resistance, morbidity, and treatment strategy. Laryngoscope 2014;124(9):2013–2016. [DOI] [PubMed] [Google Scholar]
- 5. Conterno LO, Turchi MD. Antibiotics for treating chronic osteomyelitis in adults. Cochrane Database Syst Rev 2013;9:CD004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Courson AM, Vikram HR, Barrs DM. What are the criteria for terminating treatment for necrotizing (malignant) otitis externa? Laryngoscope 2014;124:361–362. [DOI] [PubMed] [Google Scholar]
- 7. Adams A, Offiah C. Central skull base osteomyelitis as a complication of necrotizing otitis externa: Imaging findings, complications, and challenges of diagnosis. Clin Radiol 2012;67(10):e7–e16. [DOI] [PubMed] [Google Scholar]
- 8. van Kroonenburgh AMJL, van der Meer WL, Bothof RJP, van Tilburg M, van Tongeren J, Postma AA. advanced imaging techniques in skull base osteomyelitis due to malignant otitis externa. Curr Radiol Rep 2018;6(1):3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glikson E, Sagiv D, Wolf M, Shapira Y. Necrotizing otitis externa: diagnosis, treatment, and outcome in a case series. Diagn Microbiol Infect Dis 2017;87(1):74–78. [DOI] [PubMed] [Google Scholar]
- 10. Handzel O, Halperin D. Necrotizing (malignant) external otitis. Am Fam Physician 2003;68(2):309–312. [PubMed] [Google Scholar]
- 11. Al‐Noury K, Lotfy A. Computed tomography and magnetic resonance imaging findings before and after treatment of patients with malignant external otitis. Eur Arch Otorhinolaryngol 2011;268(12):1727–1734. [DOI] [PubMed] [Google Scholar]
- 12. Seabold JE, Simonson TM, Weber PC, et al. Cranial osteomyelitis: diagnosis and follow‐up with In‐111 white blood cell and Tc‐99m methylene diphosphonate bone SPECT, CT and MR imaging. Radiology 1995;196(3):779–788. [DOI] [PubMed] [Google Scholar]
- 13. Bruni C, Padovano F, Travascio L, Schillaci O, Simonetti G. Usefulness of hybrid SPECT/CT for the 99mTC‐HMPAO‐labeled leukocyte scintigraphy in a case of cranial osteomyelitis. Braz J Infect Dis 2008;12(6):558–560. [DOI] [PubMed] [Google Scholar]
- 14. Rocca M, Comin JM, Becker W, et al. A consensus protocol for white blood cells labelling with technetium‐99m hexamethylpropylene amine oxime. International Society of Radiolabeled Blood Elements (ISORBE). Eur J Nucl Med 1998;25:797–799. [DOI] [PubMed] [Google Scholar]
- 15. Glaudemans AWJM, de Vries EFJ, Vermeulen LEM, Slart RHJA, Dierckx RAJO, Signore A. A large retrospective single‐centre study to define the best image acquisition protocols and interpretation criteria for white blood cell scintigraphy with 99mTc‐HMPAO‐labelled leukocytes in musculoskeletal infections. Eur J Nucl Med Mol Imaging 2013;40(11):1760–1769. [DOI] [PubMed] [Google Scholar]
- 16. Kwon BJ, Han MH, Oh SH, Song JJ, Chang KH. MRI findings and spreading patterns of necrotizing external otitis: is a poor outcome predictable? Clin Radiol 2006;61(6):495–504. [DOI] [PubMed] [Google Scholar]
- 17. Chang PC, Fischbein NJ, Holliday RA. Central skull base osteomyelitis in patients without otitis externa: imaging findings. AJNR Am J Neuroradiol 2003;24(7):1310–1316. [PMC free article] [PubMed] [Google Scholar]
- 18. Clark MP, Pretorius PM, Byren I, Milford CA. Central or atypical skull base osteomyelitis: diagnosis and treatment. Skull Base 2009;19(4):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karantanas AH, Karantzas G, Katsiva V, Proikas K, Sandris V. CT and MRI in malignant external otitis: a report of four cases. Comput Med Imaging Graph 2003;27(1):27–34. [DOI] [PubMed] [Google Scholar]
- 20. Grandis JR, Curtin HD, Yu VL. Necrotizing (malignant) external otitis: prospective comparison of CT and MR Imaging in diagnosis and follow‐up. Radiology 1995;196(2):499–504. [DOI] [PubMed] [Google Scholar]
- 21. Chakraborty D, Bhattacharya A, Kamaleshwaran KK, Agrawal K, Gupta AK, Mittal BR. Single photon emission computed tomography/computed tomography of the skull in malignant otitis externa. Am J Otolaryngol 2012;33(1):128–129. [DOI] [PubMed] [Google Scholar]
- 22. Filippi L, Schillaci O . Usefulness of hybrid SPECT/CT in 99mTc‐HMPAO‐labeled leukocyte scintigraphy for bone and joint infections. J Nuclear Med 2006;47(12):1908–1913. [PubMed] [Google Scholar]
- 23. Driscoll CL, Lane JI. Advances in skull base imaging. Otolaryngol Clin North Am 2007;40(3):439–454 [DOI] [PubMed] [Google Scholar]
- 24. Hardoff R, Gips S, Uri N, Front A, Tamir A. Semiquantitative skull planar and SPECT bone scintigraphy in diabetic patients: differentiation of necrotizing (malignant) external otitis from severe external otitis. J Nucl Med 1994;35(3):411–415. [PubMed] [Google Scholar]
- 25. Bar‐Shalom R, Yefremov N, Guralnik L, et al. SPECT/CT Using 67Ga and 111In‐labeled leukocyte scintigraphy for diagnosis of infection. J Nucl Med 2006;47(4):587–594. [PubMed] [Google Scholar]
- 26. Liberatore M, Calandri E, Pavoni GL, et al. Reliability of white blood cell scan in the follow‐up of osteomyelitis. Biomed Pharmacother 2007;61(5):272–276. [DOI] [PubMed] [Google Scholar]
- 27. Palestro CJ. Radionuclide imaging of osteomyelitis. Semin Nucl Med 2015;45(1):32–46. [DOI] [PubMed] [Google Scholar]
- 28. Stokkel MP, Takes RP, van‐Eck‐Smit, Baatenburg de Jong RJ. The value of quantitative gallium‐67 single photon emission tomography in the clinical management of malignant external otitis. Eur J Nucl Med 1997; 24(11):1429–1432. [DOI] [PubMed] [Google Scholar]
- 29. Vouillarmet J, Morelec I, Thivolet C. Assessing diabetic foot osteomyelitis remission with white blood cell SPECT/CT imaging. Diabet Med J Br Diabet Assoc 2014;31(9):1093–1099. [DOI] [PubMed] [Google Scholar]
- 30. Simonsen L, Buhl A, Oersnes T, et al. White blood cell scintigraphy for differentiation of infection and aseptic loosening: a retrospective study of 76 painful hip prostheses. Acta Orthop 2007;78:640–647. [DOI] [PubMed] [Google Scholar]
- 31. Cheung GY, Rigby K, Wang R, et al. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog 2010;6:e1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valentini M, Gonzalez D, Al Mavridou D, Filloux A. Lifestyle transitions and adaptative pathogenesis of Pseudomonas aeruginosa. Curr Opin Microbiol 2018;41:15–20. [DOI] [PubMed] [Google Scholar]
- 33. Gotthardt M, Bleeker‐Rovers CP, Boerman OC, et al. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med Technol 2013;41:157–169. [DOI] [PubMed] [Google Scholar]
- 34. Love C, Palestro CJ. Nuclear medicine imaging of bone infections. Clin Radiol 2016;71(7):632–646. [DOI] [PubMed] [Google Scholar]
- 35. Hocine N, Faivre L, Sarda‐Mantel L. Cellular and subcellular dosimetry of radioisotopes used for PET imaging. Phys Med 2016;32:242 (Abstract). 26508016 [Google Scholar]
- 36. Vorster M, Maes A, Wiele Cv, Sathekge M. Gallium‐68 PET: A Powerful Generator‐based Alternative to Infection and Inflammation Imaging. Semin Nucl Med 2016;46(5):436–447. [DOI] [PubMed] [Google Scholar]
- 37. Sarda‐Mantel L, Saleh‐Mghir A, Welling MM, et al. Evaluation of 99mTc‐UBI 29–41 scintigraphy for specific detection of experimental Staphylococcus aureus prosthetic joint infections. Eur J Nucl Med Mol Imaging 2007;34(8):1302–1309. [DOI] [PubMed] [Google Scholar]
- 38. Brouwer CP, Sarda‐Mantel L, Meulemans A, Le Guludec D, Welling MM. The use of technetium‐99m radiolabeled human antimicrobial peptides for infection specific imaging. Mini Rev Med Chem 2008;8(10):1039–1052. [DOI] [PubMed] [Google Scholar]
- 39. Gemmel F, Van den Wyngaert H, Love C, Welling MM, Gemmel P, Palestro CJ. Prosthetic joint infections: radionuclide state‐of‐the‐art imaging. Eur J Nucl Med Mol Imaging 2012;39(5):892–909. [DOI] [PubMed] [Google Scholar]
- 40. Welling MM, Bunschoten A, Kuil J, et al. Development of a hybrid tracer for SPECT and optical imaging of bacterial infections. Bioconjug Chem 2015;26(5):839–849. [DOI] [PubMed] [Google Scholar]


