Abstract
Background
Endoscopic skull base surgery (ESBS) is a rapidly expanding field. Despite divergent reported preferences for reconstructive techniques and perioperative management, limited data exist regarding contemporary practice patterns among otolaryngologists performing ESBS. This study aims to elucidate current practice patterns, primarily the volumes of cases performed and secondarily a variety of other perioperative preferences.
Methods
An anonymous 32‐item electronic survey examining perioperative ESBS preferences was distributed to the American Rhinologic Society membership. Statistical significance between variables was determined utilizing Student t, chi‐square, and Fisher exact tests.
Results
Seventy otolaryngologists completed the survey. The effective response rate was approximately 22.5%. Sixty percent of respondents were in full‐time academic practice and 70% had completed rhinology/skull base fellowships. Annually, 43.3 mean ESBS cases were performed (29.1 private practice vs. 52.9 academic practice, P = .009). Academic practice averaged 24.1 expanded cases versus only 11 in private practice (P = .01). Of respondents, 55.7% stood on the same side as the neurosurgeon and 72.9% remained present for the entire case. Current procedural terminology coding and antibiotic regimens were widely divergent; 31.4% never placed lumbar drains preoperatively, while 41.4% did so for anticipated high‐flow cerebrospinal fluid leaks. While considerable variation in reconstructive techniques were noted, intradural defect repairs utilized vascularized flaps 86.3% of the time versus only 51.3% for extradural repairs (P < 0.001). Major complications were rare. Postoperative restrictions varied considerably, with most activity limitations between 2–8 weeks and positive airway pressure use for 2–6 weeks. Most respondents started saline irrigations 0–2 weeks postoperatively.
Conclusions
Based on responses from fellowship‐ and non‐fellowship‐trained otolaryngologists in various practice settings, there remains considerable variation in the perioperative management of patients undergoing ESBS.
Level of Evidence
5
Keywords: Endoscopic skull base surgery, skull base repair, rhinology workforce, post‐operative, statistics
INTRODUCTION
Endoscopic skull base surgery (ESBS) represents a rapidly growing field with ever‐evolving methodologies. The roots of ESBS can be traced back to the first decade of the twentieth century but experienced no rapid advancement until the 1990s.1, 2, 3, 4, 5, 6, 7 Advances in endoscopic imaging technologies, newer surgical instrumentation, and the development of multi‐specialty four‐handed techniques have allowed the scope of ESBS to advance at a remarkable rate while improving safety, efficacy, and morbidity.8, 9, 10
ESBS has since extended beyond the sella to include the cribriform, parasellar region, sphenomedullary junction, petrous apex, pterygopalatine fossa, and infratemporal fossa. With expanded approaches, the need for increasingly complex cranial base reconstruction to prevent cerebrospinal fluid (CSF) leakage and meningitis has necessarily followed. Acellular, dermal, and mucosal grafts, cartilage, bone, fat, fascia, and fibrin glue have classically been utilized in a number of layered combinations described by ESBS surgeon published data.11, 12, 13 Use of vascularized pedicled nasoseptal mucosa as described by Hadad et al. was a leap forward that permitted expanded ESBS with fewer complications, especially CSF leaks.14, 15, 16, 17 The repertoire of vascularized flaps has increased to include the inferior turbinate, temporoparietal and tunneled pericranial flaps.18, 19, 20
Performed by both private and academic otolaryngologists, there is an increasing integration of ESBS into rhinologic practices.21 Since its introduction in 2006, the Rhinology fellowship through the American Rhinologic Society (ARS) has trained endoscopic skull base surgeons whose practices reflect and grow from their mentors' experiences. With the expanding number of surgeons performing ESBS, the practice patterns among experts continues to diversify. Limited data exists in the literature21, 22, 23 describing the variety of perioperative practice preferences for ESBS. This study seeks to highlight the preoperative (antibiotic use, coding, etc.), intraoperative (positioning, adjunct procedures, etc.), and postoperative (lumbar drain use, activity restrictions, etc.) patterns of practice among the ARS membership.
MATERIALS AND METHODS
An anonymous 32‐item electronic survey examining perioperative ESBS practice patterns was distributed to ARS members, fellow‐members, and international members. ARS emeritus fellow‐members, fellows‐in‐training, residents‐in‐training, affiliate members, and medical student members were excluded from distribution. Participation was entirely voluntary; no monetary or other benefits were offered for participation. This study primarily evaluated the estimated volumes of case types/numbers and secondarily a variety of other perioperative practice patterns.
Responses from November 2016 through February 2017 were aggregated utilizing Excel 2013 software (Microsoft Corp., Redmond, Washington, U.S.A.). Statistical analysis was performed to determine if significant differences existed between proportions or means of response data. SPSS software (version 24, IBM Corp., Armonk, New York, U.S.A.) was used for all data analysis. Descriptive statistics were used to analyze respondent demographic data, case numbers, and various practice pattern proportions. Continuous variables were described with means while categorical variables were presented as frequencies/percentages. Univariate analyses were conducted using two‐tailed independent samples t tests, chi‐square tests, and Fisher exact tests as applicable. For all tests, P values < .05 were considered statistically significant.
RESULTS
A total of 70 respondents completed the electronic survey partially or in its entirety. Fifty‐one respondents completed all the required sections of the survey, while the remaining 19 completed the majority of the survey questions. Response data was utilized based on the available responses for a given survey question. The number of responses aggregated for a given question or analysis is cited throughout the results section. Queried demographic data for respondents are enumerated in Table 1.
Table 1.
Demographic Data of Respondents by Practice Type.
| Measure |
Total N = 70 |
Private Practice N = 28 (40) |
Academic Practice N = 42 (60) |
Rhinology/Skull Base Fellowship by Time in Practice; N = 49 (70) |
|---|---|---|---|---|
| Time in Practice | ||||
| <2 years | 9 (12.9) | 2 (7.14) | 7 (16.7) | 9 (100) |
| 2–5 years | 14 (20.0) | 8 (28.6) | 6 (14.3) | 13 (92.9) |
| 6–10 years | 15 (21.4) | 6 (21.4) | 9 (21.4) | 15 (100) |
| 11–15 years | 12 (17.1) | 3 (10.7) | 9 (21.4) | 8 (66.6) |
| 16–20 years | 4 (5.71) | 2 (7.14) | 2 (4.76) | 2 (50) |
| >20 years | 16 (22.9) | 7 (25) | 9 (21.4) | 2 (12.5) |
| Fellowship Training | ||||
| Rhinology/Skull Base | 49 (70.0) | 16 (57.1) | 33 (78.6) | |
| Other (H&N, etc) | 3 (4.29) | 2 (7.14) | 1 (2.38) | |
| None | 18 (25.7) | 10 (35.7) | 8 (19.0) |
Unless otherwise indicated values are expressed as number (%).
H&N = Head & Neck.
Case Numbers & Length of Stay
When comparing private practice (PP) and academic practice (AP) respondents, a significant difference was noted between both the estimated total numbers of ESBS cases and the estimated number of expanded approaches performed annually (see Table 2). A trend of increasing estimated mean length of stay in days was identified with increasing defect closure complexity (Fig. 1), but no significant differences were noted between PP and AP mean estimated length‐of‐stay practice patterns for any case type (P > .05).
Table 2.
Reported Mean Estimated Annual Case Approach Numbers by Practice Type.
| Approaches |
Total N = 69 |
Private Practice N = 28 |
Academic Practice N = 41 |
P‐value |
|---|---|---|---|---|
| Total Cases | 43.3 (41.5) | 29.1 (24.8) | 52.9 (47.7) | .009 |
| Transsphenoidal Intrasellar | 24.5 (25.9) | 18.1 (15.3) | 28.9 (30.5) | .06 |
| Expanded Approaches | 18.8 (21.1) | 11.0 (13.9) | 24.1 (23.6) | .01 |
| Transtuberculum | 4.14 (5.65) | 2.85 (5.08) | 5.02 (5.91) | .12 |
| Transcribriform | 4.09 (7.32) | 1.68 (2.29) | 5.73 (8.98) | .008 |
| Transclival | 2.45 (2.78) | 1.57 (2.12) | 3.05 (3.03) | .02 |
| Transodontoid | 0.681 (2.51) | 0.214 (0.418) | 1.00 (3.21) | .203 |
| Orbital apex | 4.00 (1.06) | 2.75 (4.76) | 4.85 (10.7) | .331 |
| Petrous apex | 0.783 (3.40) | 0.393 (0.956) | 1.05 (1.32) | .028 |
| Pterygopalatine fossa | 2.62 (3.40) | 1.50 (2.24) | 3.39 (3.85) | .022 |
Unless otherwise indicated values are expressed as mean number of cases (SD).
Bold indicates statistical significance (p < 0.05)
Figure 1.
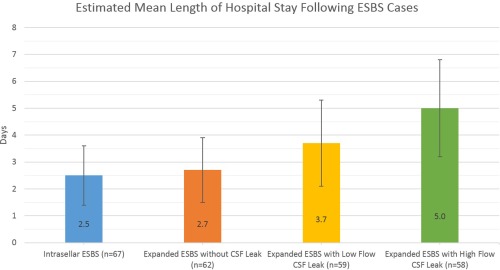
Estimated mean length of stay by respondents increased as the defect closure complexity increased. No significant differences were noted between defect types or between academic and private practice respondents (P > .05).
CSF = Cerebrospinal Fluid Leak; ESBS = Endoscopic Skull Base Surgery.
Intraoperative Positioning, Otolaryngologists' Presence, and Timing of Primary Surgeon Status
Intraoperative surgeon positioning is reported in Figure 2. No significant differences in proportions between PP and AP positioning patterns were noted (P > .05). The majority of the 70 respondents (72.9%) were present for the entire ESBS case, while 21.4% were typically present for the approach and closure while leaving during the resection; 5.7% were present for the approach only. The timing during ESBS where the neurosurgeon became the primary surgeon for intrasellar and expanded approaches is reported in Figure 3. The timing during defect closure that the otolaryngologist again became the primary surgeon is reported in Figure 4.
Figure 2.
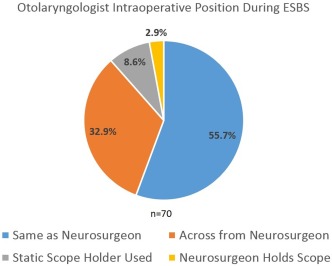
The majority of respondents stand on the same side as the neurosurgeon during ESBS. A minority of neurosurgeons holds their own scope or utilizes a static scope holder.
ESBS = endoscopic skull base surgery.
Figure 3.
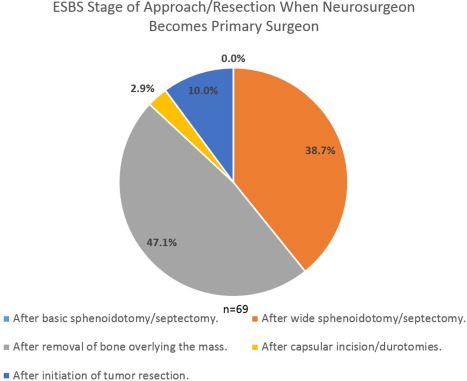
The majority of respondents hand the case over to the neurosurgeon after initiation of removal of bone overlying the mass during the approach. Only a minority state they personally perform incisions/durotomies or begin removing tumor prior to the neurosurgeon taking over.
ESBS = endoscopic skull base surgery.
Figure 4.
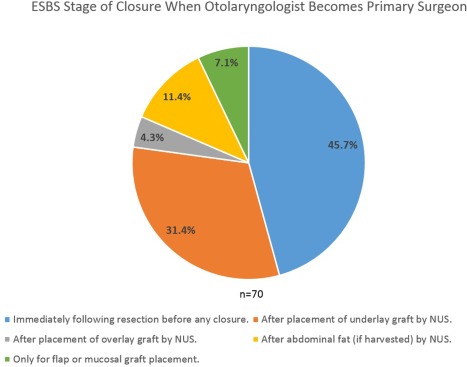
The majority of respondents take over the case immediately after completion of tumor resection. Nearly a third takes over after underlay graft placement by the neurosurgeon. A minority takes over only for final flap or graft placement.
ESBS = endoscopic skull base surgery; NUS = neurosurgeon.
Surgical Coding/Billing Practice Patterns
A lack of consensus was noted for surgical coding/billing in ESBS practice pattern queries (n = 63). Of respondents, 49.2% reported that they use endoscopic sinus codes (eg, 31255, 31287) as part of their overall coding profile, 44.4% utilize open skull base codes (eg, 61580, 61600) with or without other additional codes, and 39.7% of respondents use unlisted coding with open comparators (eg, 31299, 64999) as part of their coding profile. The relative proportion of various coding profiles is shown in Figure 5.
Figure 5.
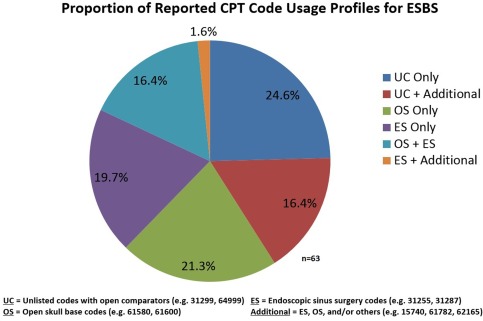
Respondents demonstrate little agreement on the variety of codes they use together to bill for ESBS cases.
CPT = current procedural terminology; ESBS = endoscopic skull base surgery.
Preoperative Antibiotic Preferences & Effect of Packing on Postoperative Antibiotics
The vast majority of respondents (n = 70) used either an intravenous first‐generation cephalosporin (41.4%) or a third‐generation cephalosporin (35.7%). A minority utilized combinations of these with gentamycin, vancomycin, clindamycin, or metronidazole. Of respondents, 7.1% used clindamycin alone. Only a single respondent stated that they used no preoperative intravenous antibiotic.
When no nasal packing was placed (n = 70) following ESBS, 45.7% prescribed no postoperative oral antibiotics; for those prescribing an oral antibiotic, the mean length for the regimen was 3.7 days (SD = 2.5). When absorbable packing was placed during ESBS (n = 69), 39.1% prescribed a 7‐day course of oral antibiotics while 24.3% still prescribed none. In the setting of nonabsorbable packing during ESBS (n = 67), 62.2% maintained the patient on postoperative oral antibiotics until the packing was removed at follow‐up; only 10.4% prescribed no antibiotics with nonabsorbable packing in place. When nonabsorbable packing was placed during ESBS (n = 46), the mean length of time that the packing was maintained was 5 days (SD = 2.6).
Lumbar Drain Usage Practice Patterns and Reported Complications
Only 2.9% (n = 70) placed lumbar drains (LDs) preoperatively for all ESBS cases (including basic pituitaries). Any time a CSF leak was anticipated, 14.3% placed LDs preoperatively, but 41.4% would only place preoperatively when a high‐flow CSF leak was anticipated. When a patient had suspected or confirmed elevated intracranial pressure (ICP), 32.9% placed LDs preoperatively. Finally, 31.4% never placed preoperative LDs.
When an LD was placed, a mean initial flow rate of 9.8 mL/hour (SD = 3.1) was calculated (n = 44). If an LD was placed and there was no ongoing evidence of a CSF leak (n = 50), continued lumbar CSF drainage was maintained a mean of 2.3 days (SD = 1.2) prior to clamping. When no ongoing evidence of a CSF leak was present, respondents (n = 50) clamped the LD for a mean of 1 day (SD = 0.35) prior to removal. When there was ongoing evidence of a CSF leak (n = 50), respondents reported conservative management with continued lumbar drainage for a mean of 2.5 days (SD = 2.2) prior to a return to the operating room for surgical exploration and repair. Overall, 38.6% reported having patients in their careers who experienced LD obstruction requiring replacement/removal and 30% whose postoperative patient course was complicated by a CSF leak from the drain site. Meningitis, drain site infection, and permanent neurologic deficit related to LD use were reported by smaller proportions of respondents (14.3%, 11.4%, and 4.3%, respectively).
Adjunct Procedures and Perceived Effects
The estimated frequencies of needed adjunct endonasal procedures performed simultaneously with ESBS is reported in Figure 6.
Figure 6.
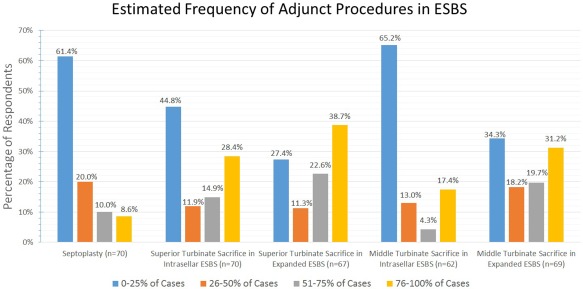
While it is infrequent for septoplasty to be required by respondents for ESBS approaches, superior and middle turbinate sacrifice are more frequently required in expanded ESBS cases than in intrasellar ESBS.
ESBS = endoscopic skull base surgery.
Nasoseptal Flap and Free Mucosal Graft Considerations
During the approach, 80.4% (of n = 56) harvested nasoseptal flaps if the need was anticipated and the remaining 19.6% harvested during closure. When harvesting the nasoseptal flap, 75% of respondents (n = 56) used cautery techniques (eg, fine tip monopolar electrocautery) for mucosal incisions and cite efficiency and hemostasis as their reason; 25% utilized cold‐steel‐only techniques (eg, sickle knife, scissors) and stated they avoid cautery for concern of olfactory preservation. A majority of respondents either left the nasoseptal flap harvest site uncovered or placed a silastic splint, while other repair types were uncommon (Fig. 7). When free mucosal grafts were harvested, 40% of respondents harvested from the middle turbinate, 33.3% from the nasal floor, and 18.3% from the septum. Estimated mean skull base repair types performed in the last year by respondents are show in Table 3.
Figure 7.
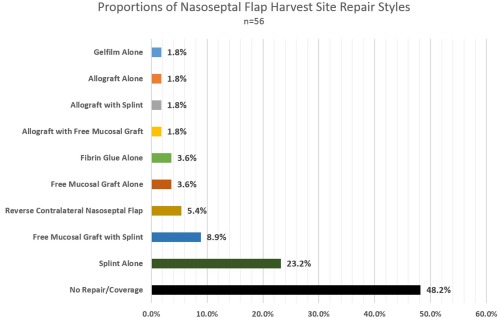
The majority of respondents perform no repair or coverage placement after nasoseptal flap harvest. The next most common coverage is a silastic splint alone.
Table 3.
Estimated Mean Skull Base Repair Type Numbers in Last Year by Practice Type.
| Repair Type |
Total N = 55 |
Private Practice N = 24 |
Academic Practice N = 31 |
P‐value |
|---|---|---|---|---|
| Nasoseptal flap | 16.4 (14.9) | 11.8 (16.9) | 19.8 (12.2) | .046 |
| Tunneled pericranial flap | 0.51 (1.56) | 0.13 (0.34) | 0.81 (2.0) | .08 |
| Transcranial pericranial flap | 0.51 (1.18) | 0.29 (1.04) | 0.68 (1.28) | .23 |
| Temporoparietal fascia flap | 0.22 (1.36) | 0.42 (2.04) | 0.06 (0.25) | .41 |
| Middle turbinate flap | 0.22 (1.36) | 0.42 (2.04) | 0.06 (0.25) | .41 |
| Inferior turbinate flap | 1.00 (3.60) | 0.54 (2.04) | 1.29 (4.46) | .45 |
Unless otherwise indicated values are expressed as mean number of cases (SD).
Bold indicates statistical significance (p < 0.05)
Preferred Skull Base Reconstruction Techniques
The most frequent reconstruction technique profiles cited for each skull base defect type are enumerated in Table 4. While infrequent, the use of “gasket seal”24 and “bilayer button”25 were reported by several respondents for all expanded intradural defects. The proportion of vascularized pedicled flaps used during intrasellar pituitary surgery was significantly higher in the intraoperative CSF leak group (39.2%) than in uncomplicated cases (9.1%); (χ2 [1, n = 106] = 13.33, P < .001). The proportion of expanded extradural cases utilizing vascularized pedicled flaps was significantly lower (51.3%) compared to expanded intradural cases (86.3%); (χ2[1, N = 236] = 33.70, P < .001).
Table 4.
Most Prevalent Reconstructive Technique Profiles by Case Type.
| Case/Defect Type | # Responses | Most Common Reconstructive Profiles |
|---|---|---|
| Purely intrasellar (No CSF leak) | 55 |
1. No repair performed (52.7%) 2. Overlay graft only (14.5%) 3. Underlay graft only (10.9%) |
| Purely intrasellar (Intraoperative CSF Leak) | 51 |
1. Combined underlay/overlay grafting (23.5%) 2. Overlay graft only or underlay graft only (19.6%) 3. Underlay graft with vascularized pedicled flap (15.7%) |
| Transtuberculum/planum extradural | 38 |
1. Underlay graft with vascularized pedicled flap (18.4%) 2. Vascularized pedicled flap alone (18.4%) 3. Combined underlay/overlay grafting (15.8%) 4. No repair performed (13.2%) |
| Transtuberculum/planum intradural | 42 |
1. Underlay graft with vascularized pedicled flap (47.6%) 2. Combined underlay/overlay grafting with vascularized pedicled flap (14.3%) 3. Gasket seal with vascularized pedicled flap (9.5%) |
| Transcribriform extradural | 39 |
1. Overlay graft only (20.5%) 2. No repair performed (17.9%) 3. Vascularized pedicled flap alone (15.4%) 4. Underlay graft with vascularized pedicled flap (12.8%) |
| Transcribriform intradural | 38 |
1. Underlay graft with vascularized pedicled flap (28.9%) 2. Combined underlay/overlay grafting with vascularized pedicled flap (23.7%) 3. Gasket seal with vascularized pedicled flap (10.5%) |
| Transclival extradural | 42 |
1. No repair performed (28.6%) 2. Vascularized pedicled flap alone (19.0%) 3. Underlay graft with vascularized pedicled flap (14.3%) 4. Combined underlay/overlay grafting (11.9%) |
| Transclival intradural | 37 |
1. Underlay graft with vascularized pedicled flap (48.6%) 2. Combined underlay/overlay grafting with vascularized pedicled flap (18.9%) 3. Combined underlay/overlay grafting (5.4%) |
CSF = Cerebrospinal Fluid.
Preferred Skull Base Repair Materials and Packing
Specific material combination use profiles were greatly divergent for various ESBS defects, especially among the expanded intradural defects. For the majority of defects, the three most frequent profiles represented less than 20% of the unique profiles. Therefore, the proportion of respondents who utilized various materials as part of their overall reconstruction is enumerated in Table 5. For purely intrasellar pituitary cases, nonabsorbable packing was used more commonly in the intraoperative CSF leak group (27.8%) than in uncomplicated pituitary cases (7.4%), (χ2 [1, n = 108] = 7.73, P = .005). Nonabsorbable packing was utilized significantly more with expanded cases with intradural defects (52.6%) than when the defect remained extradural (30.4%), (χ2 [1, n = 228] = 11.59, P = .001). Similarly, the use of any packing material was more common for intradural (87.9%) versus extradural (72.3%) expanded ESBS (χ2[1, n = 228] = 8.765, P = .003).
Table 5.
Proportion of Respondents Utilizing Various Materials in ESBS Reconstruction.
| Material Utilized | Purely Intrasellar | Transtuberculum/Transplanum | Transcribriform | Transclival | ||||
|---|---|---|---|---|---|---|---|---|
| No CSF n = 54 | +CSF Leak n = 54 | Extradural n = 39 | Intradural n = 40 | Extradural n = 36 | Intradural n = 39 | Extradural n = 37 | Intradural n = 37 | |
| No material/packing | 33.3% | 0% | 10.3% | 0% | 13.9% | 0% | 56.8% | 0% |
| Alloplastic dural substitute | 18.5% | 44.4% | 35.9% | 62.5% | 33.3% | 56.4% | 32.4% | 56.8% |
| Fascia lata | 3.7% | 5.6% | 10.3% | 20% | 5.6% | 23.1% | 5.4% | 13.5% |
| Fat | 5.6% | 48.1% | 17.9% | 37.5% | 16.7% | 35.6% | 27% | 51.4% |
| Bone | 5.6% | 18.5% | 12.8% | 17.9% | 19.4% | 20.5% | 5.4% | 10.8% |
| Cartilage | 3.7% | 13% | 2.6% | 10.3% | 8.3% | 10.3% | 2.7% | 5.4% |
| Medpor® button | 7.4% | 14.8% | 2.6% | 10.3% | 2.8% | 12.8% | 0% | 8.1% |
| Tissue glue | 22.2% | 53.7% | 43.6% | 59% | 41.7% | 56.4% | 32.4% | 56.8% |
| Absorbable packing | 51.9% | 59.3% | 66.7% | 74.3% | 58.3% | 71.8% | 56.8% | 70.3% |
| Merocel® pack with glove | 3.7% | 16.7% | 15.4% | 20% | 13.9% | 23.1% | 8.1% | 16.2% |
| Merocel® pack without glove | 3.7% | 7.4% | 15.4% | 17.5% | 16.7% | 23.1% | 18.9% | 18.9% |
| Balloon pack | 0% | 9.3% | 12.8% | 17.5% | 2.8% | 12.8% | 5.4% | 13.5% |
| Iodoform strip gauze | 0% | 1.9% | 0% | 7.5% | 0% | 5.1% | 0% | 2.7% |
CSF = cerebrospinal fluid; ESBS = endoscopic skull base surgery.
Postoperative Nasal Care and Debridement Schedule
Following intrasellar ESBS, 78.8% (n = 52) started saline sprays immediately postoperatively; the remaining all started saline sprays within two weeks. Only 25.4% (n = 51) started saline irrigations immediately after intrasellar ESBS, 37.2% started saline irrigations one week after surgery, and another 17.6% started them two weeks after surgery. Following expanded ESBS approaches, 68.7% (n = 48) began saline sprays immediately; the remaining respondents all started saline sprays within two weeks. Only 18.3% (n = 49) started saline irrigations immediately postoperatively following expanded ESBS, 36.7% started irrigations after one week, and 22.4% started irrigations after two weeks. The mean length of time postoperatively that the first nasal floor debridement was performed (n = 51) is 1.6 weeks (SD = 0.8) and for the first high nasal/skull base debridement (n = 48) is 2.8 weeks (SD = 1.2). Four respondents did not routinely perform any skull base debridements after ESBS.
Postoperative Activity Restrictions after ESBS
Various activity restrictions were surveyed and the results are reported fully in Figures 8, 9, 10, 11, 12, 13. For intrasellar ESBS, 98% of respondents (n = 51) waited at least two weeks before resumption of normal physical activities and all (n = 52) had patients refrain from driving for at least one week. Further data on swimming, flying, scuba diving, and positive airway pressure (PAP) use for various surgical cases are elaborated in Figures 10, 11, 12, 13, respectively.
Figure 8.
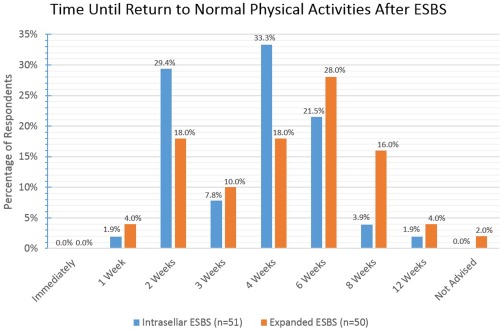
A diversity of responses were noted by respondents regarding when they allowed patients to return to normal acitivites including weight –bearing. This ranged for the most part from 2 to 8 weeks with longer periods of restriction noted for expanded ESBS cases.
ESBS = endoscopic skull base surgery.
Figure 9.
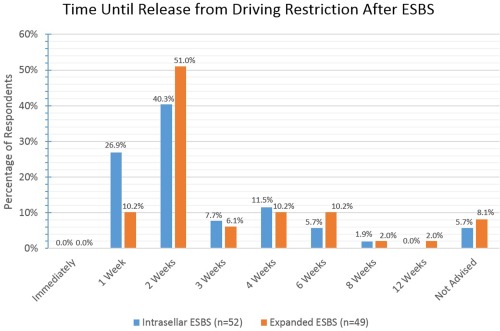
The majority of respondents allow driving by patients by the second postoperative week.
ESBS = endoscopic skull base surgery.
Figure 10.
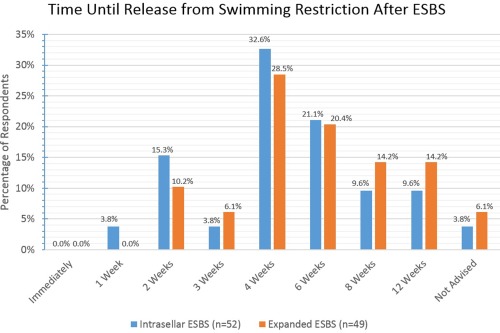
The majority of respondents make patients wait 4 to 6 weeks postoperatively, irrespective of ESBS type, before resuming swimming activities.
ESBS = endoscopic skull base surgery.
Figure 11.
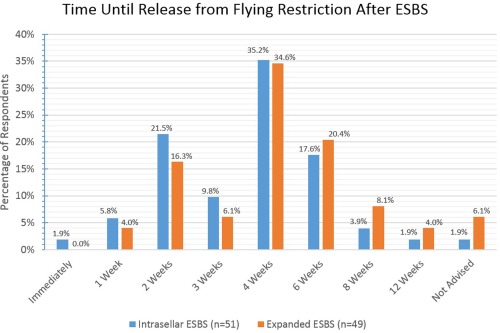
The majority of respondents allow for flying between 2 and 6 weeks postoperatively with little difference between intrasellar and expanded ESBS.
ESBS = endoscopic skull base surgery.
Figure 12.
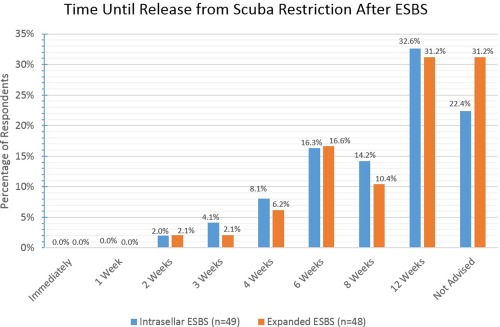
The majority of respondents require patients to wait 12 weeks to resume scuba diving activities or recommend that they never scuba dive again, regardless whether intrasellar or expanded approaches.
ESBS = endoscopic skull base surgery.
Figure 13.
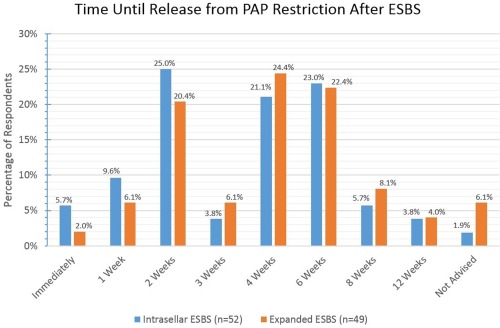
The majority of respondents allow for PAP resumption between 2 and 6 weeks postoperatively with little difference between intrasellar and expanded ESBS.
ESBS = endoscopic skull base surgery; PAP = positive airway pressure.
Provider‐Reported Postoperative Complications for ESBS
Provider‐reported frequency estimates of postoperative nasal complications are enumerated in Figure 14. The vast majority state nasal complications occur in less than 25% of their ESBS patients. Estimated numbers of severe complications within the last five years of practice were rare overall; only one respondent cited an intraoperative death having occurred. Respondents (n = 51) estimated a mean of 1.0 patient (SD = 1.8) who had suffered postoperative visual decline in the last five years. Postoperative blindness, stroke, or intraoperative carotid injury were all rare and estimated to have occurred less than once on average per respondent in the last five years.
Figure 14.
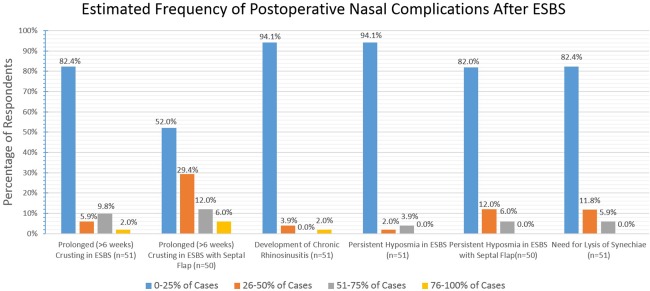
The majority of respondents report only infrequently having to address nasal complications following ESBS.
ESBS = endoscopic skull base surgery.
DISCUSSION
This survey illustrates the diversity in perioperative practices among otolaryngologists performing ESBS. Our respondents represented both private and academic practitioners with a similar proportion to a prior ARS survey by Lee et al.21 and a broad range of time in practice. Certainly, in a young subspecialty like rhinology, the inherited practice patterns of established fellowships may propagate similar practice pattern profiles. The lack of evidence‐based perioperative protocols and trials likely contribute to promoting the diversity of reconstructive profiles and postoperative restrictions we observed. However, where stronger data exist, such as for using vascularized pedicled flaps in high‐risk CSF‐leak ESBS, this study found significantly higher proportions of its use. Yet, areas such as surgeon positioning, billing/coding, antibiotic selection, and reconstructive materials demonstrate wide divergence which most likely represents personal preferences rather than data‐driven patterns.
This survey study represents the first to describe the wide range of perioperative practice patterns employed by otolaryngologists in both academic and private settings. Prior survey studies by Batra et al.22 (2013) and Lee et al.21 (2014) were limited to demographic data without reporting of preoperative, intraoperative, and postoperative practice patterns. Of the Batra et al. study22 respondents, nearly 45% were non‐otolaryngologists (neurosurgeons, neuroradiologists, etc) and 92.4% performed ESBS at academic centers which represents a completely different respondent population than this present study. Additionally, while both of these prior surveys queried coding strategies, respondents were forced to choose between four types of CPT codes which fails to adequately describe the complex CPT combinations that surgeons employ in their overall coding strategy.
It may be expected that this survey would find AP performing significantly more estimated total and expanded ESBS than their PP colleagues. The estimated case volumes in this study were consonant with a survey by Lee et al.21 In most regions, ESBS pathologies are initially referred to tertiary level neurosurgery practices. As APs are performing more overall and more expanded ESBS, it is also unsurprising that they perform more annual estimated nasoseptal flaps as this survey highlights. Respondents reported an increasing mean hospital length of stay as case complexity and risk of CSF leak increased; this trend likely bears out in most tertiary skull base practices.
Our survey found that over half of respondents were positioned on the same side (toward the head of the bed) as the neurosurgeon. While this may represent an inherited preference reflective of resident or fellowship training, it may just as likely be an effect of right‐handed predominance among surgeons. In our survey, only around 10% of neurosurgeons perform ESBS resections without an otolaryngologist driving the scope. This is consistent with the emphasis that high volume centers place on the multi‐handed, multidisciplinary team approach to ESBS.26, 27
Coding/billing represents a contentious issue among endoscopic skull base surgeons. Prior surveys document a consistently wide variation in coding patterns as well as a concern among skull base surgeons regarding adequate reimbursement.21, 22 These same surveys found that 85–87% of respondents are interested in the creation of codes specific for ESBS. Compared to these coding surveys, our respondents utilize a higher proportion of sinus codes and unlisted codes with open comparators when billing their ESBS cases.21, 22 Unlike the prior studies on this topic, our coding query identified specific coding profiles where respondents' utilization of a mixture of code types is specified.
Scant literature exists regarding antibiotic prophylaxis in ESBS and no existing data suggest the superiority of one regimen over another in ESBS.28, 29, 30, 31 Regardless of antibiotic choice, infectious complications such as meningitis remain rare and are associated with postoperative CSF leak.28, 29, 32 One prior study suggested 24 to 48 hours of a single agent covering gram‐positive organisms.32 Another study recommended two doses of cefuroxime was adequate for transsphenoidal surgery.33 The majority of our respondents utilized a regimen consistent with these recommendations. Similarly, limited data exist to suggest a definitive postoperative antibiotic regimen in the setting of nasal packing placement following endoscopic sinus surgery or ESBS.28, 34 Antibiotics have been prescribed in this situation for fear of toxic shock syndrome, but this remains an extremely rare complication.30, 34 That the majority of our respondents prescribed antibiotics when packing was placed is suggestive of this ongoing concern.
Initial ESBS experiences were associated with high‐CSF leak rates.16 Perioperative CSF lumbar drainage represents one way of potentially mitigating this risk.20, 35 With CSF leak rate reduction through use of vascularized pedicled flaps in reconstruction, there has been a reported reduction of LD usage with emphasis on placement in response to specific reconstruction, flow of CSF leak, and surgeon preference.23, 36 Our survey indicated more respondents place LDs preoperatively as the perceived risk of postoperative CSF leak increases. Contradicting this logic is the recent meta‐analysis by D'Anza et al.,35 which found that LD use was not associated with a reduced odds ratio for postoperative CSF leak. When postoperative CSF leak occurs, our respondents attempt conservative management with lumbar drainage an average of 2.5 days, consistent with published recommendations.37, 38 However, in a large series Kassam et al.38 reported a success rate of only 23.6% with LD alone for postoperative CSF leaks while the remaining 76.4% required a return to the OR for a nearly universally successful surgical closure.
The variety of skull base defects and methods of reconstruction have been amply described in the recent literature.39, 40, 41 Sigler et al.41 elaborate upon the ESBS “reconstructive ladder” wherein increasingly complex defects are addressed with pedicled intranasal or extranasal vascularized tissue reconstruction in order to mitigate the risk of CSF leak. Our survey respondents demonstrate reliance upon these principles with increasing proportions utilizing multilayer closure techniques and vascularized pedicled flaps for reconstruction of higher risk defects. Rigid reconstructive materials such as bone, cartilage, and the Medpor button (Stryker Corp, Kalamazoo, Michigan, U.S.A.) were used less frequently than fat or alloplastic dural substitutes. Tissue glue or dural sealants were used by a larger proportion of our respondents for intradural defects despite one study suggesting that they do not reduce CSF leaks.42 That a significantly higher proportion of respondents place packing after intradural repairs compared to extradural repairs speaks to the common wisdom that bolstering will reduce the risk of postoperative CSF leak.
While evidence‐based recommendations for postoperative nasal care and debridement schedules are lacking, the trends in postoperative care in our survey results correspond to the recommendations by Tien et al.30 The majority of our respondents start saline sprays in the nose immediately postoperatively and most report saline irrigations are started 1 to 2 weeks postoperatively. Initial nasal floor debridement to improve the nasal airway occurs on average around 10 days postoperatively and more extensive upper nasal cavity debridement around 3 weeks postoperatively.
There is also a lack of evidence‐based recommendations for ESBS postoperative restrictions. As spontaneous CSF leakage is associated with elevated intracranial pressure,43, 44 it is reasonable to avoid ICP‐elevating activities which may threaten the skull base repair in the immediate postoperative period. The length of time for these restrictions remains ill‐defined. In our survey, the majority release patients from activity restrictions between 2 and 6 weeks postoperatively, with increasing lengths of time for intradural repairs. The timing for resumption of positive airway pressure (PAP) devices for obstructive sleep apnea has been empirically suggested by one author to be 6 weeks based on an animal model.45 PAP use in skull base defects has been reported multiple times as a cause of life‐threatening tension pneumocephalus.45, 46, 47 Slightly less than a quarter of our respondents restarted PAP at 6 weeks for both intrasellar pituitary and expanded ESBS; a smaller but equal proportion started at 2 weeks and 4 weeks, respectively, which demonstrates the lack of consensus in PAP management following ESBS.
As with other survey studies, this present survey represents a self‐selected group of ARS members choosing to participate in the distributed survey. With a total invited cohort of 2,172 ARS members, the apparent response rate for this study would be 3.2%. However, it is unknown what proportion of ARS membership routinely performs ESBS and therefore the effective response rate is likely much higher but more difficult to quantify. The most recent data available from the Centers for Medicare and Medicaid Services (CMS) in 2015 show only 621 providers billing CPT 62,165 (removal of pituitary gland tumor using an endoscope). Based upon these CMS data and presuming 50% of these codes were billed by otolaryngologists, our effective response rate of all ESBS‐performing otolaryngologists (regardless of ARS‐membership status) would therefore approximate 22.5%. Care must be taken to avoid over‐generalizing our results onto the entire population of endoscopic skull base surgeons. Additionally, recall bias in estimated case numbers and other data points is possible. The complexity of ESBS may lead respondents to unconsciously over‐estimate their case volumes and other queried data. Responses should be considered as the estimates and/or opinions of the respondents and interpreted by coauthors of this manuscript and not the view of the ARS. Despite its limitations, this study serves as the first to highlight a sample of endoscopic skull base surgeon perioperative practice patterns for which there are scant published data.
CONCLUSION
Based on responses from fellowship‐ and non‐fellowship‐trained otolaryngologists in both academic and private settings, considerable variation remains in the perioperative management of ESBS patients. Further evidence‐based studies and guidelines are needed to inform skull base surgeons of optimal perioperative practices that will improve patient outcomes.
Funding: The distribution fee for this electronic survey to American Rhinologic Society staff membership was covered by the senior author.
Conflicts of Interest: The authors have no financial disclosures or conflicts of interest to report.
Attestation: This manuscript is not under consideration for publication by any other journals than the Laryngoscope Investigative Otolaryngology.
BIBLIOGRAPHY
- 1. Fatović‐Ferencić S, Gnjidić Z. Centenary of the first transsphenoidal surgery of the hypophysis (Hermann Schloffer 1907) and its echoes within Croatian neurosurgical practice. Wien Med Wochenschr 2007;157:618–624. [DOI] [PubMed] [Google Scholar]
- 2. Schloffer H. Erfolgreiche Operation eines Hypophysentumors auf nasalem Wege. Wien Klin Wochenschr 1907;20:621–624. [Google Scholar]
- 3. Schloffer H. Weiterer Bericht über den Fall von operiertem Hypophysentumor. Wien Klin Wochenschr 1907;20:1075–1078. [Google Scholar]
- 4. Schloffer H. Zur Frage der Operationen an der Hypophyse. Beitr Klin Chir 1906;50:767–817. [Google Scholar]
- 5. Liu JK, Cohen‐gadol AA, Laws ER, et al. Harvey Cushing and Oskar Hirsch: early forefathers of modern transsphenoidal surgery. J Neurosurg 2005;103:1096–1104. [DOI] [PubMed] [Google Scholar]
- 6. Jankowski R, Auque J, Simon C, et al. Endoscopic pituitary tumor surgery. Laryngoscope 1992;102:198–202. [DOI] [PubMed] [Google Scholar]
- 7. Jho HD, Carrau RL. Endoscopic endonasal transsphenoidal surgery: experience with 50 patients. J Neurosurg 1997;87:44–51. [DOI] [PubMed] [Google Scholar]
- 8. Casler JD, Doolittle AM, Mair EA. Endoscopic surgery of the anterior skull base. Laryngoscope 2005;115:16–24. [DOI] [PubMed] [Google Scholar]
- 9. Snyderman CH, Carrau RL, Kassam AB, et al. Endoscopic skull base surgery: principles of endonasal oncological surgery. J Surg Oncol 2008;97:658–664. [DOI] [PubMed] [Google Scholar]
- 10. Dehdashti AR, Ganna A, Witterick I, Gentili F. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations. Neurosurgery 2009;64:677–689. [DOI] [PubMed] [Google Scholar]
- 11. Snyderman CH, Kassam AB, Carrau RL, Mintz A. Endoscopic reconstruction of cranial base defects following endonasal skull base surgery. Skull Base 2007;17:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leong JL, Citardi MJ, Batra PS. Reconstruction of skull base defects after minimally invasive endoscopic resection of anterior skull base neoplasms. Am J Rhinol 2006; 20:476–482. [DOI] [PubMed] [Google Scholar]
- 13. Patel MR, Stadler ME, Snyderman CH, et al. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base 2010;20:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hadad G, Bassagasteguy L, Carrau RL. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006;116:1882–1886. [DOI] [PubMed] [Google Scholar]
- 15. Kassam AB, Thomas A, Carrau RL, et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 2008;63:44–53. [DOI] [PubMed] [Google Scholar]
- 16. Harvey RJ, Parmar P, Sacks R, Zanation AM. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope 2012;122:452–459. [DOI] [PubMed] [Google Scholar]
- 17. Zanation AM, Snyderman CH, Carrau RL, et al. Minimally invasive endoscopic pericranial flap: a new method for endonasal skull base reconstruction. Laryngoscope 2009;119:13–18. [DOI] [PubMed] [Google Scholar]
- 18. Fortes FS, Carrau RL, Snyderman CH, et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope 2007;117:970–976. [DOI] [PubMed] [Google Scholar]
- 19. Fortes FS, Carrau RL, Snyderman CH, et al. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope 2007;117:1329–1332. [DOI] [PubMed] [Google Scholar]
- 20. Zanation AM, Carrau RL, Snyderman CH, et al. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol 2009;23:518–521. [DOI] [PubMed] [Google Scholar]
- 21. Lee JT, Kingdom TT, Smith TL, et al. Practice patterns in endoscopic skull base surgery: survey of the American Rhinologic Society. Int Forum Allergy Rhinol 2014;4:124–131. [DOI] [PubMed] [Google Scholar]
- 22. Batra PS, Lee J, Barnett SL, et al. Endoscopic skull base surgery practice patterns: survey of the North American Skull Base Society. Int Forum Allergy Rhinol 2013;3:659–663. [DOI] [PubMed] [Google Scholar]
- 23. Tien DA, Stokken JK, Recinos PF, et al. Cerebrospinal fluid diversion in endoscopic skull base reconstruction: an evidence‐based approach to the use of lumbar drains. Otolaryngol Clin North Am 2016;49:119–129. [DOI] [PubMed] [Google Scholar]
- 24. Garcia‐Navarro V, Anand VK, Schwartz TH. Gasket seal closure for extended endonasal endoscopic skull base surgery: efficacy in a large case series. World Neurosurg 2013;80:563–568. [DOI] [PubMed] [Google Scholar]
- 25. Luginbuhl AJ, Campbell PG, Evans J, Rosen M. Endoscopic repair of high‐flow cranial base defects using a bilayer button. Laryngoscope 2010;120:876–880. [DOI] [PubMed] [Google Scholar]
- 26. Snyderman CH, Wang EW, Fernandez‐Miranda JC, Gardner PA. The making of a skull base team and the value of multidisciplinary approach in the management of sinonasal and ventral skull base malignancies. Otolaryngol Clin North Am 2017;50:457–465. [DOI] [PubMed] [Google Scholar]
- 27. Sindwani R, Woodard TD, Recinos PF. Building a successful endoscopic skull base and pituitary surgery practice. Otolaryngol Clin North Am 2016;49:1–8. [DOI] [PubMed] [Google Scholar]
- 28. Kono Y, Prevedello DM, Snyderman CH, et al. One thousand endoscopic skull base surgical procedures demystifying the infection potential: incidence and description of postoperative meningitis and brain abscesses. Infect Control Hosp Epidemiol 2011;32:77–83. [DOI] [PubMed] [Google Scholar]
- 29. Rosen SA, Getz AE, Kingdom T, et al. Systematic review of the effectiveness of perioperative prophylactic antibiotics for skull base surgeries. Am J Rhinol Allergy 2016;309:10–16. [DOI] [PubMed] [Google Scholar]
- 30. Tien DA, Stokken JK, Recinos PF, et al. comprehensive postoperative management after endoscopic skull base surgery. Otolaryngol Clin North Am 2016;49:253–263. [DOI] [PubMed] [Google Scholar]
- 31. Orlando R, Cappabianca P, Tosone G, et al. Retrospective analysis of a new antibiotic chemoprophylaxis regimen in 170 patients undergoing endoscopic endonasal transsphenoidal surgery. Surg Neurol 2007;68:145–148. [DOI] [PubMed] [Google Scholar]
- 32. Brown SM, Anand VK, Tabaee A, Schwartz TH. Role of perioperative antibiotics in endoscopic skull base surgery. Laryngoscope 2007;117:1529–1532. [DOI] [PubMed] [Google Scholar]
- 33. Little AS, White WL. Short‐duration, single‐agent antibiotic prophylaxis for meningitis in trans‐sphenoidal surgery. Pituitary 2011;14:335–339. [DOI] [PubMed] [Google Scholar]
- 34. Coughlan CA, Bhandarkar ND. The role of antibiotics in endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg 2015;23:47–52. [DOI] [PubMed] [Google Scholar]
- 35. D'Anza B, Tien D, Stokken JK, et al. Role of lumbar drains in contemporary endonasal skull base surgery: Meta‐analysis and systematic review. Am J Rhinol Allergy 2016;30:430–435. [DOI] [PubMed] [Google Scholar]
- 36. Ackerman PD, Spencer DA, Prabhu VC. The efficacy and safety of preoperative lumbar drain placement in anterior skull base surgery. J Neurol Surg Rep 2013;74:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Esposito F, Dusick JR, Fatemi N, Kelly DF. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Neurosurgery 2007;60(4 Suppl 2):295–304. [DOI] [PubMed] [Google Scholar]
- 38. Kassam AB, Prevedello DM, Carrau RL, et al. Endoscopic endonasal skull base surgery: analysis of complications in the authors' initial 800 patients. J Neurosurg 2011;114:1544–1568. [DOI] [PubMed] [Google Scholar]
- 39. Zuniga MG, Turner JH, Chandra RK. Updates in anterior skull base reconstruction. Curr Opin Otolaryngol Head Neck Surg 2016;24:75–82. [DOI] [PubMed] [Google Scholar]
- 40. Eloy JA, Marchiano E, Vázquez A, et al. Management of skull base defects after surgical resection of sinonasal and ventral skull base malignancies. Otolaryngol Clin North Am 2017;50:397–417. [DOI] [PubMed] [Google Scholar]
- 41. Sigler AC, D'Anza B, Lobo BC. endoscopic skull base reconstruction: an evolution of materials and methods. Otolaryngol Clin North Am 2017;50:643–653. [DOI] [PubMed] [Google Scholar]
- 42. Eloy JA, Choudhry OJ, Friedel ME, et al. Endoscopic nasoseptal flap repair of skull base defects: is addition of a dural sealant necessary? Otolaryngol Head Neck Surg 2012;147:161–166. [DOI] [PubMed] [Google Scholar]
- 43. Nelson RF, Gantz BJ, Hansen MR. The rising incidence of spontaneous cerebrospinal fluid leaks in the United States and the association with obesity and obstructive sleep apnea. Otol Neurotol 2015;36:476–480. [DOI] [PubMed] [Google Scholar]
- 44. Schlosser RJ, Woodworth BA, Wilensky EM, et al. Spontaneous cerebrospinal fluid leaks: a variant of benign intracranial hypertension. Ann Otol Rhinol Laryngol 2006;115:495–500. [DOI] [PubMed] [Google Scholar]
- 45. Kopelovich JC, de la Garza GO, Greenlee JD, et al. Pneumocephalus with BiPAP use after transsphenoidal surgery. J Clin Anesth 2012;24:415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wannemuehler TJ, Hubbell RD, Nelson RF. Tension pneumocephalus related to spontaneous skull base dehiscence in a patient on BiPAP. Otol Neurotol 2016;37:e322–e324. [DOI] [PubMed] [Google Scholar]
- 47. Jarjour NN, Wilson P. Pneumocephalus associated with nasal continuous positive airway pressure in a patient with sleep apnea syndrome. Chest 1989;96:1425–1426. [DOI] [PubMed] [Google Scholar]


