Abstract
Objective
To compare the safety and efficacy of manual and powered irrigation of the middle ear using saline or 1% baby shampoo to treat biofilm‐forming bacterial middle ear infections.
Background
Biofilms play a major role in recalcitrant otitis media and are challenging to treat. Many therapeutic strategies have been attempted and the role of topical therapies is still being investigated. Topical irrigation using saline or 1% baby shampoo and the use of a hydrodebrider have been investigated in biofilms involved in chronic rhinosinusitis and their role within the middle ear is yet to be determined.
Methods
Twenty‐two adult chinchillas underwent bilateral trans‐bullar inoculation of non‐typable biofilm forming Haemophilus influenza followed by unilateral middle ear irrigation 5 days later using saline administered via a powered hydrodebrider or manual irrigation of saline or 1% baby shampoo. Contralateral inoculated ears served as control and were not irrigated. Two days following irrigation, the bullae were harvested and processed for scanning electron microscopy to assess biofilm surface area. Auditory brainstem responses were performed before bacterial inoculation and prior to euthanasia.
Results
Manual and powered irrigation were effective in reducing the surface area of biofilm when compared to the control group. The hydrodebrider demonstrated to be more effective at eradicating biofilm than manual irrigation, especially in areas of difficult access, such as the ventral portion of the chinchillas' bullae. There was no difference in manual irrigation of saline when compared to 1% baby shampoo. Irrigations either manually or using the hydrodebrider did not affect hearing, the vestibular system or facial function.
Conclusion
Middle ear biofilms can be treated safely and effectively with rinses using either normal saline or 1% baby shampoo administered manually or with a powered hydrodebrider.
Level of Evidence
NA.
Keywords: Biofilm, otitis media with effusion, chronic otitis media, refractory otitis media, Haemophilus influenza, manual irrigation, powered irrigation, hydrodebrider, antibiotic resistant, planktonic bacteria state, biofilm matrix
INTRODUCTION
Historically, otitis media with effusion (OME) was thought to be a noninfectious “sterile” inflammatory state based on the inability to culture bacteria from most middle ear effusions. With the use of polymerase chain reaction (PCR) and DNA analysis, we now know that metabolically active, live bacteria are indeed present in as many as 50% of culture‐negative OME in the form of biofilms, with Haemophilus Influenzae being the most common pathogen.1 Studies by the CDC estimate that 65% of all human infectious processes involve biofilms,2 and mucosal biofilm formation have been identified in refractory OME. The extracellular matrix that composes the biofilm protects the bacteria against antibodies, immune‐system phagocytosis, and antibiotic penetration. In addition, bacteria living in biofilm develop adaptive genetic mutations and have a lower metabolic activity level than planktonic bacteria, further reducing their susceptibility to certain antimicrobials.3, 4
Many strategies have been proposed to eradicate mucosal biofilms in chronic infections, and they include surgery, topical antibiotics, and adjuvant therapies aimed to disrupt the biofilm life‐cycle. Treatment strategies directed at eradicating biofilms by irrigation and topical application of antibiotic,5, 6 surfactants,7 and other biofilmcidal agents instead of systemic antibiotics may prove more effective. Baby shampoo contains the surfactant agents PEG‐80 sorbitan laurate, cocamidopropyl betaine, and sodium trideceth sulfate that can act as a surfactant and decrease the viscosity and surface tension of airway mucus. It was shown to provide symptomatic improvement in patients with chronic sinusitis with pseudomonas biofilms8 but was never tested in the middle ear.
Recently, a hydrodebrider device has been developed for sinonasal cavity irrigation in the attempt to treat mucosal biofilms involved in recalcitrant rhinosinusitis. This device allows controlled delivery of a shear force to the mucosal surface of the sinonasal cavity and has proven as a useful adjunct in the disruption of the biofilm structure in a sheep model of rhinosinusitis.9
The chinchilla model to study biofilms in otitis media has been well established.10 We hereby describe our attempt to eradicate biofilms in the middle ear of chinchillas caused by non‐typable Haemophilus Influenzae using middle ear lavage with various solvents and using a prototype hydrodebrider developed to irrigate the chinchilla's middle ear. This hydrodebrider is being designed while keeping in mind its potential use in clinical trials of refractory OME.
Material and Methods
Animals
Research‐grade, healthy young adult chinchillas lanigera (Moulton Chinchilla Ranch, MN, USA) weighing approximately 500 g were used for the experiment. All animals were treated according to the experimental protocol approved by the University of Miami Animal Care and Use Committee, and in full compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Animal Welfare Act (7 U.S.C. et seq.). Upon arrival, the animals were quarantined and microbiologic cultures from each animal's nose were obtained to assure that the chinchillas were free of infection. Each chinchilla was also examined by bilateral otoscopy to rule out middle ear disease. Twenty‐two chinchillas were used in this experiment.
Audiological Analysis
Baseline hearing testing was performed utilizing auditory brainstem response (ABR) in each ear under sedation with ketamine (35 mg/kg) and xylazine (5 mg/kg) administered intramuscularly to the chinchilla. ABR tests to pure tone stimuli were performed to measure hearing threshold levels using the Intelligent Hearing Systems Hardware and Software (IHS, Miami, FL, USA). These tests were performed preoperatively and again on post‐inoculation day 7. During ABR recordings and recovery, the chinchilla was kept at body temperature with a thermocouple regulated water circulating heating pad until the animal was fully ambulatory and alert. Testing was conducted bilaterally to record responses at 0.5, 1, 4, and 16 kHz. Electrical activity was recorded using an electrode placed in the vertex of the skull, referenced to a needle electrode inserted into the neck musculature, and grounded to a second needle electrode inserted subcutaneously near the tympanic bulla. Stimulus was introduced to each ear beginning with an intensity of 70 dB SPL and decreasing in increments of 10 dB until threshold. Responses at or near threshold were confirmed on a second testing. Threshold was defined as the lowest intensity resulting in a reproducible ABR waveform, where good morphology of wave III or V response could be visually identified.
Inoculation of Biofilm‐Forming Bacteria
Each middle ear of the chinchilla was inoculated using 0.1 mL of a solution containing 105 colony‐forming units (CFU) of non‐typable Haemophilus influenzae injected through the dorsal bullae in the following manner: via a dorsal approach, an 18‐gauge needle was advanced through the skin and underlying soft tissues until it penetrated the bony wall of dorsal bulla; then, the solution was injected into the dorsal bulla through the access hole created with the 18‐gauge needle using a tuberculin syringe and a 0.5‐inch 27‐gauge needle. The anatomical continuity between the dorsal and ventral bulla of the animal allowed the spread of the infection. Inoculation was done under sedation using ketamine (35 mg/kg) and xylazine (5 mg/kg) administered intramuscularly immediately after the completion of the baseline ABR. This concentration of Haemophilus influenzae bacteria has been shown in previous studies to induce experimental otitis media and biofilm formation in the chinchilla animal model.10, 11 The bacteria were acquired from the laboratory of Christopher Post, MD, PhD, consisting of a human clinical strain of Haemophilus influenzae called “PittEE” and are known to be a biofilm‐forming strain under the aforementioned concentration.10
Manual Irrigations
Each animal had one ear randomized as the experimental ear while the contralateral ear served as control. Five days following inoculation and under appropriate sedation with ketamine and xylazine, the experimental ear of each chinchilla was accessed through a dorsal approach. Using sterile technique, the soft tissue of the skull was dissected, and a 2‐mm opening was made in the dorsal bulla using a diamond bur on an electrical drill. The experimental bullae was subsequently irrigated manually with a 22‐gauge needle using a volume of 12 mL over 10 seconds of either sterile normal saline (n = 7 bullae) or 1% baby shampoo solution (n = 7 bullae), while suctioning with a 3 French Frazier suction tip attached to a suction pump at a constant flow to avoid tympanic membrane rupture (average middle ear volume of adult chinchilla is at least 2 mL).
The inoculated, non‐irrigated side, contralateral to the side that underwent either saline or baby shampoo irrigation, was left undisturbed and served as control. We randomly chose eight non‐irrigated contralateral ears as controls from the animals that underwent either manual saline or baby shampoo irrigations. Following the irrigation/suction of the middle ear, the periosteum and skin were sutured back in a sterile fashion using a bioresolvable suture.
Hydrodebrider Irrigations
An additional 8 inoculated ears (bullae) from 8 chinchillas underwent normal saline irrigation using a prototype hydrodebrider (Medtronic Corp., Jacksonville, FL, USA) at a force of 10 bars and a constant flow rate of 1.18 mL/s on day 5 post‐inoculation. The hydrodebrider has an incorporated irrigation and suction system within the same tip. These bullae were also accessed via a dorsal approach as described above with a 12‐mm opening made utilizing a diamond bur. During the hydrodebrider irrigations, the solution was allowed to circulate in and out of the bullae for 10 seconds, through a hydrodebrider cannula that had an outer diameter of 1.47 mm, displaying five equally spaced (72 degrees) radial holes for irrigation, and one axial hole at the tip for suction. After the irrigation/suction of the middle ear, the periosteum and skin are sutured back in a sterile fashion using a bioresolvable suture (Fig. 1 and Video 1).
Figure 1.

(A) Set up of the hydrodebrider with the irrigating and suctioning tip displayed; (B) Irrigating tip of the hydrodebrided showing the 6 different flows of irrigation.
Groups
The groups consisted of one control group (group 1; 8 ears) and three intervention groups (groups 2, 3, and 4): group 2 consisted of seven ears that underwent manual irrigation with normal saline, group 3 consisted of seven ears that underwent manual irrigation with 1% baby shampoo, and group 4 consisted of eight ears that underwent irrigation with normal saline utilizing the hydrodebrider. On day 7 post‐inoculation, otoscopy was performed bilaterally to ascertain that the tympanic membranes were not disrupted by the irrigations. A final ABR prior to euthanasia on post‐inoculation day 7 was obtained under the aforementioned sedation protocol with ketamine and xylazine.
Specimen Collection and Preparation
Bilateral mucosal specimens were collected from all animals at the time of euthanasia. On day 7 after inoculation, animals were placed under deep general anesthesia by intramuscular administration of the anesthetic mixture and subsequently euthanized using 1 mL of Euthasol (Virbac AH, Inc., Fort Worth, TX, USA) administered intraperitoneally. The tympanic bullae were harvested through a dorsal approach to the skull. The bullae were split into dorsal (superior) and ventral (inferior) halves, then placed in formaldehyde (4% in phosphate‐buffered saline), and promptly transported to the imaging center for scanning electron microscopy. Four specimens were obtained from each animal (right and left dorsal, right and left ventral). At least 20 mucosal samples were collected from each of the four specimens of each animal. Samples of the formaldehyde‐fixed bullae were washed with Sorenson's phosphate buffer (0.2 M) for 15 minutes (four washes), treated with 1% osmium tetroxide for 30 minutes, dehydrated by sequential alcohol baths, and washed with hexamethyldisilavance (HMDS) four times for 15 minutes. A few drops of HMDS were then placed on the samples and the specimens were dried for 48 hours under a hood. Samples were then mounted and gold‐sputter‐coated in final preparation for scanning electron microscopy (SEM) imaging.
Scanning Electron Microscopy
Image analysis and calculations were performed by a scanning electron microscopist who was blinded to the interventions. SEM images were obtained using a Philips XL‐30 Field Emission Scanning Electron Microscope (FEI Company, Hillsboro, Oregon) at an accelerating voltage of 20 kV, and working distance of 20 mm. Images were captured using the Philips digital imaging software system and digitized as high‐resolution TIFF computer files, and then converted to high‐quality JPEG files using Photoshop CS5 (12.0) software (Adobe Systems Inc., San Jose, CA, USA). Areas containing biofilm were identified.
The presence of biofilm was confirmed by SEM. The film was routinely semi‐transparent in the electron beam at 20 kv and had a thin, often wavy appearance. Both these characteristics suggest an organic film. In addition, an Energy Dispersive Spectroscopy (EDS) elemental analysis system on the SEM was used to identify the elements present in the area under the electron beam. Confirmation of biofilm was made if no non‐organic elements were detected. Therefore, in addition to the described morphological characteristics, if elemental analysis criteria were met as well, it was concluded that the area under examination was biofilm.
Biofilm counts were quantitatively scored by determining the biofilm surface area colonization using image analysis software (Carnoy image analysis software package, http://www.kuleuven.ac.be/bio/sys/carnoy). This method has been previously validated in the literature.12 The surface area was calculated by using Heron's formula (fitting triangles, calculating area for each triangle and then adding them up).
Heron's formula13 states that the area of a triangle whose sides have lengths a, b, and c is:
An average value of biofilm‐covered surface area was then calculated from the 20 mucosal samples for each half bulla (ventral or dorsal) (Fig. 2).
Figure 2.
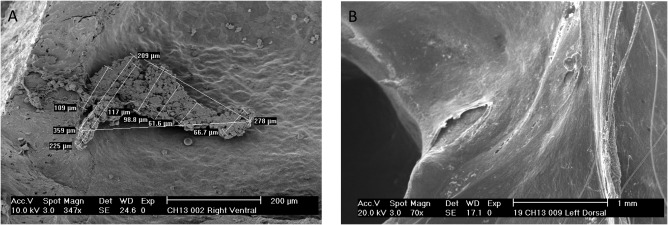
Scanning electron microscopy images. (A) Control ear specimen showing the presence of biofilm ant he method of calculation of the surface area; (B) Specimen of an irrigated ear showing absence of biofilm in the ventral surface of the bulla.
Statistical Analysis
Statistical analysis was done using JMP Pro 13 statistical package (SAS Institute Inc., Cary, NC, USA). Nonparametric pair comparisons of each group pair were made by Wilcoxon rank test because not all of the groups' data had a normal distribution.
RESULTS
Figure 3 shows representative SEM findings on each group, while Tables 1 through 4 and Figure 4 show mean and median values of the biofilm surface area for each intervention group by site (dorsal bulla and ventral bulla, respectively).
Figure 3.
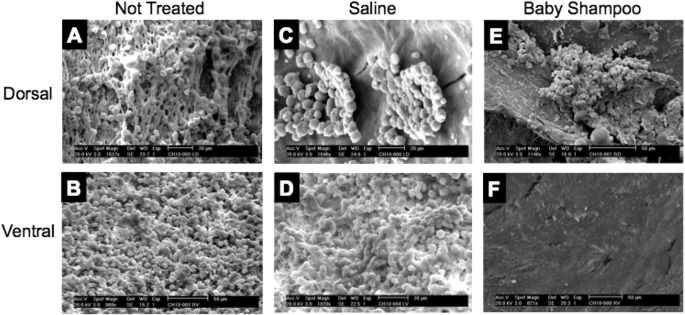
Scanning electron microscopy images for visual comparison of the presence of biofilm across the different groups, showing that irrigation favorably reduce biofilm from ventral rather than dorsal halves of the bullae. (A) Non‐treated dorsal bullae; (B) Non‐treated ventral bullae; (C) Saline treated dorsal bullae; (D) Saline treated ventral bullae; (E) Baby shampoo treated dorsal bullae; (F) Baby shampoo treated ventral bullae.
Table 1.
Median and Mean Values of the Biofilm Surface Area in the Dorsal Bulla in Control and Experimental Groups. Values were calculated from at least 20 mucosal specimens obtained from the dorsal bulla of each animal in μm2.
| Group (n) | Mean (SD) μm2 | 95% CI μm2 |
|---|---|---|
| Control (8) | 3156.61 (1790.07) | 1660–4653.2 |
| Manual saline (7) | 858.92 (492.22) | 404–1314.1 |
| Manual shampoo (7) | 3137.22 (3807.31) | −384–6658.4 |
| Hydrodebrider saline (8) | 355.76 (506.16) | −67–778.9 |
CI = confidence interval; SD = standard deviation.
Table 4.
Descriptive Statistics of Nonparametric Comparisons for each pair using Wilcoxon Rank Test in the Ventral Bullae.
| Comparison | Mean (SE) differences in μm2 | 95% CI μm2 | P‐value |
|---|---|---|---|
| Shampoo vs. saline | 1.143 (2.236) | −2594.83–1920.33 | .609 |
| Saline vs. control | 4.42 (2.315) | −3442.90–227.00 | .056 |
| Hydrodebrider vs. saline | 4.955 (2.315) | −3134.66–(−193.29) | .0323* |
| Shampoo vs. control | 5.223 (2.315) | −3471.98–(−578.18) | .024* |
| Hydrodebrider vs. shampoo | 5.759 (2.315) | −2265.44–(−180.6) | .0128* |
| Hydrodebrider vs. control | 7.875 (2.380) | −4123.01–(−2600.81) | .0009*** |
CI = confidence interval; SE = standard error. * P < .05; *** P < .01.
Figure 4.
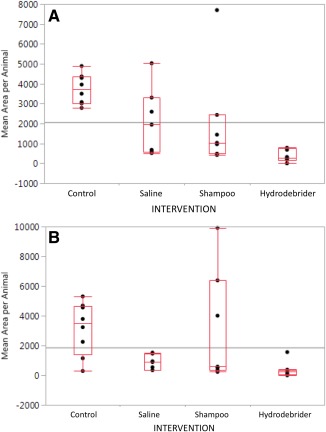
Box‐plot graph with points showing mean surface area of the biofilm per group in the dorsal bulla (A) and the ventral bulla (B).
Table 2.
Median and Mean Values of the Biofilm Surface Area in the Ventral Bulla in Control and Experimental Groups. Values were calculated from at least 20 mucosal specimens obtained from the ventral bulla of each animal in μm2.
| Group (n) | Mean (SD) μm2 | 95% CI μm2 |
|---|---|---|
| Control (8) | 3736.55 (756.7) | 3104–4369.2 |
| Manual saline (7) | 2094.91 (1688.96) | 533–3656.9 |
| Manual shampoo (7) | 2068.77 (2573.73) | −312–4449.1 |
| Hydrodebrider saline (8) | 395.60 (303.48) | 142–649.3 |
CI = confidence interval; SD = standard deviation.
In the dorsal bulla, the biofilm surface area value of animals treated by manual saline irrigation and hydrodebrider saline irrigation were lower than that of the control animals (P = .0323 and P = .0038, respectively). The biofilm surface area value of the hydrodebrider group was also significantly lower than that of the animals treated by manual shampoo irrigations (P = .0321). There was no difference between the values of the manual saline irrigation and the manual shampoo irrigation groups (P = .751), between the manual saline irrigation and the hydrodebrider irrigation groups (P = .0560), and between the manual shampoo and control groups (P = .6854).
In the ventral bulla, the group of animals treated by hydrodebrider saline irrigations had a lower value of mean biofilm surface area than the control (P = .0009), manual saline irrigation (P = .0323) and manual shampoo irrigation groups (P = .0128). The manual shampoo irrigation group had a lower mean surface area compared to the control group (P = .024). There were no differences between manual shampoo and manual saline irrigation (P = .0609), and between manual saline irrigation and control (P = .0562) (Table 5).
Table 5.
Summary of Results.
| P‐value Comparison | ||
|---|---|---|
| Compared Groups | Dorsal Surface | Ventral Surface |
| Hydrodebrider vs. control | .0038** | .0009*** |
| Hydrodebrider vs. saline | .056 | .0323 * |
| Hydrodebrider vs. shampoo | .0321 * | .0128 * |
| Saline vs. control | .0323 * | .056 |
| Saline vs. shampoo | .701 | .609 |
| Shampoo vs. control | .6854 | .024 * |
* P < .05; ** P < .01; *** P < .001.
There were no instances of tympanic membrane perforations, and no animals were observed to exhibit signs of facial weakness or vestibular deficit.
Regarding the hearing results, inoculation of bullae with bacteria resulted in an elevation of the pure‐tone thresholds when compared to baseline in all four tested frequencies in all the tested ears (control group = 3 ears, saline manual irrigation group = 3 ears, hydrodebrider group = 3 ears), as expected after a middle ear effusion formed in response to the inoculation. Experimental treatment with manual or hydrodebrider irrigation did not result in worse (higher) pure‐tone thresholds than in control (untreated) ears (Table 6).
Table 6.
Hearing Thresholds Before Transbullar Bacteria Inoculation and After Irrigation Treatment in dBHL. Results are shown in dBHL.
| Baseline | Contralateral Ears (Control) | Manual Saline | Hydrodebrider | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 Hz | 4000 Hz | 16000 Hz | 500 Hz | 4000 Hz | 16000 Hz | 500 Hz | 4000 Hz | 16000 Hz | 500 Hz | 4000 Hz | 16000 Hz | |
| Animal 1 | 20 | 25 | 25 | 30 | 35 | 35 | 45 | 60 | 45 | 45 | 35 | 45 |
| Animal 2 | 25 | 25 | 30 | 45 | 55 | 45 | 30 | 35 | 40 | 60 | 40 | 40 |
| Animal 3 | 25 | 20 | 20 | 45 | 45 | 55 | 40 | 25 | 60 | 55 | ||
DISCUSSION
Over the past two decades there has been an increasing interest in the role of biofilm formation in chronic and recurrent otolaryngological infections, mainly involving the paranasal sinuses and the middle ear.14 Biofilms have been shown to be present in human middle ears with chronic suppurative otitis media.15, 16, 17
Recent data showed that systemic antibiotherapy is failing at eradicating biofilms in OME.18 Bacteria within biofilms are significantly more tolerant to antimicrobial treatment when compared to planktonic state bacteria. This enhanced antibiotic resistance by bacteria in biofilms is thought to results from a reduced metabolic state, induction of biofilm specific genes, the presence of a sub‐population of persister cells, and a decreased ability of the antibiotic to penetrate the biofilm matrix.19 Persister cells comprise a subpopulation of the bacterial biofilm that are metabolically inactive and tolerant to antibiotics.20 Subsequently, they have been implicated in the recalcitrance of chronic infection since antibiotics kill the majority of cells but persisters remain viable and repopulate the biofilm when the level of antibiotics drops.20
Treatment strategies directed at eradicating biofilms by irrigation and topical application of antibiotic, surfactants and other biofilmcidal agents are gaining popularity in clinical practice. There exist some reports of the use of antimicrobial and biofilmcidal products to eradicate biofilm in sinonasal cavities, but not in the middle ear cavity. A prospective, randomized study of patients having undergone functional endoscopic sinus surgery who then underwent sinus cavity irrigations manually with either saline or baby shampoo showed improved patients' symptoms, olfaction scores, and decreased edema and polypoid degeneration of sinus cavities irrigated with baby shampoo, a chemical surfactant thought to disrupt biofilm integrity.8
The impetus in developing a powered hydrodebrider for clinical practice stemmed from reports showing that power irrigation can increase the removal of bacteria by a factor of at least 100 when compared to bulb syringe in a staphylococcus biofilm coated implants.21 A hydrodebrider developed by Medtronic (Medtronic Corp., Jacksonville, FL, USA) to irrigate the sinonasal cavities has shown to improve the lasting effects of biofilm removal in a sheep model of rhinosinusitis with staphylococcus aureus without completely eradicating it.9 The hydrodebrider device is thought to work by providing a controlled shear force which helps mechanically disrupt the mucosal biofilm extracellular polymeric substance (EPS) matrix.9 Thus, it was imperative to test the hydrodebrider in the middle ear and assess its efficacy at biofilm removal in an otitis media model.
The main finding of this study was that the hydrobedriber saline irrigation system was effective in removing biofilm from the tympanic bulla of chinchillas, especially in anatomical recesses that are difficult to reach, such as the ventral aspect when accessing the bulla from a dorsal approach as it was done in our experiments. It was precisely in the ventral bulla where the benefits of the hydrodebrider were more evident. Although the flow of the irrigation was kept constant between the manual irrigation and the hydrodebrider system, the hydrodebrider outperformed manual irrigation because it projects a multidirectional flow and the flow of the irrigation is recirculated by incorporating a suction channel. Furthermore, the hydrodebrider group was the only group that produced samples with total eradication of biofilm.
The hydrodebrider was designed for use in the middle ear. The diameter of the cannula allows for its placement through either a small myringoplasty or a ventilation tube with an opening of 1.5 mm, and the device is suitable for use during endoscopic or microscopic otologic surgery. The hydrodebrider has an incorporated irrigation and suction system within the same tip. Simultaneous suctioning during irrigation is important to allow recirculation and prevent pooling of solution which will affect contact between the irrigation stream and the mucosal surface. The multidirectional irrigation flow that results from the distribution in the cannula tip of the five radial and one axial holes allows the irrigation to reach hidden recesses.
Analyses of the control specimens revealed that biofilm growth was non‐uniform and denser in dorsal specimens. We also observed biofilm formation favoring dorsal mucosa over ventral mucosa in control samples. The surgical opening and subsequent healing of the dorsal aspect of the bulla might have an effect on the formation and support of biofilm colonies adjacent to the surgical site. The variability of biofilm formation prior to the irrigation treatment between the dorsal and ventral areas of the bulla, and among different animals, is a limitation of this study. An ideal experimental model would assure an equal biofilm burden in all groups before irrigation, but this may not be achievable. Consequently, differences in the effectiveness of eradicating biofilm between groups must be interpreted with this limitation in mind.
Although normal saline and baby shampoo irrigations appear to reduce the biofilm surface area without complete eradication, only normal saline irrigations achieved statistical significance in reduction of biofilm surface area when compared to the baby shampoo or the control specimen group. The lack of statistically significant reduction of biofilm surface area in the baby shampoo group compared to the control samples and the superiority of irrigations using normal saline over baby shampoo may be due to the limitations resulting from different biofilm burden (as discussed above) and sampling bias. Another potential factor is the mechanical effect of irrigations regardless of the solvent that breaks down the EPS matrix of biofilms. The more viscous nature of the baby shampoo vis‐à‐vis normal saline may contribute to the baby shampoo's decreased effective recirculation in the bullas.
Our ABR data was consistent with a conductive hearing loss from the middle ear infection and effusion that resulted after bacterial inoculation of the bulla. There was no significant difference between the control and the experimental ears suggesting that the experimental irrigations in this study do not seem to produce additional hearing loss. Furthermore, there were no animals with obvious vestibular toxicity or facial nerve weakness observed throughout the experiment suggesting no inner ear toxicity or neural toxicity from the irrigations.
This pilot study helped assess the safety and efficacy of a middle ear hydrodebrider prototype model. In the future, additional studies with greater subject numbers will be conducted to examine the optimal type and concentration of solvent and most effective parameters for hydrodebrider irrigations to eradicate and inhibit biofilms formation in the middle ear as well as reevaluating potential inner ear toxicity. Although more experimental studies are necessary, this study could be used to inform future preclinical and clinical studies of the applicability and usefulness of hydrodebridement of the middle in refractory otitis media.
CONCLUSION
Our data suggests that biofilm formation in OME can be reduced by the relatively simple measure of middle ear lavage using either normal saline or 1% baby shampoo (Tables 3, 4, 5). The use of a hydrodebrider device with controlled shear force on the mucosal surface is safe and efficient at biofilm removal in the chinchilla's middle ear. Additional studies will be required to further validate these results and assess their long‐term safety and efficiency.
Table 3.
Comparison for each pair using Wilcoxon Rank Test in the Dorsal Bullae.
| Comparison | Mean (SE) differences in μm2 | 95% CI μm2 | P‐value |
|---|---|---|---|
| Shampoo vs. saline | 0.857 (2.236) | −878.32–6058.38 | .701 |
| Shampoo vs. control | −0.938 (2.315) | −4111.56–4609.95 | .685 |
| Hydrodebrider vs. saline | −4.42 (2.312) | −1220.36–27.54 | .056 |
| Hydrodebrider vs. shampoo | −4.955 (2.312) | −6385.20–(−10.88) | .0321* |
| Saline vs. control | −4.955 (2.314) | −4148.3–(−203.62) | .0323* |
| Hydrodebrider vs. control | −6.875 (2.379) | −4557.66–(−789.31) | .0038** |
CI = confidence interval; SE = standard error. * P < .05; ** P < .01.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Supporting Information Legends
Video 1. Mechanical debridement of the middle ear using the hydrodebrider (powered irrigation). The video shows how the animal's bullae is approached dorsally, dissecting the soft tissues, and then drilling a central hole through which the tip of the irrigation mechanisms was introduced for the irrigation. A final image of the animal's ossicular chain was showed followed by surgical closure of the soft tissue by plane.
ACKNOWLEDGEMENT
Patricia Blackwelder, PhD, University of Miami Center for Advanced Microscopy (UMCAM)/Chemistry, University of Miami, Coral Gables, Florida.
Funding: Medtronic (Research grant)
Oral Presentation: American Academy of Otolaryngology–Head and Neck Surgery Annual Meeting, 2012, Washington, DC, U.S.A.
BIBLIOGRAPHY
- 1. Van Hoecke H, De Paepe AS, Lambert E, et al. Haemophilus influenzae biofilm formation in chronic otitis media with effusion. Eur Arch Otorhinolaryngol 2016;273:3553–3560. [DOI] [PubMed] [Google Scholar]
- 2. Potera C. Forging a link between biofilms and disease. Science 1999;283:1837–1839. [DOI] [PubMed] [Google Scholar]
- 3. Cavaliere R, Ball JL, Turnbull L, Whitchurch CB. The biofilm matrix destabilizers, EDTA and DNaseI, enhance the susceptibility of nontypeable Hemophilus influenzae biofilms to treatment with ampicillin and ciprofloxacin. Microbiologyopen 2014;3:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berlanga M, Gomez‐Perez L, Guerrero R. Biofilm formation and antibiotic susceptibility in dispersed cells versus planktonic cells from clinical, industry and environmental origins. Antonie Van Leeuwenhoek 2017;110(12):1691–1704. [DOI] [PubMed] [Google Scholar]
- 5. Mohmmed SA, Vianna ME, Penny MR, Hilton ST, Knowles JC. The effect of sodium hypochlorite concentration and irrigation needle extension on biofilm removal from a simulated root canal model. Aust Endod J 2017;43(3):102–109. [DOI] [PubMed] [Google Scholar]
- 6. Mohmmed SA, Vianna ME, Penny MR, Hilton ST, Mordan N, Knowles JC. Confocal laser scanning, scanning electron, and transmission electron microscopy investigation of Enterococcus faecalis biofilm degradation using passive and active sodium hypochlorite irrigation within a simulated root canal model. Microbiologyopen 2017. doi: 10.1002/mbo3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. E Silva SS, Carvalho JWP, Aires CP, Nitschke M. Disruption of Staphylococcus aureus biofilms using rhamnolipid biosurfactants. J Dairy Sci 2017;100:7864–7873. [DOI] [PubMed] [Google Scholar]
- 8. Chiu AG, Palmer JN, Woodworth BA, et al. Baby shampoo nasal irrigations for the symptomatic post‐functional endoscopic sinus surgery patient. Am J Rhinol 2008;22(1):34–37. [DOI] [PubMed] [Google Scholar]
- 9. Valentine R, Jervis‐Bardy J, Psaltis A, Tan LW, Wormald PJ. Efficacy of using a hydrodebrider and of citric acid/zwitterionic surfactant on a Staphylococcus aureus bacterial biofilm in the sheep model of rhinosinusitis. Am J Rhinol Allergy 2011;25:323–326. [DOI] [PubMed] [Google Scholar]
- 10. Post JC. Direct evidence of bacterial biofilms in otitis media. Laryngoscope 2001;111:2083–2094. [DOI] [PubMed] [Google Scholar]
- 11. Ehrlich GD, Veeh R, Wang X, et al. Mucosal biofilm formation on middle‐ear mucosa in the chinchilla model of otitis media. JAMA 2002;287:1710–1715. [DOI] [PubMed] [Google Scholar]
- 12. Coticchia JM, Sugawa C, Tran VR, Gurrola J, Kowalski E, Carron MA. Presence and density of Helicobacter pylori biofilms in human gastric mucosa in patients with peptic ulcer disease. J Gastrointest Surg 2006;10:883–889. [DOI] [PubMed] [Google Scholar]
- 13. Kendig K. Is a 2000‐year‐old formula still keeping some secrets? Am Math Month 2000;107:402–415. [Google Scholar]
- 14. Post JC, Hiller NL, Nistico L, Stoodley P, Ehrlich GD. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg 2007;15:347–351. [DOI] [PubMed] [Google Scholar]
- 15. Homoe P, Bjarnsholt T, Wessman M, Sorensen HC, Johansen HK. Morphological evidence of biofilm formation in Greenlanders with chronic suppurative otitis media. Eur Arch Otorhinolaryngol 2009;266:1533–1538. [DOI] [PubMed] [Google Scholar]
- 16. Lee MR, Pawlowski KS, Luong A, Furze AD, Roland PS. Biofilm presence in humans with chronic suppurative otitis media. Otolaryngol Head Neck Surg 2009;141:567–571. [DOI] [PubMed] [Google Scholar]
- 17. Wessman M, Bjarnsholt T, Eickhardt‐Sorensen SR, Johansen HK, Homoe P. Mucosal biofilm detection in chronic otitis media: a study of middle ear biopsies from Greenlandic patients. Eur Arch Otorhinolaryngol 2015;272:1079–1085. [DOI] [PubMed] [Google Scholar]
- 18. Belfield K, Bayston R, Birchall JP, Daniel M. Do orally administered antibiotics reach concentrations in the middle ear sufficient to eradicate planktonic and biofilm bacteria? A review. Int J Pediatr Otorhinolaryngol 2015;79:296–300. [DOI] [PubMed] [Google Scholar]
- 19. Reimche JL, Kirse DJ, Whigham AS, Swords WE. Resistance of non‐typeable Haemophilus influenzae biofilms is independent of biofilm size. Pathog Dis 2017;75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soo VW, Wood TK. Antitoxin MqsA represses curli formation through the master biofilm regulator CsgD. Sci Rep 2013;3:3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anglen JO, Apostoles S, Christensen G, Gainor B. The efficacy of various irrigation solutions in removing slime‐producing Staphylococcus. J Orthop Trauma 1994;8:390–396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Supporting Information Legends
Video 1. Mechanical debridement of the middle ear using the hydrodebrider (powered irrigation). The video shows how the animal's bullae is approached dorsally, dissecting the soft tissues, and then drilling a central hole through which the tip of the irrigation mechanisms was introduced for the irrigation. A final image of the animal's ossicular chain was showed followed by surgical closure of the soft tissue by plane.


