Graphical abstract
Keywords: Copper, Finkelstein reaction, Ionic liquid, Halogen exchange, Aryl halides
Abstract
We have developed a general method for reverse aromatic Finkelstein reactions. Good reaction yields were obtained when aryl iodides or aryl bromides were treated with copper halide salts as promoters in a 1-butyl-3-methylimidazolium bromide ([BMIM]Br) ionic liquid (IL) solvent at 140 °C for 8 h. Preliminary investigation supported that the copper salts were also the halide sources in halogen exchange reactions. The optimized conditions are applicable to a variety of substrates and have excellent functional group tolerance. Additionally, the [BMIM]Br solvent showed good stability for at least 10 consecutive runs. Results indicated that the [BMIM]Br solvent was recyclable for reverse aromatic Finkelstein reactions.
Introduction
Aryl halides are widely used in organic synthesis to form carbon-carbon and carbon-heteroatom bonds under metal catalysis such as in Heck, Sonogashira, Suzuki, and Ullmann coupling reactions [1]. They are also highly versatile synthetic intermediates for many applications in agrochemicals, pharmaceuticals, and materials [2], [3]. Therefore, the development of convenient and efficient methods for the selective synthesis of aryl and heteroaryl halides has attracted increasing attention [4], [5], [6], [7]. Traditional methods involved two common preparatory routes: direct halogenation via a Friedel-Crafts reaction and a nucleophilic aromatic substitution reaction (SNAr) of diazonium salts [8]. However, these methods suffer from several drawbacks including poor functional group tolerance, harsh conditions, lengthy procedures, and regioselectivity issues. Recently, transition metal-catalyzed or transition metal-mediated halogen exchange has been emerging as a promising pathway [7], [9], [10]. Aryl iodides are generally more reactive in organic transformations. Although aryl chlorides or aryl bromides are relatively more inert, they are much more commonly found in pharmaceuticals and agrochemicals, in which they are introduced to modify the physical and biological properties of aromatic rings [11]. Furthermore, the utilization of gaseous chlorine (Cl2) and bromine (Br2) in halogenation often required special caution with regards to handling and safety. In contrast, iodine chemistry has recently attracted much attention due to its polyvalence, good selective reactivity, and ease of use [12], [13]. Thus, many intermediates in the synthetic sequences contain iodine substituents. Converting these iodinated compounds to corresponding chlorinated or brominated ones was occasionally required [14]. Therefore, it would be useful to have a general method for the interconversion of different halogen derivatives. Particularly, the aromatic Finkelstein reaction for converting aryl chlorides or bromides into the corresponding more reactive aryl iodides has gained increasing attention [15]. Lately, many studies under nickel, copper, or palladium catalysis have been intensively reported [15], [16], [17], [18], [19], [20]. However, research in converting iodides to the corresponding bromides or chlorides is rare. The first report from Cramer using stoichiometric NiCl2 as a promoter for aryl chloride synthesis from aryl bromides was followed and further developed by Leadbeater and co-worker [18], [19]. A photocatalytic substitution of aryl bromides by chlorides using FeCl3 as a promoter was recently described [20]. These existing reaction routes suffered from either harsh conditions, the utilization of amide solvents, low yields, or not being industrially accessible. Thus, the development of more practical, milder, and greener methods, especially using recyclable solvents and less toxic transition metals, should be targeted.
The utilization of ionic liquids (ILs) has been investigated by a great number of researchers in the past few decades [21], [22], [23]. The increase in the number of publications involving their use has been attributed to their unique properties, such as the ease of product separation, the reduction of the emission of toxic compounds, the facilitation of catalyst recovery, and reusability [24], [25]. With respect to catalysis, ILs have often been used in catalytic organic reactions to enhance the reaction rates and selectivity due to their ability to dissolve transition metal complexes [26]. Specifically, ILs were frequently employed in palladium-catalyzed cross coupling reactions [27]. Besides acting as the reaction solvent, ILs were proposed to play an important role as a coordinating agent. This often resulted in ligand-free conditions when ILs were employed [28]. However, these advantages of ILs are not intensively exploited with other first-row transition metal catalysts. Herein, we report the implementation of ILs in reverse aromatic Finkelstein reactions. Notably, a copper salt is used for the first time as a promoter for the halogen exchange transformation (Fig. 1). Additionally, the ILs could be separated from the reaction mixture and reused at least 10 times without detectable changes in their structure and activity.
Fig. 1.
The differentiation of this work.
Experimental
Synthesis of the ILs
In a typical reaction for the preparation of [BMIM]Br, 1-methylimidazole (20.5 g, 0.25 mol) was mixed with 1-bromobutane (38.1 g, 0.28 mol) in a 250 mL round bottom flask equipped with a reflux condenser. The mixture was then irradiated in a microwave oven (Sanyo, EM S2086W, 800 W) at 80 W and stirred vigorously during the reaction time by a magnetic stirrer. The irradiation was paused every 10 s to prevent overheating. The irradiation was repeated for a total time of 3 min. After completion, the resulting mixture was cooled to room temperature. The starting materials and undesired products were extracted with ethyl acetate (3 × 100 mL), followed by diethyl ether (3 × 100 mL). The residue of volatile solvents was removed by rotary vacuum evaporation at 50 °C to deliver 52.8 g of product (97% yield). Procedures for the preparation of other ILs were detailed in Supporting Information (Section S2).
Catalytic studies
Aryl halide (1 mmol) and copper (I) halide (1.2 mmol) were added into a 4 mL vial. To this vial, the IL solvent (1 mL) was added. The resulting reaction mixture was stirred at 140 °C for 8 h. After completion, the reaction mixture was quenched with water (15 mL). The organic layer was extracted by ethyl acetate (3 × 25 mL), dried over anhydrous Na2SO4, and evaporated to remove organic solvent. The residue was subjected to flash chromatography, followed by elution with the appropriate solvent to elute the products. Product identity was confirmed by gas chromatography-mass spectroscopy (GC–MS) and nuclear magnetic resonance (NMR). For solvent recycling, after quenching with H2O and diethyl ether, the resulting aqueous solution was subjected to vacuum distillation to remove the water, leaving the [BMIM]Br ionic liquid. The recovered ionic liquid was then reused in further reactions under identical conditions to those of the first run.
Results and discussion
It is worth mentioning that the mechanism of the aromatic Finkelstein reaction has been extensively investigated under copper and palladium catalysts [7]. Specifically, the oxidative addition of aryl bromides or aryl chlorides required an additional ligand, and the halide exchange from bromides/chlorides to iodides in metal complexes is quite facile. Previous studies from Stack and Ribas showed that trends in the rate of C–X bond reductive elimination from Cu(III) complexes are controlled by the relative carbon–halogen bond strengths, which are as follows: C–Cl > C–Br > C–I [29], [30]. We hypothesize that the reverse Finkelstein reaction would favor the oxidative addition and reductive elimination, while the halide exchange could be facilitated by using the copper halide promoters as halide sources.
In optimization screening, halide replacement reactions of 4-iodoacetophenone with CuBr were performed with respect to the IL type, temperature, and amount of promoter (Table 1). By taking advantage of coordination property of ILs, no additional ligand was utilized during this process. Optimal results were obtained in [BMIM]Br at 140 °C with 1.2 equiv. of CuBr salt, and a 93% GC yield of the corresponding aryl bromide was achieved (entry 1). Increasing the hydrophobicity of the IL by using 1-hexyl-3-methylimidazolium bromide ([HMIM]Br) or 1-octyl-3-methylimidazolium bromide ([OMIM]Br) resulted in a greater amount of dehalogenation by-product and a lower efficiency (entries 2, 3). A similar trend was observed when hexafluorophosphate (PF6) and tetrafluoroborate (BF4) were introduced as anions in ILs (entries 4, 5). Using less or more than 1.2 equiv. of promoter did not afford better yields (entries 6–8). Unlike the regular aromatic Finkelstein reaction, reverse halogen exchange did not afford a reasonable amount of the desired product with a catalytic amount of copper salt, even in the presence of bromide salts (entry 9). Reactions conducted at 130 °C and 150 °C provided 36% and 76% yields, respectively (entries 10, 11). A substantial amount of dehalogenation product was detected when the reaction temperature increased, and 8 h is the optimal reaction time (entries 12, 13).
Table 1.
| Entry | Type of IL | [CuBr] (equiv.) | Temperature (°C) | (1)/(2) ratio | Yield (%)b |
|---|---|---|---|---|---|
| 1 | [BMIM]Br | 1.2 | 140 | 21.3 | 93 |
| 2 | [HMIM]Br | 1.2 | 140 | 16.8 | 73 |
| 3 | [OMIM]Br | 1.2 | 140 | 15.2 | 81 |
| 4 | [BMIM]PF6 | 1.2 | 140 | 11.4 | 84 |
| 5 | [BMIM]BF4 | 1.2 | 140 | 13.7 | 79 |
| 6 | [BMIM]Br | 0.5 | 140 | 3.1 | 41 |
| 7 | [BMIM]Br | 0.85 | 140 | 8.8 | 63 |
| 8 | [BMIM]Br | 1.4 | 140 | 21.9 | 94 |
| 9c | [BMIM]Br | 0.1 | 140 | 0.48 | 12 |
| 10 | [BMIM]Br | 1.2 | 130 | 22.0 | 36 |
| 11 | [BMIM]Br | 1.2 | 150 | 5.1 | 76 |
| 12d | [BMIM]Br | 1.2 | 140 | 21.8 | 92 |
| 13e | [BMIM]Br | 1.2 | 140 | 22.6 | 65 |
Volume of solvent 1.0 mL, 1.0 mmol scale, 8 h.
GC yields (Supporting Information, Section S3).
Reaction in the presence of KBr (1.5 equiv.).
Reaction in 12 h.
Reaction in 6 h. See the supporting information for more details (Section S4).
To highlight the excellent property of [BMIM]Br for the transformation, reactions in other common solvents were carried out (Table 2). In fact, the solvent was found to have an important role with regards to the reaction efficiency. Notably, polar aprotic solvents such as dimethyl formaldehyde (DMF), N-methyl-2-pyrrolidone (NMP), and dimethyl sulfoxide (DMSO) are ineffective with <75% yield, and a significant amount of by-product was generated (entries 2–4). Reactions in a protic solvent (n-BuOH) or non-polar solvent also generated the desired product in low yields (entries 5–7). Experiments to compare the activity of CuBr with other bromide salts were conducted (entries 8–13). As expected, CuBr2 was less effective, presumably due to its difficulty in oxidative addition [29], [30]. Interestingly, other first-row transition metal salts and KBr produced no detectable amount of the desired product. To further confirm the bromide source for the halide exchange, several control experiments were performed (entries 14–16). Replacing the bromide with a chloride anion in ILs did not affect the formation of the aryl bromide product. In the presence of [BMIM]Br, only 5% of halide replacement product was detected when Cu(OAc)2 was employed. Furthermore, aryl chloride was mostly obtained in the reaction conducted using CuCl as a promoter and [BMIM]Br as the solvent. Although isotopic labeling is needed to further support the halide source, it is likely that reductive elimination occurred with the halogen originating from the copper salt.
Table 2.
| Entry | Solvent | Promoter | (1)/(2) ratio | Yield (%)b |
|---|---|---|---|---|
| 1 | [BMIM]Br | CuBr | 21.3 | 93 |
| 2 | DMF | CuBr | 4.1 | 56 |
| 3 | NMP | CuBr | 5.9 | 74 |
| 4 | DMSO | CuBr | 8.9 | 32 |
| 5 | n-BuOH | CuBr | 0.7 | 15 |
| 6 | Diglyme | CuBr | 1.1 | 36 |
| 7 | Mesitylene | CuBr | N.D | <2 |
| 8 | [BMIB]Br | CuBr2 | 18.6 | 53 |
| 9 | [BMIB]Br | KBr | N.D | <2 |
| 10 | BMIB]Br | NiBr2 | N.D | <2 |
| 11 | BMIB]Br | FeBr3 | N.D | <2 |
| 12 | BMIB]Br | AgBr | N.D | <2 |
| 13 | BMIB]Br | ZnBr2 | N.D | <2 |
| 14 | [BMIB]Cl | CuBr | 17.9 | 90 |
| 15 | [BMIB]Br | Cu(OAc)2 | 0.24 | 5 |
| 16 | [BMIB]Br | CuCl | 19.9 | 87c |
N.D: not determined.
Volume of solvent 1.0 mL, 1.0 mmol scale.
GC yield.
4-chloroacetophenone product. N.D: not determined.
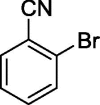
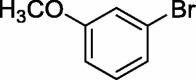
To assess the generality of the optimized conditions, a variety of aryl halide derivatives were utilized in the exchange reaction. The isolated product yields are presented in Table 3. Aryl iodides bearing electron withdrawing groups and electron donating groups are reactive, and the bromide products were obtained in good yields (entries 1–5). It should be noted that previous studies on a Ni-promoted reverse aromatic Finkelstein reaction were not efficient with electron-rich aryl halides. Conditions are not limited to para-substituted substrates. The bromination of 3-iodoacetophone, 2-iodobenzonitrile, and 3-iodoanisole afforded products in reasonable yields (entries 6–8). The reactions are highly functional-group tolerant, with nitro, ester, cyano, and carbonyl functionalities all compatible with the reaction conditions. Furthermore, halogen exchange with chlorine is also possible, and 4-chloroacetophenone and 1-chloronaphthalene were achieved in 86% and 72% yields, respectively (entries 9, 10). Selective mono- or di-bromination exchange product can be achieved with modified reaction conditions (entry 11). Interestingly, aryl bromides can be chlorinated efficiently, and the corresponding aryl chlorides were formed in reasonable yields (entries 12, 13).
Table 3.
| Entry | Reactant | Product | Yield (%) |
|---|---|---|---|
| 1 | 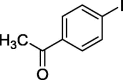 |
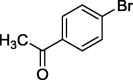 |
89 |
| 2 | 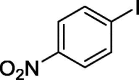 |
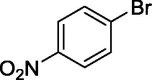 |
71 |
| 3 | 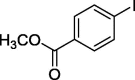 |
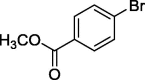 |
81 |
| 4 | 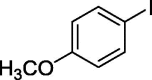 |
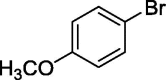 |
74 |
| 5 | 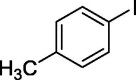 |
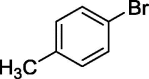 |
64 |
| 6 | 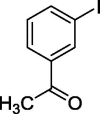 |
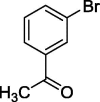 |
78 |
| 7 | 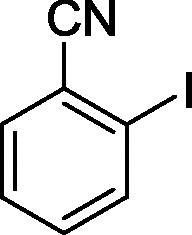 |
 |
61 |
| 8 | 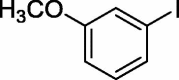 |
 |
67 |
| 9 | 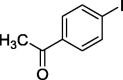 |
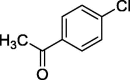 |
82 |
| 10 | 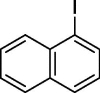 |
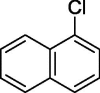 |
76 |
| 11 | 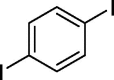 |
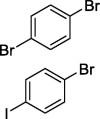 |
70b 64c |
| 12 | 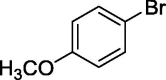 |
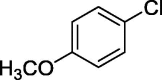 |
58 |
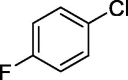
| 13 | 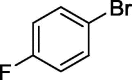 |
 |
42 |
Volume of solvent 1.0 mL, 1.0 mmol scale.
2.15 equiv. of CuBr, 14 h.
Reaction in 6 h.
To identify the effectiveness of the methodology, more difficult substrates were tested. Interestingly, the chlorination of an aryl iodide containing a bromide functionality is selectively accessible. The optimal conditions are also applicable for vinyl halides, and the corresponding product synthesized for the first time by this pathway was isolated in a 67% yield (Scheme 1). Moreover, heteroaryl halides can undergo halogen exchanges. Thus, the chlorination of 4-iodopyridine and bromination of 3-iodoindole give the desired products in acceptable yields.
Scheme 1.
Selective halogen exchange and reactions of vinyl- and heteroaryl iodides.
With respect to efforts to reduce chemical consumption, the easy recycling of ILs makes use of their negligible solubility in non-polar organic solvents such as diethyl ether. This allowed reaction products as well as unreacted starting materials to be extracted by diethyl ether while ILs were completely dissolved in water. Recovered ILs after evaporating the water were then applied in the next runs under identical conditions (Fig. 2). The results revealed that [BMIM]Br could be utilized efficiently in at least 10 consecutive runs. Indeed, a yield of approximately 90% was still obtained in the 10th run. GC–MS analysis of reused [BMIM]Br indicated the stability of the IL under long-term exposure to the reported conditions (Fig. S4). Besides the utilization of less toxic copper salt promoters, this methodology supports the classification of ionic liquids as environmentally benign solvents.
Fig. 2.

Recycling study of [BMIM]Br.
Conclusions
In summary, an efficient route for a reverse aromatic Finkelstein reaction has been developed. The optimal conditions involved the use of recyclable [BMIM]Br solvent and copper halide salts as promoters and halide sources at 140 °C in 8 h. A wide range of substrates are applicable in conjunction with good functional group compatibility. This method also shows the use of ionic liquids as solvents for transition metal-mediated reactions.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.01-2014.76.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jare.2017.12.006.
Appendix A. Supplementary material
References
- 1.Hassan J., Sevignon M., Gozzi C., Schulz E., Lemaire M. Aryl−aryl bond formation one century after the discovery of the ullmann reaction. Chem Rev. 2012;102:1359–1469. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 2.Ametamey S.M., Honer M., Schubiger P.A. Molecular imaging with PET. Chem Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 3.Seevers R.H., Counsell R.E. Radioiodination techniques for small organic molecules. Chem Rev. 1982;82:575–590. [Google Scholar]
- 4.Schmidt R., Stolle A., Ondruschka B. Aromatic substitution in ball mills: formation of aryl chlorides and bromides using potassium peroxomonosulfate and NaX. Green Chem. 2012;14:1673–1679. [Google Scholar]
- 5.Shen X., Hyde A.M., Buchwald S.L. Palladium-catalyzed conversion of aryl and vinyl triflates to bromides and chlorides. J Am Chem Soc. 2010;132:14076–14078. doi: 10.1021/ja107481a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H., Hynes J. Copper-catalyzed chlorination of functionalized arylboronic acids. Org Lett. 2010;12:1192–1195. doi: 10.1021/ol9029337. [DOI] [PubMed] [Google Scholar]
- 7.Petrone D.A., Ye J., Lautens M. Modern transition-metal-catalyzed carbon–halogen bond formation. Chem Rev. 2016;116:8003–8104. doi: 10.1021/acs.chemrev.6b00089. [DOI] [PubMed] [Google Scholar]
- 8.Boyd R.W., Morrison R. Prentice Hall; Englewood Cliffs, N.J.: 1992. Organic chemistry. [Google Scholar]
- 9.Zanon J., Klapars A., Buchwald S.L. Copper-catalyzed domino halide exchange-cyanation of aryl bromides. J Am Chem Soc. 2003;125:2890–2891. doi: 10.1021/ja0299708. [DOI] [PubMed] [Google Scholar]
- 10.Yang S.H., Li C.S., Cheng C.H. Halide exchange reactions between aryl halides and alkali halides catalyzed by nickel metal. J Org Chem. 1987;52:691–694. [Google Scholar]
- 11.Magano J., Dunetz J.R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem Rev. 2011;111:2177–2250. doi: 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]
- 12.Saiz-Lopez A., Plane J.M.C., Baker A.R., Carpenter L.J., Glasow R.V., Martín J.C.G. Atmospheric chemistry of iodine. Chem Rev. 2012;112:1773–1804. doi: 10.1021/cr200029u. [DOI] [PubMed] [Google Scholar]
- 13.Kaiho T. John Wiley & Son. Inc; 2015. Iodine chemistry and application. [Google Scholar]
- 14.Sakakura A., Ukai A., Ishihara K. Enantioselective halocyclization of polyprenoids induced by nucleophilic phosphoramidites. Nature. 2007;445:900–903. doi: 10.1038/nature05553. [DOI] [PubMed] [Google Scholar]
- 15.Sheppard T.D. Metal-catalysed halogen exchange reactions of aryl halides. Org Biomol Chem. 2009;7:1043–1052. doi: 10.1039/b818155a. [DOI] [PubMed] [Google Scholar]
- 16.Cant A.A., Bhalla R., Pimlott S.L., Sutherland A. Nickel-catalysed aromatic Finkelstein reaction of aryland heteroaryl bromides. Chem Commun. 2012;48:3993–3995. doi: 10.1039/c2cc30956d. [DOI] [PubMed] [Google Scholar]
- 17.Klapars A., Buchwald S.L. Copper-catalyzed halogen exchange in aryl halides: an aromatic finkelstein reaction. J Am Chem Soc. 2002;124:14844–14845. doi: 10.1021/ja028865v. [DOI] [PubMed] [Google Scholar]
- 18.Cramer R., Coulson D.R. Nickel-catalyzed displacement reactions of aryl halides. J Org Chem. 1975;40:2267–2273. [Google Scholar]
- 19.Arvela K.K., Leadbeater N.E. Fast and easy halide exchange in aryl halides. Synlett. 2003;8:1145–1148. [Google Scholar]
- 20.Wang Y., Li L., Ji H., Ma W., Chen C., Zhao J. Iron(III)-mediated photocatalytic selective substitution of aryl bromine by chlorine with high chloride utilization efficiency. Chem Commun. 2014;50:2344–2346. doi: 10.1039/c3cc48246d. [DOI] [PubMed] [Google Scholar]
- 21.Rogers R.D., Seddon K.R. Ionic liquids–solvents of the future? Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- 22.Holbrey JD, Turner MB, Rogers RD. Ionic liquids as green solvents. ACS Symposium Series, vol. 856, Chapter 1, 2–12.
- 23.Lei Z., Chen B., Koo Y.M., MacFarlane D.R. Introduction: ionic liquids. Chem Rev. 2017;117:6633–6635. doi: 10.1021/acs.chemrev.7b00246. [DOI] [PubMed] [Google Scholar]
- 24.Jain N., Kumar N., Chauhan S., Chauhan S.M.S. Chemical and biochemical transformations in ionic liquids. Tetrahedron. 2005;61:1015–1060. [Google Scholar]
- 25.Pârvulescu V.I., Hardacre C. Catalysis in ionic liquids. Chem Rev. 2007;107:2615–2665. doi: 10.1021/cr050948h. [DOI] [PubMed] [Google Scholar]
- 26.Vekariya R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J Mol Liq. 2017;227:44–60. [Google Scholar]
- 27.Prechtl M.H., Scholten J.D., Dupont J. Carbon-carbon cross coupling reactions in ionic liquids catalysed by palladium metal nanoparticles. Molecules. 2010;15:3441–3461. doi: 10.3390/molecules15053441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivier-Bourbigou H., Magna L. Ionic liquids: perspectives for organic and catalytic reactions. J Mol Catal A. 2002;182:419–437. [Google Scholar]
- 29.Casitas A., Canta M., Sola M., Costas M., Ribas X. Nucleophilic aryl fluorination and aryl halide exchange mediated by a CuI/CuIII catalytic cycle. J Am Chem Soc. 2011;133:19386–19392. doi: 10.1021/ja2058567. [DOI] [PubMed] [Google Scholar]
- 30.Lin B.L., Kang P., Stack T.D.P. Unexpected Ccarbene−X (X: I, Br, Cl) reductive elimination from n-heterocyclic carbene copper halide complexes under oxidative conditions. Organometallics. 2010;29:3683–3685. doi: 10.1021/om1005726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








