Graphical abstract
Keywords: Pentas, Lanceolata, Rubiaceae, Anthraquinone, Iridoid, Antiplasmodial, Healing
Abstract
The genus Pentas belongs to the Rubiaceae family, which contains approximately 40 species. Several Pentas species were reported to be used as a folk treatment by African indigenous people in treating some diseases such as malaria, tapeworms, dysentery, gonorrhea, syphilis and snake poisoning. This article covers the period from 1962 to 2017 and presents an overview of the biological activity of different Pentas species and describes their phytochemical traits. As a conclusion, the main secondary metabolites from Pentas species are quinones, highly oxygenated chromene-based structures, and iridoids. Pentas species are widely used in folk medicine but they have to be more investigated for their medicinal properties.
Introduction
The genus Pentas belongs to the botanical plant family Rubiaceae. It consists of about 40 species, many of them used widely by indigenous people in Africa as medicinal plants. It is a flowering plant found mainly as an herb or shrub (P. bussi and P. nobilis), herb or subshrub (P. lanceolata and P. zanzibarica) or subshrub only (P. paviflora). The stem length varies between 60 and 2 m in the case of subshrubs and between 2 and 4 m if a shrub. The shape of the leaves is ovate, oblong, lanceolate or elliptic, while the flower shape is dismorphus, subsessile or unimorphous [1].
This genus is commonly used in the treatment of tropical and other diseases such as malaria (P. micrantha and P. longiflora) [2], [3], tapeworms (P. longiflora), itchy rashes and pimples [4] (P. longiflora and P. decora), gonorrhea, syphilis and dysentery (P. brussei), cough (P. micrantha) [4], dysmenorrhea, headache and pyrexia (P. purpurea) [5], hepatitis B [6], mental illness and epilepsy (P. schimperiana) [7], lymphadenitis, abdominal cramps, ascariasis, snake poisoning, retained placenta and some veterinary diseases (P. lanceolata) [8], [9].
Iridoids and highly oxygenated compounds have been shown to be the most common secondary metabolites of this genus. These plants have not been intensively studied to determine their biological characteristics. Several reports have found that some of their biological activity is antimalarial and antimicrobial [10], [11], [12], [13]. However, P. lanceolata is the only species that has been tested for analgesic and wound-healing properties, whereas very few examples were studied as having antitumor characteristics [11], [14], [15], [16]. The secondary metabolites that were identified in this genus are a common feature of the Rubiaceae family; however, there are some examples that have only been expressed in this genus [17]. This review endeavors to provide a comprehensive and up-to-date compilation of documented biological activities and the phytochemistry of the Pentas genus.
Phytochemical screening of Pentas species
The chemistry of Pentas species does not exhibit great diversity. The common active constituents of Pentas species can be considered chemotaxonomic markers. The main groups of secondary metabolites that were isolated are simple phenolic compounds, naphthoquinones, napthohydroquinones, anthraquinones, and iridoids. Furthermore, few examples of alkaloids, triterpenes, sterols, and chromenes were identified. The isolated compounds, structures, species, solvents of extraction and extracted organs are compiled in the Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8) which are displayed below.
Table 1.
Simple phenolics identified in P. lanceolata.
| Isolated compound | Structure | Species | Extract/Organ | Ref. |
|---|---|---|---|---|
| 4-Hydroxycinnamic acid 1 | 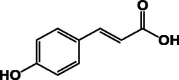 |
P. lanceolata | MeOH/Colleters | [18] |
| Thymol 2 | 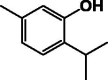 |
Table 2.
Naphthoquinones (3–7) isolated from P. longiflora.
Table 3.
Naphthohydroquinones (8–12) isolated from Pentas species.
| Isolated compound | Structure | Species | Extract/Organ | Refs. |
|---|---|---|---|---|
| Busseihydroquinone A 8 R1 = H, R2 = OH, R3 = OCH3, R4 = CH3, R5 = H |
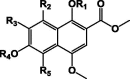 |
P. bussei | Crystallized out as needles from (DCM/MeOH)/Root | [23] |
| Methyl 8-hydroxy-1,4,6,7-tetramethoxy-2-naphthoate 9 R1 = CH3, R2 = OH, R3 = OCH3, R4 = CH3, R5 = H |
Hexane/Root | [25] | ||
| Parvinaphthols A 10 R1 = H, R2 = OH, R3 = OH, R4 = CH3, R5 = H |
P. parvifolia | (DCM/MeOH)/Root | [24] | |
| Parvinaphthols B 11 R1 = H, R2 = H, R3 = H, R4 = H, R5 = OH | ||||
| 1,4,5-Trihydroxy-3-methoxy-6-(3,7,11,15,19-pentamethyleicosa-2,6,10,14,18-pentaenyl)naphthalene 12 |  |
EtOAc/Root | [25] |
Table 4.
Chromene-based structures (13–29) separated from Pentas species.
| Isolated compound | Structure | Species | Extract/Organ | Refs. |
|---|---|---|---|---|
| Scopoletin 13 | 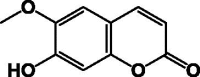 |
P. longiflora | EtOAc/Root | [22] |
| Methyl 5,10-dihydroxy-7-methoxy-3-methyl-3-(4-methyl-3-pentenyl)-3H-benzo[f]chromene-9-carboxylate 14 | 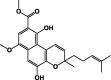 |
P. bussei | Hexane/Root | [27] |
| P. parvifolia | [25] | |||
| Methyl 5,10-dihydroxy-7-methoxy-1,1,3a-trimethyl-1a,2,3,3a,10c,10d-hexahydro-1H-4-oxacyclobuta[3,4]indeno[5,6-a]naphthalene-9-carboxylate 15 | 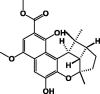 |
P. bussei | ||
| 9-Methoxy-2-methyl-2-(4-methyl-3-pentenyl)-2H-benzo[h]-chromene-7,10-diol 16 |  |
P. bussei,P. parvifolia |
||
| 9-Methoxy-2,2-dimethyl-2H-benzo[h]chromene-7,10-diol 17 | 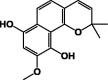 |
|||
| Busseihydroquinone B 18 | 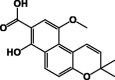 |
P. bussei | (DCM/MeOH)/Root | [23] |
| P. parvifolia | DCM/Root | [25] | ||
| Busseihydroquinone C 19 | 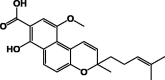 |
P. bussei | (DCM/MeOH)/Root | [23] |
| Busseihydroquinone D 20 | 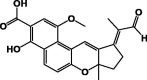 |
|||
| Mollugin 21 | 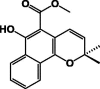 |
P. longiflora | Hexane, (DCM/MeOH) /Root | [22], [28] |
| P. lanceolata | MeOH/Colleter | [18] | ||
| 3-Hydroxymollugin 22 |  |
P. longiflora | Hexane/Root | [22] |
| 3-Methoxymollugin 23 | 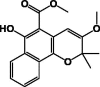 |
DCM/Root | ||
| trans-3,4-Dihydroxy-3,4-dihydromollugin 24 | 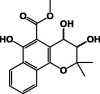 |
Hexane/Root | ||
| cis-3,4-Dihydroxy-3,4-dihydromollugin 25 | ||||
| Parvinaphthols C 26 R = Me |
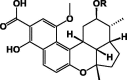 |
1P. parvifolia | 2 (DCM/MeOH)/Root | 3[24] |
| Busseihydroquinone E 27 R = Et |
P. bussei | |||
| [(3α,3′α,4β,4′β)-3,3′]-Dimethoxy-cis- [4,4′-bis(3,4,5,10-tetrahydro-1H-naphtho[2,3-c]pyran)]-5,5′,10,10′-tetraone 28 |  |
P. longiflora | Hexane/Root | [22] |
| Busseihydroquinone E 29 | 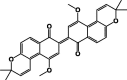 |
3.1P. parvifolia | 3.2 (DCM/MeOH)/Root | 3.2[24] |
Table 5.
Anthraquinones (30–42) that are abundant in different species of Pentas.
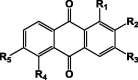 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Isolated compound | Derivatives |
Species | Extract/Organ | Refs. | ||||
| R1 | R2 | R3 | R4 | R5 | ||||
| Tectoquinone 30 | H | CH3 | H | H | H | P. micrantha | MeOH, (DCM/MeOH)/Root | [11] |
| P. lanceolata | (DCM/MeOH)/Root | [10] | ||||||
| Rubiadin 31 | OH | CH3 | OH | H | H | P. micrantha | MeOH,(DCM/MeOH)/Root | [11] |
| P. zanzibarica | MeOH/Stem | [22] | ||||||
| P. lanceolata | (DCM/MeOH)/Root | [10] | ||||||
| Rubiadin-1-methyl ether 32 | OCH3 | CH3 | OH | H | H | P. micrantha | MeOH, (DCM/MeOH)/Root | [11] |
| P. zanzibarica | Methanol/Stem | [22] | ||||||
| P. lanceolata | (DCM/MeOH)/Root | [10] | ||||||
| Nordamnacanthal 33 | OH | CHO | OH | H | H | [11] | ||
| Damnacanthal 34 | OCH3 | CHO | OH | H | H | P. micrantha | MeOH, (DCM/MeOH)/Root | [11] |
| P. zanzibarica | MeOH/Stem | [22] | ||||||
| P. lanceolata | (DCM/MeOH)/Root | [10] | ||||||
| Lucidin-ω-methyl ether 35 | OH | CH2OCH3 | OH | H | H | P. micrantha | MeOH, (DCM/MeOH) /Root | [11] |
| P. lanceolata | (DCM/MeOH)/Root | [10] | ||||||
| Damnacanthol 36 | OCH3 | CH2OH | OH | H | H | P. micrantha | MeOH, (DCM/MeOH)/Root | [11] |
| P. lanceolata | (DCM/MeOH)/Root | [10] | ||||||
| 5,6-Dihydroxylucidin-11-O-methyl ether 37 | OH | CH2OCH3 | OH | OH | OH | P. micrantha | MeOH, (DCM/MeOH)/Root | [11] |
| 5–6-Dihydroxydamnacanthol 38 | OCH3 | CH2OH | OH | OH | OH | [11] | ||
| P. lanceolata | (DCM/MeOH)/Root | [10] | ||||||
| Munjistin ethyl ester 39 | OH | COOCH3 | OH | H | H | P. micrantha | MeOH, (DCM/MeOH) /Root | [11] |
| 40 | H | OCH3 | CH3 | H | H | P. longiflora | DCM/Root | [25] |
| 41 | CH3 | H | OH | H | H | |||
| 42 | H | CH2OH | H | H | H | P. schimperi | EtOAc/Stem bark | [30] |
Table 6.
Anthraquinones glycosides (43–46) and anthraquinone dimers (47, 48) that are distributed in different Pentas species.
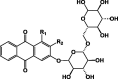 | |||||
|---|---|---|---|---|---|
| Isolated compound | Derivatives |
Species | Extract/Organ | Refs. | |
| R1 | R2 | ||||
| Rubiadin-1-methylether-3-O-β-primeveroside 43 | OCH3 | CH3 | P. bussei | EtOAc/Root | [25] |
| P. lanceolata | MeOH/Root, 50% EtOH/Leaves |
||||
| P. zanzibarica | MeOH/Stem | [29] | |||
| Rubiadin-3-O-β-primeveroside 44 | OH | CH3 | P. parvifolia | MeOH/Root | [25] |
| P. zanzibarica | MeOH/Stem | [29] | |||
| Damnacanthol-3-O-β-primeveroside 45 | OCH3 | CH2OH | P. parvifolia | MeOH/Root | [25] |
| P. bussei | |||||
| P. zanzibarica | MeOH/Stem | [29] | |||
| Lucidin-3-O-β-primeveroside 46 | OH | CH2OH | P. parvifolia | MeOH/Root | [25] |
| P. bussei | |||||
| P. zanzibarica | MeOH/Stem | [29] | |||
| Schimperiquinones A 47 R1 = OH, R2 = CH3 |
 |
P. schimperi | EtOAc/Stem bark | [30] | |
| Schimperiquinones B 48 R1 = H, R2 = OH | |||||
Table 7.
Iridoids from P. lanceolata.
| Isolated compound | Structure | Species | Extract/Organ | Refs. |
|---|---|---|---|---|
| Asperuloside 49 |  |
P. lanceolata | MeOH/Aerial parts | [32] |
| MeOH/Colleter | [18] | |||
| EtOH/Entire plant | [33], [34] | |||
| Asperulosidic acid 50 |  |
MeOH/Stem and leaves | [32] | |
| EtOH/Entire plant | [33], [34] | |||
| Tudoside 51 | 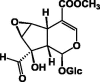 |
MeOH/Colleter | [18] | |
| EtOH/Entire plant | [28] | |||
| 13R-epi-Gaertneroside 52 | 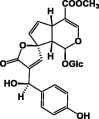 |
P. lanceolate | MeOH/Aerial parts | [32] |
| 13R-epi-Epoxygaertneroside 53 | 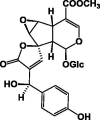 |
EtOH/Entire plant | [28] | |
| E-Uenfoside54 | 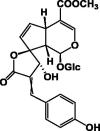 |
|||
| Z-Uenfoside55 | MeOH/Aerial parts | [32] | ||
| EtOH/Entire plant | [28] | |||
| Loganin 56 | 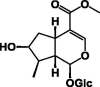 |
MeOH/Colleter | [18] | |
| Deacetyl-asperulosidic acid 57 | 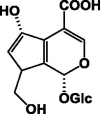 |
EtOH/Entire plant | [28] | |
| Ixoside 58 | 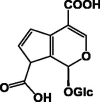 |
|||
| Griselinoside 59 | 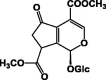 |
|||
| 6β,7β-Epoxysplendoside 60 | 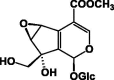 |
|||
| 61 |  |
EtOH/Entire plant | [28] | |
| 13R-Methoxy-epi-gaertneroside 62 |  |
P. lanceolate | 80% Aqueous MeOH/Aerial parts | [35] |
| 13S-Methoxy-epi-gaertneroside 63 |
Table 8.
Terpenes, sterols, Saponin and Oxindole alkaloids identified in P. lanceolata.
| Isolated compound | Structure | Species | Extract/Organ | Refs. |
|---|---|---|---|---|
| Oleanolic acid 64 R1, R2 = CH3 |
 |
P. lanceolata | MeOH/Colleter | [17], [18] |
| Ursolic acid 65 R1 = H, R2, R3 = CH3 | ||||
| Campesterol 66 | 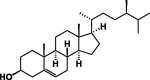 |
P. lanceolata | MeOH/Colleter | [17], [18] |
| β-Stigmasterol 67 | 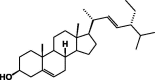 |
|||
| Caryophyllene 68 |  |
|||
| 3-O-β-fucosyl-quinovic acid 69 | 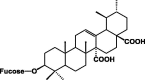 |
50% EtOH/Leaves | [36] | |
| Quermiside 70 | 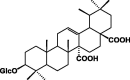 |
|||
| Speciophylline 71 | 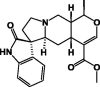 |
100% EtOH/Leaves | ||
| 72 | 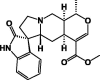 |
Simple phenolic compounds
Two examples of simple phenolics (1 and 2) were identified in the colleters of P. lanceolata by GC–Ms chromatography in a greater amount than in the stipules without colleters (Table 1) [18].
Naphthoquinones
P. longiflora was the only source among the genus Pentas from which naphthoquinones (3–7) were separated. Pantagolin 3 [19] and isagarin 5 were identified for the first time in the roots of P. longiflora, whereas psychorubrin 4 is a common constituent of other Rubiaceae species: Psychotria camponutans [20] and Mitracarpus frigidus (Table 2) [17].
Naphthohydroquinones
Busseihydroquinone A 8 [23] and the very recently discovered parvinaphthols A 10 and B 11 [24] were named after P. bussei and P. parvifolia, respectively. They are as well as the naphthohydroquinones (9 and 11) have been identified only in Pentas species (Table 3).
Chromene-based structures
This class of compounds is widespread in different species of Pentas as well as the other members of Rubiaceae. Compounds 14–17, 25 and 28 were discovered as novel compounds in 2003 in P. longiflora, P. bussei, and P. parvifolia. Additionally, an isolation of known compounds 21–24 from the root of P. longiflora [22], [25] was reported; these were similarly identified in another plant of Rubiaceae (Rubia cordifolia) [26]. Scopoletin 13 is a very common coumarin found broadly in many genera of Rubiaceae [17] (Table 4).
Anthraquinones
The anthraquinones are the major class of secondary metabolites in Pentas. They are also commonly found as mixtures of closely related pigments in the Rubiaceae family. Some members of this family have been used for centuries as a source of natural dye for textiles [17]. Many Pentas species produced anthraquinones in the form of aglycone (30–42) (Table 5) [10], [11], [22], [25], [21] or as glycosides (43–46) (Table 6) [24], [25], [29]. Two dimeric structures of anthraquinone named schimperiquinones, A 47 and schimperiquinones B 48 (Table 6), were isolated from P. schimperi as novel structures in 2014 [30]. Anthraquinones seem to be very important to the antiplasmodial activity expressed by Pentas [10].
Iridoids
Iridoids are monoterpenoid cyclopentanopyran type glycosides [31], which are common constituents of P. lanceolata. The first study to identify iridoids in P. lanceolata was performed by Schripsema and his coworkers in 2007 [32]. In this study, seven iridoid glycosides were identified from the aerial parts of P. lanceolata. Furthermore, asperuloside 49 and asperulosidic acid 50, which are characteristic iridoids of Rubiaceae, and five iridoids 51–55 were isolated (Table 7) [32]. The ethanolic extract of P. lanceolata (Forssk.) Deflers was analyzed. A total of 12 compounds were identified, and ten of them were iridoid glucosides. Among these, compounds 57–60 were identified for the first time in P. lanceolata in addition to a new iridoid 61 (Table 7) [28]. Recently, two new iridoids, namely, 13R-methoxy-epi-gaertneroside 56 and 13S-methoxy-epi-gaertneroside 57, were identified by way of bio-guided sub-fractionation. They were identified in the immunomodulatory active sub-fractions of P. lanceolata (Table 7) [35].
Terpenes, sterols, saponins, and alkaloids
These classes of secondary metabolites are not common in Pentas species. They have only been isolated from P. lanceolata. These are triterpenes (oleanolic 58 and ursolic acids 59), sterols (campesterol 60, β-stigmasterol 61) and sesquiterpene (caryophyllene 62) was found in the colleters of P. lanceolata (Table 8) [17], [18]. The identified alkaloids 71 and 72 were an oxindole skeleton (Table 8) [36].
Biological activities of Pentas species
Antiplasmodial activity
Endale and his coworker discussed the antiplasmodial activities of P. longiflora and P. lanceolata. They mentioned that the dichloromethane/methanol (1:1) extract of the roots indicated in vitro antiplasmodial activity against chloroquine-resistant (W2) (IC50: 0.93 ± 0.16 μg/mL) and chloroquine-sensitive (D6) strains (IC50: 0.99 ± 0.09 μg/mL) of Plasmodium falciparum [10]. Pentalongin 3 and psychorubrin 4 (Table 2) were tested against the same strains, W2 and D6, in the same study. The IC50 values of the first were 0.27 ± 0.09 and 0.23 ± 0.08 μg/mL, respectively, and for compound 4 (Table 2) were 0.91 ± 0.15 and 0.82 ± 0.24 μg/mL, respectively [10]. However, all of the previous results were lower than the reference compounds, which were chloroquine and mefloquine [10]. In 2013, those researchers found that the crude methanol root extract of P. micrantha, which is used as an antimalarial in East Africa, exhibited moderate antiplasmodial activity against W2 (IC50: 3.37 ± 0.74 μg/mL) and D6 (IC50: 4.00 ± 1.86 μg/mL) strains. Anthraquinones 30–36 and 38–39 (Table 5) were examined for the same strains, but they were not active [11].
Antimicrobial properties
P. decora was used traditionally in Western Uganda as an antifungal [12]. This common medicinal usage encouraged Ahumuza et al. to analyze the plant to determine whether this traditional use has a scientific basis or not. The ethanolic extract of P. decora leaves was studied for four fungal strains: Epidermophyton floccosum, Microsporum canis, Trichophyton rubrum and Candida albicans. The inhibitory zone of 2000 mg/mL of the plant extract was 4.8 ± 0.4 and 3.7 ± 0.2 mm against C. albicans and M. canis, respectively, while the other two fungal strains were not sensitive. Both results were greater than that of clotrimazole. They attributed the results to the presence of alkaloids and terpenoids, which are well-known to be biologically active in the treatment of fungal infections [12]. The ethanolic extract of P. longiflora (100, 500 and 100 µg/mL in 95% ethanol) was tested among another 19 extracts of some medicinal Rwandese plants against Mycobacteria. It inhibited the growth of M. simiue and M. avium at a concentration of 1000 µg/mL, whereas M. tuberculosis was less sensitive to it [13].
Wound healing
The ethanol flower extract of P. lanceolata was evaluated for its effect on wound healing. This was assessed using an excision wound model. Significant increments in granulation tissue weight, tensile strength, glycosaminoglycan, and hydroxyproline content were found. A group of rats treated with the extract at 150 mg/kg/day for 10 days via the oral route showed incremental improvement in the wound contraction relative to the untreated one, which may be due to increased collagen deposition, alignment, and maturation [14].
Analgesic effect
Suman et al. reported that n-hexane of leaves of P. lanceolata exhibited significant activity in relieving the pain from the acetic acid-induced writhing method [15]. The percentage of inhibitory activity was 61.91% at a dose of 200 mg/kg of the extract, whereas it was 75% at 150 mg/kg of aspirin.
Immunomodulatory activity
Ethyl acetate and n-butanol extracts of P. lanceolata and 13R-epi-gaertneroside 52 (Table 7) were discovered to be immunostimulants at both the humoral and cellular levels. This evaluation was performed on specific-pathogen-free chickens vaccinated against Newcastle disease (ND) virus. Increases in lymphocytes and macrophages were observed in the blood of poultry. These fractions (Ethyl acetate and n-butanol extracts of P. lanceolata), in addition to compound 52 (Table 7), appeared to decrease the mortality from ND in chickens [35].
Antitumor activity
Minimal literature has found a cytotoxic effect in the Pentas species. The methanolic root extract of P. micrantha and anthraquinones 30–36 and 38–39 (Table 5) revealed low cytotoxicity on the breast cancer cell line MCF-7 [11]. The compounds busseihydroquinone E 29 (Table 4), busseihydroquinone C 19 (Table 4), and rubiadin-1-methyl ether 32 (Table 5) exhibited the most potent cytotoxic activity within a survey done for some quinones separated from the roots of P. parvifolia and P. bussei. They had IC50 values of 62.3, 48.4 and 54.4 μM against the MDA-MB-231 ER-negative human breast cancer cell line, respectively [24]. Damnacanthal 34 (Table 5) proved to have a moderate influence on CCRF-CEM leukemia cells (IC50: 3.12 ± 0.27 μM) and against the drug-resistant cell line MDA-MB-231-BCRP (IC50: 7.02 ± 0.51 μM) by apoptosis in comparison with doxorubicin. This antiproliferative activity was attributed to reactive oxygen species (ROS) production and mitochondrial membrane potential (MMP) disruption [16].
Conclusions and future perspective
The main active constituents that were purified from Pentas are quinones, highly oxygenated chromene-based structures, and iridoids. P. lanceolata has represented the sole source of iridoids, whereas the naphthoquinones have been attributed exclusively to P. longiflora until now. Pentas species are widely used in folk medicine in many tropical regions. However, more attention should be paid to this plant in terms of its medicinal properties. The most interesting medicinal use of Pentas is antimalarial (which is attributed to the anthraquinones) and wound-healing activity; however, it did not show very promising antitumor activity. Further investigation should be conducted to evaluate this plant group with biological assays to address this research gap.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Heba-Tollah M. I. Sweelam, graduated from Al Azhar University, Faculty of Science, Botany Department. She obtained her Master’s degree in the field of plant physiology. She is currently working as an assistant researcher in the National Research Centre (NRC), Pharmaceutical, and Drug Industries Division, Chemistry of Natural Compounds Department. She has experience in the quantification and analysis of different plant constituents such as carbohydrates, proteins, lipids, volatile oil, and macro- and microelements. She has expertise in the phytochemical screening of some medicinal plants for plant metabolites, extraction, fractionation, and isolation of some bioactive compounds by several chromatographic techniques. She is also practicing different tissue culture techniques and increasing the content of bioactive compounds in regenerated plants.

Howaida I. Abd-Alla, Ph.D., specializes in metabolomics natural products chemistry and completed her Ph.D. at the University of Cairo in 2004. After spending time as a postdoctoral fellow at Laboratoire des Interactions Moléculaires et Réactivité Chimique et Photochimique UMR CNRS 5623, Université de Toulouse, France, she became a professor in the Chemistry of Natural Compounds Department, National Research Centre, Egypt. Currently, Prof. Dr. Abd-Alla works as the head of the department where her research focuses primarily on isolation, purification and identification of natural compounds from medicinal plants, bacteria and marine organisms using advanced techniques for identification (1D and 2D NMR analysis), synthesis of derivatives of natural products, and bioactive assays in vivo and in vitro in natural products for use in treating different diseases.

Ahmed B. Abdelwahab, Ph.D., graduated from the faculty of pharmacy, Menia University. He conducted his Master’s dissertation in the field of medicinal chemistry. He underwent a training period with the group of Prof. Dr. H. Laatsch, at the Institute of Organic and Biomolecular Chemistry, Goettingen, Germany. He worked as an Assistant Researcher in the Chemistry of Natural Compounds Department, National Research Centre, Egypt. He obtained his Ph.D. from Université de Lorraine, Metz, France, under the supervision of Prof. G. Kirsch. He worked in a project funded by the Plant Advanced Technologies (PAT) Company, Nancy, France, to find a new commercial pathway for the synthesis of Coronalone.

Mahmoud M. Gabr, Ph.D., is a former full professor of plant physiology in the Department of Botany, Faculty of Science, Cairo University.

Gilbert Kirsch, Ph.D., has been trained as an organic chemist at the Universities of Strasbourg and Metz. He started his academic career in 1973 at the University of Metz (now University of Lorraine) where he currently holds a position of Emeritus Professor of Organic Chemistry. He completed a postdoc at Oak Ridge National Laboratory (TN) in the Nuclear Medicine Group and was also an invited scientist at Kodak (Rochester, NY) at the University of Minho (Portugal), Emory University (Atlanta, GA) and Sapienza University in Rome. He has published approximately 300 papers, chapters in Patai’s Functional group series, in Houben-Weyl, in Wiley's Chemistry of Heterocyclic Compounds and in Springer’s Selenium and Tellurium Chemistry and was an editor for Springer's book about “Recent advances in redox active plant and microbial products”.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Hortorium L.H.B. Macmillan; 1976. Hortus third: a concise dictionary of plants cultivated in the United States and Canada. [Google Scholar]
- 2.Njoroge G.N., Bussmann R.W. Diversity and utilization of antimalarial ethnophytotherapeutic remedies among the Kikuyus (Central Kenya) J Ethnobiol Ethnomedicine. 2006;2:8. doi: 10.1186/1746-4269-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kokwaro J.O. University of Nairobi Press; 2009. Medicinal plants of East Africa. [Google Scholar]
- 4.Kokwaro JO. Medicinal plants of East Africa, East African Literature Bureau, Nairobi; 1976. p. 223.
- 5.Watt J.M., Breyer-Brandwijk M.G. E. & S Livingstone; 1962. The medicinal and poisonous plants of southern and eastern Africa: being an account of their medicinal and other uses, chemical composition, pharmacological effects and toxicology in man and animal. [Google Scholar]
- 6.Focho D.A., Ndam W.T., Fonge B.A. Medicinal plants of Aguambu-Bamumbu in the Lebialem highlands, southwest province of Cameroon. Afr J Pharm Pharmacol. 2009;3:001–013. [Google Scholar]
- 7.Mesfin F., Demissew S., Teklehaymanot T. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia. J Ethnobiol Ethnomedicine. 2009;5:28. doi: 10.1186/1746-4269-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giday M., Asfaw Z., Woldu Z. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J Ethnopharmacol. 2009;124:513–521. doi: 10.1016/j.jep.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Bekalo T.H., Woodmatas S.D., Woldemariam Z.A. An ethnobotanical study of medicinal plants used by local people in the lowlands of Konta Special Woreda, southern nations, nationalities and peoples regional state, Ethiopia. J Ethnobiol Ethnomedicine. 2009;5:26. doi: 10.1186/1746-4269-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endale M., Alao J.P., Akala H.M., Rono N.K., Eyase F.L., Derese S. Antiplasmodial quinones from Pentas longiflora and Pentas lanceolata. Planta Med. 2012;78:31–35. doi: 10.1055/s-0031-1280179. [DOI] [PubMed] [Google Scholar]
- 11.Endale M., Ekberg A., Alao J.P., Akala H.M., Ndakala A., Sunnerhagen P. Anthraquinones of the Roots of Pentas micrantha. Molecules. 2013;18:311–321. doi: 10.3390/molecules18010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahumuza T., Kirimuhuzya C. Qualitative (phytochemical) analysis and antifungal activity of Pentas decora (De wild), a plant used traditionally to treat skin fungal infections in Western Uganda. Res Pharm Biotechnol. 2011;3:75–84. [Google Scholar]
- 13.van Puyvelde L., Ntawukiliyayo J.D., Portaels F., Hakizamungu E. In vitro inhibition of mycobacteria by Rwandese medicinal plants. Phytother Res. 1994;8:65–69. [Google Scholar]
- 14.Nayak B.S., Vinutha B., Geetha B., Sudha B. Experimental evaluation of Pentas lanceolata flowers for wound healing activity in rats. Fitoterapia. 2005;76:671–675. doi: 10.1016/j.fitote.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Suman D., Vishwanadham Y., Kumaraswamy T., Shirisha P., Hemalatha K. Phytochemical evaluation and analgesic activity of Pentas lanceolata leaves. Nat Prod Chem Res. 2014;2 [Google Scholar]
- 16.Kuete V., Donfack A.R.N., Mbaveng A.T., Zeino M., Tane P., Efferth T. Cytotoxicity of anthraquinones from the roots of Pentas schimperi towards multi-factorial drug-resistant cancer cells. Invest New Drugs. 2015;33:861–869. doi: 10.1007/s10637-015-0268-9. [DOI] [PubMed] [Google Scholar]
- 17.Martins D., Nunez C.V. Secondary metabolites from Rubiaceae species. Mol Basel Switz. 2015;20:13422–13495. doi: 10.3390/molecules200713422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muravnik L.E., Kostina O.V., Shavarda A.L. Development, structure and secretion compounds of stipule colleters in Pentas lanceolata (Rubiaceae) South Afr J Bot. 2014;93:27–36. [Google Scholar]
- 19.Hari L., De Buyck L.F., De Pootert H.L. Naphthoquinoid pigments from Pentas longiflora. Phytochemistry. 1991;30:1726–1727. [Google Scholar]
- 20.Hayashi T., Smith F.T., Lee K.H. Antitumor agents. 89. Psychorubrin, a new cytotoxic naphthoquinone from Psychotria rubra and its structure-activity relationships. J Med Chem. 1987;30:2005–2008. doi: 10.1021/jm00394a013. [DOI] [PubMed] [Google Scholar]
- 21.Van Puyvelde L., El Hady S., De Kimpe N., Feneau-Dupont J., Declercq J.-P. Isagarin, a new type of tetracyclic naphthoquinone from the roots of Pentas longiflora. J Nat Prod. 1998;61:1020–1021. doi: 10.1021/np970589o. [DOI] [PubMed] [Google Scholar]
- 22.El-Hady S., Bukuru J., Kesteleyn B., Van Puyvelde L., Van T.N., De Kimpe N. New pyranonaphthoquinone and pyranonaphthohydroquinone from the roots of Pentas longiflora. J Nat Prod. 2002;65:1377–1379. doi: 10.1021/np020110e. [DOI] [PubMed] [Google Scholar]
- 23.Endale M., Ekberg A., Akala H.M., Alao J.P., Sunnerhagen P., Yenesew A. Busseihydroquinones A-D from the roots of Pentas bussei. J Nat Prod. 2012;75:1299–1304. doi: 10.1021/np3002223. [DOI] [PubMed] [Google Scholar]
- 24.Abdissa N., Pan F., Gruhonjic A., Gräfenstein J., Fitzpatrick P.A., Landberg G. Naphthalene derivatives from the roots of Pentas parvifolia and Pentas bussei. J Nat Prod. 2016;79:2181–2187. doi: 10.1021/acs.jnatprod.6b00178. [DOI] [PubMed] [Google Scholar]
- 25.Bukuru J. Isolation and structural elucidation of natural products from Pentas bussei K. Krause, Pentas lanceolata (Forsk.) Deflers and Pentas parvifolia Hiern (Rubiaceae). dissertation. Ghent University; 2003.
- 26.Itokawa H., Ibraheim Z.Z., Qiao Y.F., Takeya K. Anthraquinones, naphthohydroquinones and naphthohydroquinone dimers from Rubia cordifolia and their cytotoxic activity. Chem Pharm Bull (Tokyo) 1993;41:1869–1872. doi: 10.1248/cpb.41.1869. [DOI] [PubMed] [Google Scholar]
- 27.Bukuru J.F., Van T.N., Van Puyvelde L., Mathenge S.G., Mudida F.P., De Kimpe N. A benzochromene from the roots of Pentas bussei. J Nat Prod. 2002;65:783–785. doi: 10.1021/np0101604. [DOI] [PubMed] [Google Scholar]
- 28.Van Puyvelde L., Geysen D., Ayobangira F.-X., Hakizamungu E., Nshimiyimana A., Kalisa A. Screening of medicinal plants of Rwanda for acaricidal activity. J Ethnopharmacol. 1985;13:209–215. doi: 10.1016/0378-8741(85)90008-x. [DOI] [PubMed] [Google Scholar]
- 29.Kusamba C., Federici F., De Vicente Y., Galeffi C. The anthraquinones of Pentas zanzibarica. Fitoterapia. 1993;64:18–22. [Google Scholar]
- 30.Donfack A.R.N., Tala M.F., Wabo H.K., Jerz G., Zeng G.-Z., Winterhalter P. Two new anthraquinone dimers from the stem bark of Pentas schimperi (Rubiaceae) Phytochem Lett. 2014;8:55–58. [Google Scholar]
- 31.Dinda B., Chowdhury D.R., Mohanta B.C. Naturally occurring iridoids, secoiridoids and their bioactivity. An updated review, part 3. Chem Pharm Bull (Tokyo) 2009;57:765–796. doi: 10.1248/cpb.57.765. [DOI] [PubMed] [Google Scholar]
- 32.Schripsema J., Caprini G.P., van der Heijden R., Bino R., de Vos R., Dagnino D. Iridoids from Pentas lanceolata. J Nat Prod. 2007;70:1495–1498. doi: 10.1021/np070116+. [DOI] [PubMed] [Google Scholar]
- 33.Jensen S.R., Nielsen B.J. Iridoid glucosides in fouquieriaceae. Phytochemistry. 1982;21:1623–1629. [Google Scholar]
- 34.Venditti A., Guarcini L., Ballero M., Bianco A. Iridoid glucosides from Pentas lanceolata (Forssk.) Deflers growing on the Island of Sardinia. Plant Syst Evol. 2015;301:685–690. [Google Scholar]
- 35.Abd-Alla H.I., Sweelam H.M., Mohamed T.A., Gabr M.M., El-Safty M.M., Hegazy M.-E.F. Efficacy of extracts and iridoid glucosides from Pentas lanceolata on humoral and cell-mediated immune response of viral vaccine. Med Chem Res. 2017;26:2196–2204. [Google Scholar]
- 36.Kamurthy H., Dontha S., Duggi S., Sudhakar M. Phytochemical screening on Pentas lanceolata leaves-Isolation of saponin and anthracene glycosides and alkaloids. Am J Ethnomedicine. 2014;1:206–215. [Google Scholar]