Graphical abstract
Jet fuel production using thermal cracking of biodiesel.
Keywords: Bio jet fuel, Palm oil, Blended bio-jet, Biodiesel, Empirical equations, Economic evaluation
Abstract
Distillate of upgraded palm biodiesel was blended in different volume percentages (5, 10, 15, and 20%) with jet A-1. The mixture can be used as a replacement for petroleum Jet fuel. Physical properties of blends were measured and compared with those of jet A-1. Empirical equations were developed to predict the properties of blended fuel, including density, kinematic viscosity, freezing point, H/C ratio, and acid value. The statistical analysis indicated that the proposed equations predictions agree well with the experimental data. The predicted model shows an (R2) between 0.99–0.98, indicating good fitting between the experimental data and proposed model. The distillate of upgraded palm biodiesel was miscible with the kerosene jet A-1 in all volume fractions under study 5–20%. The economic analysis shows that the production cost per unit of the produced bio jet fuel was much higher than the selling price of the petroleum jet fuel. This price difference is due to the raw materials cost; as the palm oil used is nearly three times that of crude oil. The economic evaluation study reveals that the operating cost of prepared bio jet equals to 2360 $/ton, which is a promising result.
Introduction
Substitution of conventional jet A-1 can be achieved by adding a10% of bio jet to petroleum jet fuel [1]. The aviation industry is responsible for about 2% of global CO2 emissions, which is a greenhouse gas. The aviation community has called for a reduction of emissions [2]. The aviation industry power sources are limited unlike other methods of transportation [3]. The International Air Transport Association (IATA) has set a target of diminishing emissions by 50% in 2050 [1]. In order to limit emissions of CO2 emissions from the aviation sectors, the European Commission, the European Parliament, and the European Council decided to include international aviation in the existing European Union’s CO2 Emissions Trading Scheme (EU ETS) in December 2008. This policy means that any airplane will land at or depart from any airport in EU should be included in the EU ETS since 2012 [2]. Therefore, greenhouse gases emissions from aviation sector are under international control [3].
The United Nations has set a goal for the international aviation sector to achieve carbon neutral growth at 2020 [4]. Synthetic paraffinic kerosene is produced via Fisher-Tropch procedure (SPK-FT) as the first alternative jet fuel [4], [5]. SPK-FT can be mixed with petroleum jet fuel up to 50%, due to low aromatic compounds content in SPE-FT. Jet fuel with low levels of aromatic compounds may cause problems in aircraft fuel system seals [6].
Green House Gas emissions can be potentially reduced by using alternative fuel Such as bio-based jet-fuel [7]. A reduction in GHG emissions will increase the flexibility in aviation operations [8], [9]. Bio-SPK made from plants, such as Jatropha, algae, and Camelina, can deliver a clean burn, which may result in improving fuel efficiency and less wear on engine components [10], [11]. Sustainable aviation fuels have a crucial role in completely decreasing emissions growth. Due to continuous improvement in technology and economics of jet fuel, its usage will increase considerably in the future [12]. This in turn would reduce carbon footprint of the industry up to 80% [13] and number of pounds of waste [14]. The most promising alternative aviation fuels are the synthetically produced jet fuels from upgraded bio oils [15], [16], [17].
The financial overall performance is a vital parameter in assessing process viability to research the assignment’s profitability. The financial performance of a biodiesel plant (e.g., fixed capital and manufacturing cost, and the breakeven factor) can be determined once certain factors are identified, such as plant capacity, conversion method, raw material price, and chemical expenses. A 2013 study achieved by means of the Midwest Aviation Sustainable Biofuels Initiative (MASBI) “fueling a sustainable future for aviation” shows that a financial incentive US$ 2.0 consistent with gallon of bio-jet fuel is needed to compete with contemporary fossil jet gas charge. This calculation assumes a noticeably optimistic price of feedstock. This study estimates that for a more conservative cost development of feedstock, the incentive would be around US$ 2.7 for a gallon of bio-jet gas. A 3% blend could as a result increase the mixed jet gasoline charge by 2.5%, if the underlying bio-jet gas price is round US$ 40 per ton. Underneath these situations, the US marketplace could require incentives totaling US$ 540 million yearly for every 1% of mixing (on the basis of an annual intake of 20 billion gallons of jet gasoline a year by the USA Navy and Business Aviation (MASBI) report [18]. An international mixing of 1% would require annual incentives of the order US$ 1.8 billion.
The aim of this study was therefore to formulate a system of equations to characterize the blend of a distillate from upgraded palm biodiesel with jet A-1. The experimental results from our previous work are used in this study [19]. The economics of producing bio-jet gasoline was investigated on a business scale primarily based on experimental information.
Material and methods
Transesterification of palm oil to biodiesel and jet fuel production
The biodiesel was produced in a batch stirred tank reactor using KOH as homogeneous catalyst (0.7%, w/v) and methanol (20%, v/v) with palm oil at 70 °C for 2 h as shown in Fig. 1a. The reactor was sealed and equipped with a reflux condenser. Then, the produced methyl ester was separated from glycerol and washed with 5% warm acetic acid. Creating bio-jet fuel range hydrocarbon from palm biodiesel was prepared through conventional transesterification process [20]. Produced biodiesel was upgraded using heterogeneous catalyst (Zinc aluminate) on bench scale as shown in Fig. 1b [21]. Upgraded biodiesel was distilled and the distillate was blended with different volumetric ratios of jet A-1 [19]. All experimental values of density, kinematic viscosity, acid value, and freezing point were measured according to the standard test methods illustrated in Table 1. Ratio of H/C was calculated after determination of C, H, N, O, and S, using elemental analyzer (Elemental Vero-El, Germany).
Fig. 1.
(a) Block flow sheet of biodiesel production from palm oil, based on 360 mL biodiesel production capacity per batch, and (b) Block flow sheet of Bio-jet production based on 360 mL biodiesel reactant capacity /batch.
Table 1.
Physical properties of measured and predicted values for blends of upgraded palm biodiesel with jet A-1.
| Various blend ratio | Density, g/mL ASTM D-4052 |
Kinematic viscosity, mm2/s ASTM D-445 |
Freezing Point, °C ASTM D-7153 |
H/C ratio |
Acid value, mg KOH/g ASTM D-664 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp. value | Pred. Value | Exp. value | Pred. Value | Exp. value | Pred. Value | Exp. value | Pred. Value | Exp. value | Pred. Value | |
| 5% | 0.802 | 0.802 | 2.95 | 2.81 | −27 | −26.5 | 3.63 | 3.58 | 0.23 | 0.226 |
| 10% | 0.817 | 0.817 | 1.7 | 1.9 | −11 | −12.3 | 3.17 | 3.18 | 0.12 | 0.13 |
| 15% | 0.82 | 0.82 | 1.72 | 1.57 | −6 | −4.6 | 3 | 3.02 | 0.3 | 0.29 |
| 20% | 0.822 | 0.822 | 1.74 | 1.77 | −3 | −3.4 | 3.1 | 3.08 | 0.7 | 0.7 |
Mathematical modeling of jet fuel properties
A system of equations was developed as a function of bio-jet fuel volume fraction in a jet fuel and upgraded biodiesel fuel blend. The equations can be used to predict properties of jet fuel and bio-jet fuel blend up to 20% volume fraction of the bio-jet fuel. The experimental values were measured in our lab as described in the experimental section and in Table 1; these properties may change slightly depending on the palm oil source. The blend properties that can be predicted using this system of equations are density, kinematic viscosity, freezing point, hydrogen to carbon ratio (H/C), and acid value. These equations are developed to predict the properties of the blend, which will minimize cost and materials to investigate the properties of a certain blend composition. Parameters are fitted by minimizing error between experimental data and model output, using least square method. Equations in literature, used to fit experimental data for blending bio-fuels and petroleum fuels, were tried but poor fitting was observed [22], [23], [24]. Throughout this work, several polynomials were developed to predict blend properties.
Feasibility study of bio jet fuel
Feasibility evaluation is an extensively used method for improving studies to acquire economically feasible final results. Economic modeling may be used to assess and evaluate alternate procedures, assist in defining the mission scale and scope for economic value, and measure uncertainty of project technical and financial risks. It introduces and describes the way of examined feasibility examine and the findings of this investigation may be used.
The following steps are undertaken to perform the analysis in this study
-
•
Undergoes the process in concern in the laboratory; then collect the optimum experimental conditions.
-
•
Design process model using Aspen HYSYS™ process engineering software provided by Aspen Tech., Inc., USA [25].
-
•
Sizing the process’s equipment according to principles outlined in the literature [26], [27], [28].
-
•
Determine capital investments and operating cost.
-
•
Finally, calculate production cost of the main product.
Results and discussion
Mathematical modeling of bio jet fuel physical properties
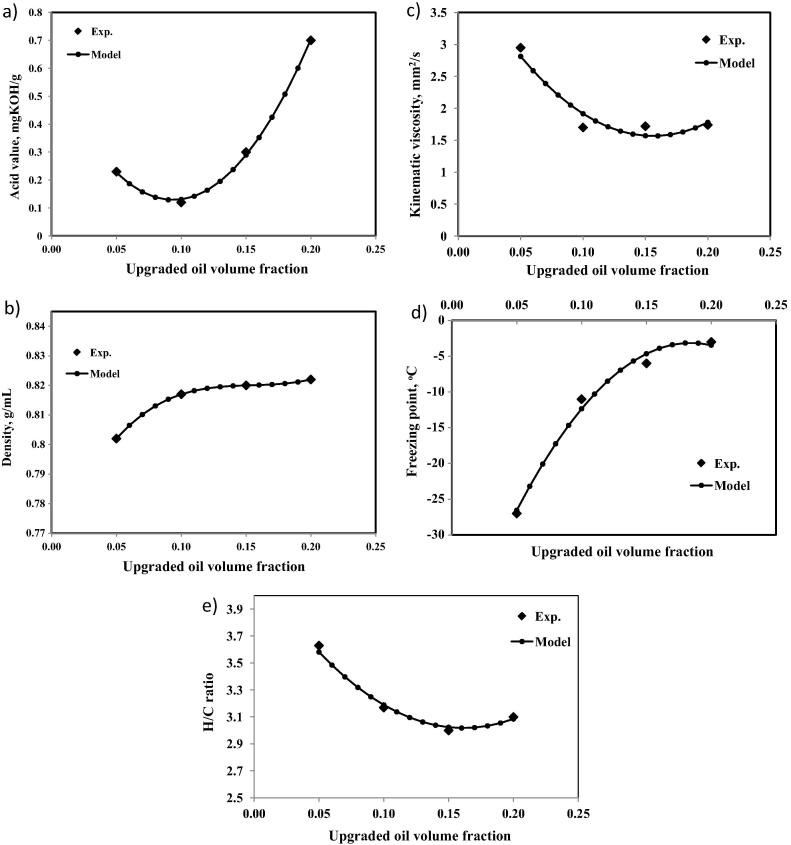
Fig. 2(a–e) shows measured and predicted values of density, kinematic viscosity, freezing point, H/C ratio, and acid value as shown in Eqs. (1), (2), (3), (4), (5), respectively, for blends of upgraded palm biodiesel with Jet A-1 at various volumetric percentages. It is clear that all values of different ranges of binary blends were closer to the optimum value or not far from the acceptable range of Jet fuel A-1. A comparison between the model predictions and experimentally measured values of the bio-jet physical properties indicates a good agreement between experimental results and model predictions, as confirmed by R2 values of 0.99. As shown, the viscosities of binary blends from jet A-1 and upgraded palm methyl ester increases with increasing the volume of ester in the blends, only viscosities of 3–5% are acceptable. Freezing points are still out of the permissible range, it needs part per million of hydrocarbon additives as stated in previous work [24]. H/C molar ratio may increase during reaction if n-paraffin is increased in the bio-jet fuel.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
where ρ is the density in g/mL, γ is the Kinematic viscosity in mm2/s, α is the freezing point in °C, H/C is the ratio of hydrogen to carbon, Acid value is calculated in mg KOH/g and is the volume fraction of bio-jet fuel in a blend with A type Jet fuel.
Fig. 2.
Comparison between model predictions and experimentally measured of binary blends (a) acid value, (b) density, (c) kinematic viscosity, (d) freezing point and (e) H/C ratio.
The interpretation shown in the above equations demonstrates the adequacy of these equations to represent data, having observed R2 equal to 0.99–0.98. A comparison between the experimental and model predictions is shown in Table 1. The developed equations are relations between the investigated different volume percentages of blended fuels with their different characteristics. Therefore, it can be used to identify the optimum mixture for different applications within the range of 0–0.2 vol% of bio-jet fuel.
Techno-economic feasibility study
Process model
The process simulation software, Aspen HYSYS™ V8.4 developed by AspenTech Inc., USA was used to construct the process model. The first step in setting up a process model was to define the chemical components. Triolein (C57H104O6) is considered as the triglyceride obtained from vegetable oil as the most common triglyceride in palm oil next to tri-palmitic. Accordingly, methyl oleate (C19H36O2) was taken as the bio-oil product, which then upgraded to bio-jet fuel. For those components not available in the library, such as catalysts, they were defined using “the Hypo Manager” tool in HYSYS™.
The NRTL thermodynamic model was used in this study, to accommodate methanol, which is a highly polar component [29]. The operating conditions were obtained from laboratory experiments (see Table 2). The alkali catalyzed transesterification process was used to convert palm oil into bio-oil; which is thermally cracked to jet fuel. This study was based on 100,000 ton/year of bio-oil production. This economic evaluation was based on the some assumptions. Operating hours are set at 8000 h/year.
Table 2.
Design basics and laboratory data.
| Parameter | Value |
|---|---|
| Design basics | |
| Qbio-oil (ton/year) | 100,000 |
| Hrs of operation/year | 8000 |
| % solvent recovery | 99.9 |
| Transesterification reaction | |
| Feedstock | Palm oil |
| Oil (mole) | 1 |
| Methanol (mole) | 6 |
| Catalyst | KOH |
| Catalyst (wt.%) | 0.7 |
| Rxn time (min) | 120 |
| Rxn temp (°C) | 70 |
| Rxnpres (kpa) | 100 |
| % oil conversion | 95 |
| Thermal cracking reaction | |
| Catalyst | Zn(AlO2)2 |
| Zn(AlO2)2 (gm) | 2.5 |
| Bio-oil (mL) | 100 |
| Rxn time (min) | 180 |
| Rxn temp (°C) | 300 |
| Rxnpres (kpa) | 330 |
| Jet fuel additive | |
| Additive | 2-Methoxyethanol |
| 2-Methoxyethanol (mL) | 4 |
| Jet fuel (L) | 1 |
High pressure and superheated steam are used for heating, while water was used for cooling. All chemical costs, including raw materials, catalysts, and products are given in Table 3, according to international market prices. The process was evaluated based on total capital investment (TCI), total manufacturing cost (TMC), return on investment (ROI%), and breakeven point. The assessment performed in this work was classified as a ‘‘preliminary estimate” with a range of expected accuracy from +30% to −20% [30]. While the results of such a study will likely not reflect the final cost of constructing a chemical plant, the technique is useful for providing a relative comparison of competing processes.
Table 3.
Costs of raw materials, utilities and products used in the process.
| Item | Cost ($) |
|---|---|
| Raw materials | |
| Palm oil ($/ton) | 740 |
| Methanol ($/ton) | 250 |
| KOH ($/ton) | 1150 |
| Acetic acid($/ton) | 550 |
| H3PO4 ($/ton) | 900 |
| Zn(AlO2)2 ($/ton) | 47,000 |
| 2-Methoxyethanol ($/ton) | 2000 |
| Products | |
| Jet fuel ($/ton) | 436.2 |
| Biojet ($/ton) | 2620 |
| No. 2 Diesel ($/ton) | 520 |
| No. 2 fuel oil ($/ton) | 600 |
| Glycerol ($/ton) | 800 |
| K3PO4 ($/ton) | 1750 |
| Utilities | |
| HPS ($/ton), 41 barg & 254 °C | 31.04 |
| Superheated HPS ($/ton), 41 barg & 500 °C | 39.66 |
| Cooling water ($/m3), 30 to 45 °C | 0.015 |
| Electricity ($/kw.h) | 0.06 |
| Waste treatment | |
| Hazardous ($/ton) | 200 |
| Non-hazardous ($/ton) | 36 |
The biojet fuel production from palm oil process was divided into three steps: transesterification (Fig. 3), product purification (Fig. 4), and thermal cracking (Fig. 5). The main processing units include reactors, distillation column, heat exchangers, pumps, and separators. Because detailed kinetic information was not available, a simple reactor model with 97% oil conversion to FAME was used to describe the transesterification reaction. The reactor considered as a continuous stirred tank reactor (CSTR) with a mounted jacket to provide the necessary heat. Multi-stage distillation was used for methanol recovery. The bio-oil was separated from glycerol, using mixer-separator combination using acetic acid. The glycerol was purified to +99 wt% and the bio-oil was thermally cracked to obtain the jet fuel. The thermal cracking was performed at 300 °C and 3.3 bar. The products were separated using a distillation tower into three main products: jet fuel (C8–C15) 48.7 vol%, diesel (C16–C19) 7.3 vol%, and heavy oil (>C19) 44 vol%.
Fig. 3.
Transesterification flow sheet; where (M100) make-up alcohol/catslyst mixer, (M200) recycled alcohol/catalyst and fresh alcohol mixer, (P100) recycled alcohol pump, (R100) transesterification reactor and (T100) methanol recovery distillation tower.
Fig. 4.
Product purification flow sheet; where (P200) transesterification products pump, (E200) transesterification products cooler, (M300) acid washing mixer, (V100) glycerol/bio-oil separator, (R200) neutralization reactor, (X200) settling tank, and (T300) glycerol purification distillation tower.
Fig. 5.
Thermal cracking flow sheet; where (P300) bio-oil pump, (R300) thermal cracking reacto, (X300) settling tank, (T400) products fractionation tower and (M500) jet fuel/additive mixer.
Total capital investment (TCI), total manufacturing cost, production cost, and rate of return on investment
The total capital investment (TCI) is needed to make the plant ready for startup and it includes the costs of equipment, installation, piping, instrumentation, electrical, building, utilities, storage, site development, auxiliary buildings, design, contractor's fee, and contingency [30] in addition to the working capital investment (WCI) that was set to be 8% of TCI. Table 4 shows the total capital investment beside the purchased costs of main equipment. The purchased cost of the main equipment was calculated using the charts and tables provided by Turton [30].
Table 4.
Equipment cost, fixed capital cost and total capital investment.
| Item | Cost ($) |
|---|---|
| Reactors | |
| Transesterification (R100) | 434,000 |
| Neutralization (R200) | 17,200 |
| Petroleum shift (R300) | 629,000 |
| Columns | |
| Methanol recovery (T100) | 377,100 |
| FAME purification (T200) | 0 |
| Glycerol purification (T300) | 322,500 |
| Product fractionation (T400) | 586,000 |
| Other | |
| Pumps | 40,800 |
| Heat Exchangers | 183,600 |
| Mixers | 58,500 |
| Gravity separators | 38,910 |
| Total bare module cost, CBM | 2,687,610 |
| Contingency fee, CCF = 0.18CBM | 483,770 |
| Total module cost, CTM = CBM + CCF | 3,171,380 |
| Auxiliary facility cost, CAC = 0.3CBM | 806,283 |
| Fixed capital cost, CFC = CTM + CAC | 3,977,663 |
| Working capital cost, CWC = 0.15CFC | 596,649 |
| Total capital investment, CTC = CFC + CWC | 4,574,312 |
In order to sell a product and to decide its price, manufacturing cost must be calculated and by adding profits, the selling price was determined. The manufacturing cost shown in Table 5 includes costs of raw materials, miscellaneous, utilities, shipping and packaging, labor, supervision, plant overhead, depreciation, interest, insurance, rent, royalties, and maintenance. The indirect manufacturing cost (IDMC) was set to be 20% of TMC. Net profit and ROI% for using palm oil as a feedstock are shown in Table 5.
Table 5.
Total manufacturing cost.
| Item | Cost ($) |
|---|---|
| Direct manufacturing cost | |
| Raw materials, CRM | |
| Palm oil | 77,894,737 |
| Methanol | 2,717,027 |
| KOH | 847,368 |
| Acetic acid | 176,000 |
| H3PO4 | 386,084 |
| Zn(AlO2)2 | 273,348 |
| 2-Methoxyethanol | 541,655 |
| Utilities, CUT | |
| Electricity | 2398 |
| H.P.S. | 1,834,232 |
| Superheated H.P.S | 10,338,748 |
| Cooling water | 32,240 |
| Waste treatment CWT | |
| Non-hazardous | 65,501 |
| Hazardous | 0 |
| Operating labors, COL | 1,021,500 |
| Direct supervisory and clerical labors, 18% of COL | 183,870 |
| Maintenance and repairs, 6% of CFC | 238,660 |
| Operating supplies, 15% of maintenance and repairs | 35,799 |
| Laboratory charges, 15% of COL | 153,225 |
| Patents and royalties | 3,642,610 |
| Subtotal | 100,385,001 |
| Fixed manufacturing costs | |
| Depreciation, ADEP | 397,766 |
| Plant overhead costs, 60% of the sum of operating labor, supervision and maintenance | 866,418 |
| Local taxes and insurance, 3.2% of CFC | 127,285 |
| Subtotal | 1,391,469 |
| General manufacturing expenses | |
| Administrative costs, 15% of the sum of operating labor, supervision and maintenance | 216,604 |
| Distribution and selling cost | 13,356,236 |
| Research and development | 6,071,016 |
| Subtotal | 19,643,857 |
| Total cost of manufacturing (COM) | 121,420,328 |
To calculate the production cost of the jet fuel, its total production capacity was divided by the total manufacturing cost per year. The production cost was 2360 $/ton of bio-jet fuel. Comparing the production cost with the price of petroleum jet fuel (436 $/ton), it is clear that bio-jet fuel price was much higher. To get ROI%, the net profit must be calculated. Different scenarios are analyzed for different biojet fuel selling price in relation to petroleum jet A-1 fuel selling price as shown in Table 6.The market trends for renewable jet fuel show that its selling price can be six times the price of the petroleum one(or even more) [28], [30].
Table 6.
Net profit and ROI%.
| Item | Petroleum jet price | 2 × petroleum jet price | 3 × petroleum jet price | 4 × petroleum jet price |
|---|---|---|---|---|
| Cost ($) | Cost ($) | Cost ($) | Cost ($) | |
| Products | ||||
| Jet fuel | 22,402,170 | 44,804,339 | 67,206,509 | 89,608,679 |
| Diesel | 4,276,409 | 4,276,409 | 4,276,409 | 4,276,409 |
| Heavy oil | 30,426,881 | 30,426,881 | 30,426,881 | 30,426,881 |
| Glycerol | 8,320,911 | 8,320,911 | 8,320,911 | 8,320,911 |
| K3PO4 | 1,626,935 | 1,626,935 | 1,626,935 | 1,626,935 |
| Total Revenue, AR | 67,053,306 | 89,455,475 | 111,857,645 | 134,259,815 |
| Annual net profit, ANP = AR-COM | −54,367,022 | −31,964,852 | −9,562,683 | 12,839,487 |
| Income taxes, AIT = 30% of ANP | −16,310,107 | −9,589,456 | −2,868,805 | 3,851,846 |
| After tax net profit, ANNP = ANP-AIT | −38,056,915 | −22,375,397 | −6,693,878 | 8,987,641 |
| After tax rate of return on investment, ROI% = (ANNP + ADEP)/CFCI * 100 | −946.766 | −552.526 | −158.287 | 215.953 |
| Payback period (years), PB = CFCI/ANNP | −0.105 | −0.178 | −0.594 | 0.443 |
Conclusions
-
•
Mathematical model are developed based on experimental results from our previous work on blends of upgraded palm biodiesel and jet A-1 with different volumetric percentages to predict blend characteristics. The model accuracy has been evaluated based on the coefficient of determination (R2), which ranged between 0.99–0.98. Excellent fitting between the experimental results and model prediction is observed.
-
•
An economical study of producing bio-jet fuel from palm oil was conducted. The production cost is 2360 $/ton of bio-jet fuel.
-
•
The main reason of the price difference between the production cost per unit of the renewable jet fuel produced and the petroleum jet fuel selling price is the cost of raw materials; as palm oil used to produce bio jet fuel costs nearly 3 times of the crude oil.
-
•
By using market selling price to calculate the net profit, the economic indicators for bio-jet production are very promising; as the ROI% equaled to 1010%.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgement
The authors thankfully appreciate the support of the Science and Technology Development Fund (STDF) – Egypt.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Jong S., Antonissen K., Hoefnagels R., Lonza L., Wang M., Faaij A. Life-cycle analysis of greenhouse gas emissions from renewable jet fuel production. Biotechnol Biofuels. 2017;10(64):1–18. doi: 10.1186/s13068-017-0739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anger A., Kohle J. Including aviation emissions in the EU ETS: much ado about nothing? A review. Transp Policy. 2017;2010:38–46. [Google Scholar]
- 3.Colvile R.N., Hutchinson E.J., Mindell J.S., Warren R.F. The transport sector as a source of air pollution. Atmos Environ. 2001;35:1537–1565. [Google Scholar]
- 4.Gossling S. Carbon neutral destinations: a conceptual analysis. JOST. 2009;17(1):38–46. [Google Scholar]
- 5.ASTM D1655. Standard specification for aviation turbine fuel containing synthesized hydrocarbon. ASTM; 2006.
- 6.Ebbinghaus A., Wiesen P. Aircraft fuels and their effect upon engine emissions. Air Space Europe. 2001;3(1–2):101–103. [Google Scholar]
- 7.Ridoutt B.G., Pfister S. A revised approach to water foot printing to make transparent the impacts of consumption and production on global freshwater scarcity. Glob Environ Change. 2010;20:113–120. [Google Scholar]
- 8.Hoefnagels R., Smeets E., Faaij A. Greenhouse gas footprints of different biofuel production systems. Renew Sustain Energy Rev. 2010;14:1661–1694. [Google Scholar]
- 9.Shonnard D.R., Williams L., Kalnes T.N. Camelina-derived jet fuel and diesel: sustainable advanced biofuels. Environ Prog Sustain Energy. 2010;29:383–392. [Google Scholar]
- 10.Baratieri M., Baggio P., Fiori L., Grigiante M. Biomass as an energy source: thermodynamic constraints on the performance of the conversion process. Bioresour Technol. 2008;99:7063–7073. doi: 10.1016/j.biortech.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Paul W. Griffin, Geoffrey P. Hammond and Jonathan B. Norman. Industrial energy use and carbon emissions reduction: a UK perspective. Advanced review. WIREs energy and environment. Wiley & Sons; 2016.
- 12.Afroughsabet V., Biolzi L., Ozbakkaloglu T. High-performance fiber-reinforced concrete: a review. J. Mater. Sci. 2016;51:6517–6551. [Google Scholar]
- 13.Dagaut P., Bakali A.E., Ristori A. The combustion of kerosene: experimental results and kinetic modeling using 1- to 3-component surrogate model fuels. Fuel. 2006;85:944–956. [Google Scholar]
- 14.Kumar K., Sung C., Hui X. Laminar flame speeds and extinction limits of conventional and alternative jet fuels. Fuel. 2011;90:1004–1011. [Google Scholar]
- 15.Kumar K., Sung C.A. comparative experimental study of the auto ignition characteristics of alternative and conventional jet fuel/oxidizer mixtures. Fuel. 2010;89:2853–2863. [Google Scholar]
- 16.Meghdad S. Experimental and numerical studies for soot formation in laminar coflow diffusion flames of Jet A-1 and synthetic jet fuels. PhD thesis. University of Toronto; 2013.
- 17.El-Diwani G., Hawash S.I., Kamal N. Development and evaluation of biodiesel fuel and byproducts from Jatropha oil. Int J Environ Sci Tech. 2009;6(2):219–224. [Google Scholar]
- 18.http://www.masbi.org/content/assets/MASBI_Report.pdf.
- 19.Hawash SI, Abdelkader E, Ashraf Amin, El-ArabyR, El-Diwani G. Investigation of metallic oxide catalyst role for up grading biodiesel to bio jet fuel range hydrocarbon. ARPN-JEAS: 12(7); 2017.
- 20.Abdelkader E, El-Araby R, El-Diwani G, Hawash SI. Blended bio-aviation fuel from up – graded palm biodiesel and jet A-1; submitted for publication.
- 21.El-Diwani G., Hawash S.I., Kamal N. Development and evaluation of biodiesel fuel and byproducts from jatropha oil. IJEST Int J Environ Sci Technol. 2009;6(2):219–224. [Google Scholar]
- 22.Mustafa ET, Van Gerpen Jon H. The kinematic viscosity of biodiesel and its blends with diesel fuel. JAOCS: 76 (12); 1999.
- 23.MejiaJ D, Salgado N, Orrego CE. Effect of blends of diesel and palm-castor biodiesels on viscosity, cloud point and flash point industrial crops and products: 43; 2013: p. 791–7.
- 24.Ertan A., Mustafa C. Determination of the density and the viscosities of biodiesel-diesel fuel blend. Renew Energy. 2008;33:2623–2630. [Google Scholar]
- 25.AspenTech Inc., Hysys 2004.2: Simulation Basis. Cambridge (USA): Aspen Technology, Inc.; 2005.
- 26.Sinnott RK. Coulson and Richardson’s Chemical Engineering, Volume 6: Chemical Engineering Design. 4th ed. London, UK: Butterworth-Heinemann; 2005.
- 27.McCabe W.L., Smith J.C., Harriott P. 5th ed. McGraw-Hill; New York (USA): 1993. Unit operations of chemical engineering. [Google Scholar]
- 28.Kern D.Q. McGraw-Hill; New York (USA): 1950. Process heat transfer. [Google Scholar]
- 29.El-Galad M.I., El-Khatib K.M., Zaher F.A. Economic feasibility study of biodiesel production by direct esterification of fatty acids from the oil and soap industrial sector. Egypt J Pet. 2015;24:455–460. [Google Scholar]
- 30.Deane P, Shea RO, Gallachoir B, Insight E. Biofuels for aviation, rapid response energy brief; April 2015.