Abstract
Background & objectives:
Fatty acids may affect the expression of genes, and this process is influenced by sex hormones. Cytokines are involved in the pathogenesis of non-alcoholic fatty liver disease (NAFLD), so this study was aimed to assess the association of erythrocyte membrane fatty acids with three cytokines and markers of hepatic injury in NAFLD patients and to explore whether these associations were the same in both sexes.
Methods:
In this cross-sectional study, 62 consecutive patients (32 men and 30 women) with NAFLD during the study period. Tumour necrosis factor-α (TNF-α), interleukin 6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), aspartate aminotransferase and alanine aminotransferase were measured in a fasting serum sample, and Fibroscan was conducted for each individual. Gas chromatography was used to measure erythrocyte membrane fatty acids. Univariate and multiple linear regressions were used to analyze data.
Results:
In men, IL-6 had a significant (P <0.05) positive association with total ω-3 polyunsaturated fatty acids (PUFAs). In women, TNF-α had a significant positive association with total ω-3 (P <0.05) and ω-6 (P <0.01) PUFAs, IL-6 had a significant (P <0.05) positive association with total monounsaturated fatty acids and MCP-1 had a significant positive association with total trans-fatty acids (P <0.05). No significant associations were observed between erythrocyte membrane fatty acids and liver enzymes or Fibroscan report in both sexes. In this study, women were significantly older than men [51 (42.75-55) vs 35.5 (29-52), P <0.01], so the associations were adjusted for age and other confounders.
Interpretation & conclusions:
Erythrocyte membrane fatty acid profile was not associated with serum liver enzymes or Fibroscan reports in NAFLD patients, but it had significant associations with serum TNF-α, IL-6 and MCP-1 and these associations were probably sex dependent.
Keywords: Cytokine, erythrocyte membrane, fatty acid, hepatic injury, NAFLD, non-alcoholic fatty liver
Non-alcoholic fatty liver disease (NAFLD), which is generally believed a hepatic manifestation of metabolic syndrome is considerably increasing worldwide because of dietary and lifestyle changes1. It may progress to non-alcoholic steatohepatitis (NASH) or cirrhosis, and hepatocellular carcinoma and the disease process is strongly influenced by proinflammatory cytokines2. Tumour necrosis factor-α (TNF-α), interleukin 6 (IL-6) and monocyte chemoattractant protein -1 (MCP-1) are among the major cytokines studied in the pathogenesis of NAFLD. Studies indicate that TNF-α is involved in every aspect of NAFLD including insulin resistance (IR), liver steatosis, necrosis, apoptosis and fibrosis3. Even healthy individuals with higher TNF-α level are at higher risk to develop NAFLD4. Adipose tissue and liver are among the major sites of TNF-α secretion that makes it an important factor in the pathogenesis of NAFLD.
MCP-1 is a chemotactic factor for monocytes. Previous studies indicate that increased expression of MCP-1 is associated with increased macrophage infiltration into the hepatic tissue and is associated with hepatic steatosis and IR5. Patients with NAFLD have a higher serum level of MCP-1 compared to healthy controls, and it has the highest value in NASH6. Numerous in vivo and in vitro studies have investigated the role of IL-6 in the pathogenesis of IR, liver injury as well as NAFLD. This cytokine could induce hepatic IR and is significantly higher in patients with NAFLD compared to other chronic liver diseases especially in the presence of advanced histopathology findings7.
The fatty acid profile could affect cytokine levels in the body. It could alter gene expressions, for example, it has been shown that the type of fatty acids consumed, may alter gene expressions, mainly through interaction with transcription factors8. Plasma/serum fatty acid profile has been found to be in close association with pathogenesis of many diseases including NAFLD9. On the other hand, sex hormones also affect cytokine gene expressions and fatty acid metabolism10.
The erythrocyte membrane fatty acid (EMFA) profile is a good predictor of tissue fatty acid profile, which is the result of dietary supply, fatty acid metabolism in the body, and genetic factors11. This is partly due to the long half-life (120 days) of erythrocytes. Therefore, erythrocyte membrane fatty acid profile is an appropriate biomarker for investigating the association between fatty acids and the pathology of diseases including NAFLD. The aim of this study was thus, to assess the association of erythrocyte membrane fatty acid profile with serum cytokine levels (TNF-α, IL-6 and MCP-1) and markers of hepatic injury (serum liver enzymes and Fibroscan results) in patients with NAFLD. It was also explored whether these associations were the same in both sexes.
Material & Methods
In this cross-sectional study, 62 consecutive patients (32 men, 30 women) who were newly (within preceding one month) diagnosed with NAFLD and referred to the Liver Disease Clinic in Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran, during June-August in 2015, were included. Patients were included if they were 18 yr or older, had steatosis based on ultrasonographic findings and controlled attenuation parameter (CAP) value above 180 (5%) in Fibroscan, had a stable body weight (±2%) and physical activity for at least three months before the study. The exclusion criteria were liver stiffness measurement (LSM) >10 in Fibroscan report (LSM is a marker of liver fibrosis and as the liver is a major site of cytokine extraction, advanced liver fibrosis may change their serum levels), having diabetes [fasting blood sugar (FBS) ≥126 mg/dl or using blood glucose lowering drugs], using lipid lowering drugs, having drug abuse, exposure to chemical pollutions, using steatogenic or hepatotoxic drugs (such as amiodarone, calcium channel blockers, perhexiline maleate, tamoxifen, chloroquine, methotrexate, corticosteroids, synthetic estrogens), using antioxidants or polyunsaturated fatty acid (PUFA) supplements in the six months before the study, having other acute or chronic liver diseases (such as viral hepatitis or cirrhosis), using drugs that affect weight (such as antidepressants, antipsychotics and hormone therapy), having any endocrine diseases which affect weight (such as hyperprolactinemia, Cushing's syndrome, thyroid disorders and congenital adrenal hyperplasia), having kidney or heart diseases or having a history of alcohol intake (>20 g/day).
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran, and all participants gave written informed consent.
Definition of fatty liver in sonography: In sonography, fatty liver was diagnosed with an increase in hepatic echogenicity using renal echogenicity as a reference, the presence of enhancement and the lack of differentiation of periportal and bile duct walls reinforcement because of great hyperechogenicity of the parenchyma12.
Fibroscan: Fibroscan (Echosens, Paris, France) is an ultrasound-based vibration-controlled transient elastography (VCTE™) device to assess liver stiffness (measured in kPa, correlated to fibrosis) in a non-invasive method by pressing a probe in between the ribs. It also quantifies steatosis at the same time, using the CAP which measures ultrasound attenuation and is correlated with the decrease in amplitude of ultrasound waves as they spread through the liver. The final CAP value is the median of individual measurements and ranges from 100 to 400 decibels per meter (dB/m)13. In this study, 10 LSM were performed. The median number from these 10 measurements was compared with the designated values on the Fibroscan Scoring Card.
Information on medical history and alcohol intake: At baseline, a medical history questionnaire was filled out for each participant. Alcohol intake was also estimated using, the type of alcohol consumed, its volume and the frequency of consumption. Fatty liver was considered non-alcoholic if the patient consumed <20 g of alcohol/day.
Anthropometric measurements: Weight, height and waist circumference of the participants were measured by a trained dietician according to the standard protocols14. Body mass index (BMI) was calculated as body weight (in kilograms) divided by the square of height (in meters). Waist-to-hip ratio was calculated as waist circumference divided by hip circumference.
Biochemical measurements: A 12 h fasting venous blood sample (15 ml) was taken from each participant to measure biochemical parameters. FBS, serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined by AutoAnalyzer Alpha Classic (Pars Azmon Kits, Tehran, Iran) and serum insulin level by enzyme-linked immunosorbent assay (ELISA) method (Diaplus Inc. Kit, North York, ON, Canada). Hepatitis B surface antigen (HBsAg), hepatitis B surface antibody, hepatitis B core antibody, hepatitis C virus (HCV) antibody and antinuclear antibodies (ANA) were evaluated using the third-generation ELISA method by Acon kits (Acon lab, San Diego, USA). Serum IL-6, TNF-α and MCP-1 levels were determined by commercially available ELISA kits (Human TNF-alpha Platinum ELISA, Human IL-6 Platinum ELISA and Human MCP-1 Platinum ELISA, eBioscience, Inc., San Diego, USA) according to the manufacturer's instructions.
IR was assessed by homeostasis model assessment-IR (HOMA-IR) index15 as follows:
HOMA-IR=[glucose (mg/dl) × Insulin (mU/l)]/405.
Fatty acid measurement in erythrocyte membrane: Venous blood samples (15 ml) were centrifuged at 3000 g for 10 min at 4°C. Erythrocytes were separated. Then an equal volume of physiological saline (sodium chloride 0.9%) was added to the erythrocytes, shaken and then centrifuged at 3000 g for 10 min at 4°C. Erythrocytes were washed according to the above procedure for three times. The washed erythrocytes were aliquoted and stored at −80°C; 200 μl of washed erythrocytes was evaporated to dryness under nitrogen gas. Boron trifluoride-methanol solution 14 per cent (2 cc) and methanol (1 ml) were added to the dried erythrocytes and heated in ban marry for 10 min at 60°C. Then, 2 ml of n-hexane were added to each tube and shaken for two minutes. After settling, the n-hexane layer that contained the methylated fatty acids was transferred into another tube, and the solvent was removed by evaporation. The residue was re-dissolved in 50 μl n-hexane, completely mixed and then 1 μl of this solution was used for gas chromatograph. YL6500 gas chromatograph (Young Lin instrument company, Korea) equipped with a 60 m×0.25 mm (film thickness 0.2 μm) capillary column (TR-CN100, Teknokroma) and flame ionization detector was used to measure EMFA profile. The analytic conditions were as follows: a volume injection of 1 μl, carrier gas hydrogen (2 ml/min), injector temperature of 240°C, flame ionization detection at 280°C, split ratio of 1:20 and an oven temperature from 90° to 220°C with a stepped temperature programme within a total run time of 47 min. Thirty seven fatty acid methyl esters were separated, using Supelco 37-Component fatty acid methyl ester standard Mix (Sigma-Aldrich Co. LLC, USA). Young Lin Autochro3000 chromatograph data system, Version 2.0.15 (Young Lin Co. Ltd, Anyang, South Korea) was used for quantification and identification of peaks. Fatty acids were expressed as the percentage of fatty acids curve area from the total fatty acids curve areas in the chromatogram. The EMFA profile is reported as the percentage of total saturated fatty acids (SFAs), total monounsaturated fatty acids (MUFAs), total ω-6 PUFAs, total ω-3 PUFAs, ω-6 to ω-3 fatty acid ratio and total trans-fatty acids (TFAs).
Statistical analysis: Data were presented as mean±standard deviation or median (interquartile range: IQR) for parametric and non-parametric variables, respectively. Shapiro–Wilks test was used to check the normality of the continuous variables and arithmetic transformations were performed if necessary. Continuous variables were compared between the two sexes by Student's t test. Univariate linear regression was used to evaluate the relationship between EMFA profile (Total SFA, MUFA, ω-6 PUFA, ω-3 PUFA, ω-6 to ω-3 ratio and TFAs) as independent variables and serum cytokines (TNF-α, IL-6 and MCP-1) and markers of hepatic injury (LSM, CAP, serum AST and ALT) as dependent variables. Variables with P <0.2 in univariate linear regression, were entered into multiple-linear regression. In multiple-linear regression (backward method), all these relationships were adjusted for factors, which could affect EMFA, serum cytokine levels or markers of hepatic injury including age, BMI and HOMA index. P <0.05 in multiple linear regression was considered significant. SPSS statistics software version 23 (IBM SPSS Statistics, Armonk, NY, USA) was used to analyze data.
Results
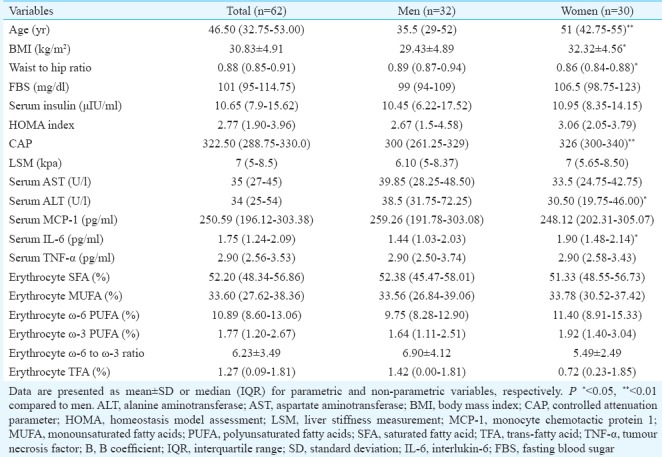
Table I shows the baseline characteristics of participants. In this study, 62 patients with NAFLD were enrolled, of whom 32 were men (age range: 23-61 yr) and 30 were women (age range: 18-67 yr). Among women, 16 were postmenopausal and 14 were premenopausal. Women were significantly older (P <0.01), had higher BMI (P <0.05), CAP (P <0.01) and serum IL-6 level (P <0.05). Waist-to-hip ratio and serum ALT level were significantly (P <0.05) higher in men. Serum AST, TNF-α, MCP-1, LSM, FBS, fasting serum insulin, HOMA index and erythrocyte membrane fatty acid profile were not significantly different between men and women.
Table I.
Baseline characteristics of participants
Association of serum cytokine levels with erythrocyte membrane fatty acids in patients with NAFLD (Tables II and III): In total analysis, serum TNF-α had a significant (P <0.05) negative association with MUFAs and significant (P <0.05) positive associations with ω-6 and ω-3 PUFAs of erythrocyte membrane in both univariate and multiple analysis. Serum MCP-1 had a significant (P <0.05) positive association with TFAs of erythrocyte membrane in both univariate and multiple analysis. Serum IL-6 was not associated with any of erythrocyte membrane fatty acids.
Table II.
Univariate linear regression analysis of serum cytokines with erythrocyte membrane fatty acids in patients with non-alcoholic fatty liver
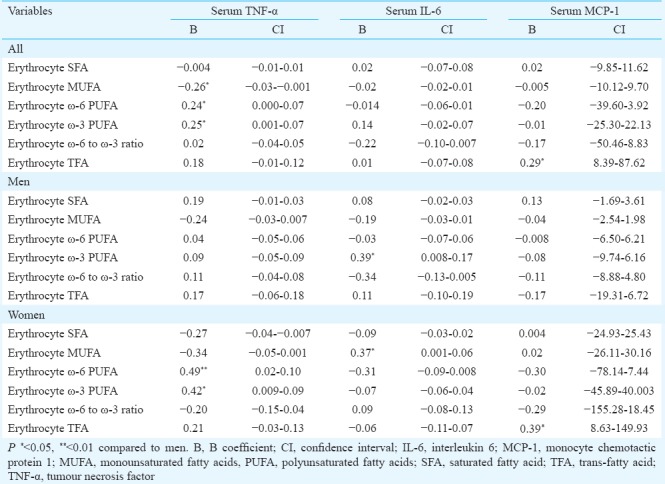
Table III.
Multiple linear regression analysis of serum cytokines with erythrocyte membrane fatty acids in patients with non-alcoholic fatty liver
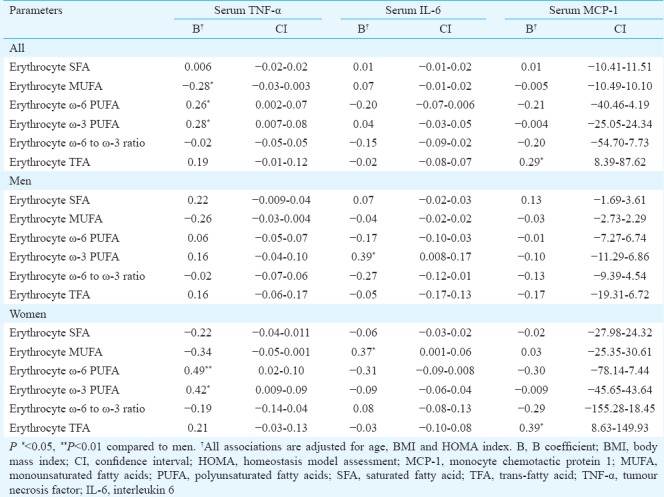
In men, the only significant association was between serum IL-6 and ω-3 PUFAs of erythrocyte membrane. Serum IL-6 had a significant (P <0.05) positive association with ω-3 PUFAs of erythrocyte membrane in both univariate and multiple analyses. Serum TNF-α and MCP-1 were not associated with any of erythrocyte membrane fatty acids either in univariate or multiple analyses.
In women, serum TNF-α had a significant positive association with ω-3 (P <0.05) and ω-6 (P <0.01) PUFAs of erythrocyte membrane, serum IL-6 had a significant (P <0.05) positive association with MUFAs of erythrocyte membrane and serum MCP-1 had a significant (P <0.05) positive association with TFAs of erythrocyte membrane. These associations were significant in both univariate and multiple analyses.
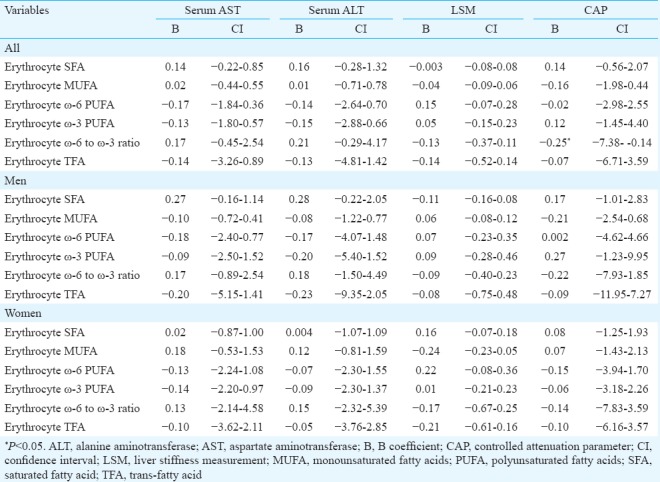
Association of markers of hepatic injury with erythrocyte membrane fatty acids in patients with NAFLD (Table IV): In total analysis, CAP had a significant (P <0.05) negative association with ω-6 to ω-3 ratio of erythrocyte membrane in univariate analysis but in multiple analysis, this association did not remain significant (not shown in the Table). There was no other significant associations between erythrocyte membrane fatty acids and markers of hepatic injury in total analysis or each sex separately.
Table IV.
Univariate linear regression analysis of Fibroscan report and serum liver enzymes with erythrocyte membrane fatty acids in patients with non-alcoholic fatty liver
Discussion
In this study, there was not any significant association between markers of hepatic injury and erythrocyte membrane fatty acid profile in either men or women. This was in accordance with another study which did not find any significant difference in erythrocyte membrane fatty acids between NASH and simple steatosis16. However, serum cytokines had significant associations with erythrocyte membrane fatty acid profile. In total analysis, serum TNF-α had a significant positive association with ω-6 PUFAs and a significant negative association with MUFAs. As TNF-α is known as an inflammatory cytokine, its positive association with ω-6 PUFAs and its negative association with MUFAs are in accordance with the known inflammatory role of ω-6 PUFAs17 and the anti-inflammatory role of MUFAs18.
There was also a significant positive association between ω-3 PUFAs of erythrocyte membrane and serum TNF-α level which was in contrast with the suggested anti-inflammatory role for ω-3 fatty acids19, but another study also reported higher ω-3 PUFAs in erythrocyte membrane of obese individuals compared to the control group20. The contrast may be the result of different tissues used to assess fatty acids or different study populations regarding age, sex, BMI or race (genetic differences) which influence PUFA metabolism.
In this study, ω-6 to ω-3 ratio of erythrocyte had no significant associations with serum cytokines while both of these had significant associations with serum TNF-α separately. Deckelbaum21 also suggested that total amount of each one of these fatty acids had more utility than their ratio. The ratio could mask the very low or high intakes of each one of them21.
TFAs of erythrocyte membrane showed a significant positive association with serum MCP-1 in this study. A positive association between membrane levels of trans-fatty acids and systemic inflammatory responses has also been reported in established heart disease22.
The significant positive association of serum TNF-α with ω-6 and ω-3 PUFAs and the significant positive association of serum MCP-1 with TFAs of erythrocyte membrane existed only in women. In addition to these associations, serum IL-6 also had a significant positive association with MUFAs of erythrocyte membrane in this group. Although this is in contrast with the anti-inflammatory role of MUFAs, it has been reported that higher MUFAs in erythrocyte membranes are a predictor of postmenopausal breast cancer23 and simultaneously, cumulative exposure to IL-6 increases the risk of its recurrence24. In men, the association of erythrocyte membrane fatty acid profile and the serum cytokines was limited to the significant positive association between ω-3 PUFAs and serum IL-6 level.
Based on these findings, it can be concluded that EMFA profile has significant associations with the serum level of TNF-α, IL-6 and MCP-1 and these associations are probably sex-dependent and more significant in women. This could be explained by the difference in body fat mass, its distribution and the effect of sex hormones on cytokine gene expressions and fatty acid metabolism25. For instance, oestrogen could increase docosahexaenoic acid synthesis in women26, so the power of association between ω-3 PUFAs and inflammatory markers may be different between men and women. Feltham et al10 also showed that the influence of fatty acids on gene expression was sex dependent in a mouse model.
In the present study, 53 per cent of the women were postmenopausal but due to higher adipose tissue (as an important source of estradiol) in women, and higher steroid levels in adipose tissue of postmenopausal women compared to their plasma levels27, postmenopausal status did not cover the sex-dependent association between fatty acids and cytokines. Though menopause is associated with increased serum cytokine levels, the levels are still comparable to those of men28. Therefore, the effect of menopausal status on the observed sex-dependent association between fatty acids and cytokines is probably low.
In the present study, all the relationships were adjusted for factors, which could affect erythrocyte membrane fatty acids, serum cytokines or hepatic injury (including age, BMI and HOMA index), so that the independence of these associations could be confirmed. This study also had some limitations. The cross-sectional nature of this study did not allow establishing causal relationships, so it was more a hypothesis-generating study. A larger sample size with biopsy-proven pathologic findings (to separate simple steatosis from NASH) may further clarify these associations. Although all the associations in this study were adjusted for age, BMI and HOMA index, it is suggested that men and women to be carefully matched for confounding variables in future studies.
In conclusion, erythrocyte membrane fatty acid profile was not associated with markers of hepatic injury in NAFLD, but it showed significant associations with serum TNF-α, IL-6 and MCP-1 levels and these associations were probably sex dependent.
Acknowledgment
The authors express their gratitude to Keivan Virology Lab, Tehran, Iran.
Footnotes
Financial support & sponsorship: Authors acknowledge Iran University of Medical Sciences, Tehran, Iran, for financial support to this study
Conflicts of Interest: None.
References
- 1.Amirkalali B, Poustchi H, Keyvani H, Khansari MR, Ajdarkosh H, Maadi M, et al. Prevalence of non-alcoholic fatty liver disease and its predictors in North of Iran. Iran J Public Health. 2014;43:1275–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Stojsavljević S, Gomerčić Palčić M, Virović Jukić L, Smirčić Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:18070–91. doi: 10.3748/wjg.v20.i48.18070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JK, Feng ZW, Li YC, Li QY, Tao XY. Association of tumor necrosis factor-α gene promoter polymorphism at sites -308 and -238 with non-alcoholic fatty liver disease: A meta-analysis. J Gastroenterol Hepatol. 2012;27:670–6. doi: 10.1111/j.1440-1746.2011.06978.x. [DOI] [PubMed] [Google Scholar]
- 4.Seo YY, Cho YK, Bae JC, Seo MH, Park SE, Rhee EJ, et al. Tumor necrosis factor-α as a predictor for the development of nonalcoholic fatty liver disease: A 4-year follow-up study. Endocrinol Metab (Seoul) 2013;28:41–5. doi: 10.3803/EnM.2013.28.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nio Y, Yamauchi T, Iwabu M, Okada-Iwabu M, Funata M, Yamaguchi M, et al. Monocyte chemoattractant protein-1 (MCP-1) deficiency enhances alternatively activated M2 macrophages and ameliorates insulin resistance and fatty liver in lipoatrophic diabetic A-ZIP transgenic mice. Diabetologia. 2012;55:3350–8. doi: 10.1007/s00125-012-2710-2. [DOI] [PubMed] [Google Scholar]
- 6.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–74. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Prakash S, Chhabra S, Singla V, Madan K, Gupta SD, et al. Association of pro-inflammatory cytokines, adipokines & oxidative stress with insulin resistance & non-alcoholic fatty liver disease. Indian J Med Res. 2012;136:229–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Adv Nutr. 2012;3:127–34. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, et al. Increase in long-chain polyunsaturated fatty acid n – 6/n – 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106:635–43. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 10.Feltham B, Balogun K, Cheema S. Omega-3 polyunsaturated fatty acids have sex-specific effects on the phospholipid fatty acid composition and neurotrophin expression in the brain of C57BL/6 mice. FASEB J. 2015;29(1 Suppl):401.8. [Google Scholar]
- 11.Takkunen MJ, de Mello VD, Schwab US, Šgren JJ, Kuusisto J, Uusitupa MI, et al. Associations of erythrocyte membrane fatty acids with the concentrations of C-reactive protein, interleukin 1 receptor antagonist and adiponectin in 1373 men. Prostaglandins Leukot Essent Fatty Acids. 2014;91:169–74. doi: 10.1016/j.plefa.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal AJ American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–25. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 13.Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): A novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–35. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 14.McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric Reference Data for Children and Adults: US Population, 1999-2002. Adv Data. 2005;361:1–5. [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Allard JP, Aghdassi E, Mohammed S, Raman M, Avand G, Arendt BM, et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): A cross-sectional study. J Hepatol. 2008;48:300–7. doi: 10.1016/j.jhep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75:197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Monfort-Pires M, Ferreira SRG. Modification in a single meal is sufficient to provoke benefits in inflammatory responses of individuals at low-to-moderate cardiometabolic risk. Clin Nutr. 2016;35:1242–50. doi: 10.1016/j.clnu.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Calder PC. Long-chain fatty acids and inflammation. Proc Nutr Soc. 2012;71:284–9. doi: 10.1017/S0029665112000067. [DOI] [PubMed] [Google Scholar]
- 20.Del Genio G, Ferreri C, Marfella R, Pournaras D, Le Roux CW, del Genio F, et al. Morbid obesity is associated to altered fatty acid profile of erythrocyte membranes. J Diabetes Metab. 2015;6:582–5. [Google Scholar]
- 21.Deckelbaum RJ. N-6 and n-3 fatty acids and atherosclerosis: Ratios or amounts? Arterioscler Thromb Vasc Biol. 2010;30:2325–6. doi: 10.1161/ATVBAHA.110.214353. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC, et al. Trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80:1521–5. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pala V, Krogh V, Muti P, Chajès V, Riboli E, Micheli A, et al. Erythrocyte membrane fatty acids and subsequent breast cancer: A prospective Italian study. J Natl Cancer Inst. 2001;93:1088–95. doi: 10.1093/jnci/93.14.1088. [DOI] [PubMed] [Google Scholar]
- 24.Cole SW. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;27:3418–9. doi: 10.1200/JCO.2009.21.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cartier A, Côté M, Lemieux I, Pérusse L, Tremblay A, Bouchard C, et al. Sex differences in inflammatory markers: What is the contribution of visceral adiposity? Am J Clin Nutr. 2009;89:1307–14. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- 26.Kafkas S, Dost T, Ozkayran H, Yenisey C, Tuncyurek P, Birincioglu M, et al. Effect of estrogen therapy on adipocytokines in ovariectomized-aged rats. J Obstet Gynaecol Res. 2012;38:231–8. doi: 10.1111/j.1447-0756.2011.01696.x. [DOI] [PubMed] [Google Scholar]
- 27.Szymczak J, Milewicz A, Thijssen JH, Blankenstein MA, Daroszewski J. Concentration of sex steroids in adipose tissue after menopause. Steroids. 1998;63:319–21. doi: 10.1016/s0039-128x(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 28.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]


