Abstract
Post-menopausal osteoporosis (PO) is one of the major health issues associated with menopause-related oestrogen withdrawal. Despite the intense research and the relevant progress achieved in the last two decades, the pathogenic mechanism underlying PO is still poorly understood. As a consequence of this gap in the knowledge, such disorder and the related complications are still difficult to be effectively prevented. A wealth of experimental and epidemiological/clinical evidence suggests that the endocrine change associated to menopausal transition might lead to a derangement of redox homeostasis, that is, the prelude to the health-threaten condition of oxidative stress (OxS). In turn, this (bio)chemical stress has been widely hypothesized to contribute, most likely in synergy with inflammation, to the development of menopause-related diseases, including PO. The main aim of this review is to discuss the current literature evidence on the association between post-menopausal oestrogen withdrawal, OxS and PO. It is also aimed to provide a critical overview of the most significant epidemiological studies on the effects of dietary antioxidants on bone health and to devise a strategy to overcome the limitations emerged and controversial results.
Keywords: Menopause, oestrogens, oxidative stress, post-menopausal osteoporosis, reactive oxygen species, redox homeostasis
Introduction
Post-menopausal osteoporosis (PO) is one of the major health problems associated with menopause-related oestrogen deprivation1. The disease is characterized by a reduced bone mass and an impairment of bone architecture, with both leading to an increased risk in fragility fractures (FFs). FFs, in turn, are significantly associated to a high rate of disability and mortality1,2.
According to an epidemiological study, 22 million women and 5.5 million men living in the European Union suffered from PO in 2010, with more than 3.5 million new FFs sustained3. The incidence of osteoporosis in Asia is also steadily increasing mostly because this region is witnessing a rapid rise in elderly population. In particular, India is expected to have the highest total increase in people aged over 50 yr (from 120 million in 2013 to 620 million in 2050)4. Moreover, the female population is estimated to go through menopause earlier than Caucasians (mean age 46 vs 51 yr). This fact, along with the expected increase in female life expectancy (from 68 to 73 yr in the period 2011-2021), will extend the time span of post-menopausal period and, hence, the likelihood of related disturbances1,4.
Since PO is a worldwide health issue, an effort should be made to better clarify the underlying pathogenic mechanism(s) so as to devise effective preventive strategies. One of the most intriguing hypotheses focuses on the pivotal role of oxidative stress (OxS) as a link between gonadal failure and clinical onset and progression of PO5,6,7. By definition, OxS is the result of disruption of a finely tuned balance between antioxidants and oxidants, with the latter group including reactive oxygen species (ROS), reactive nitrogen species (RNS) and other less abundant substances8,9. The main aim of this review is to illustrate and discuss the current evidence on the association between post-menopausal oestrogen withdrawal, uncontrolled ROS generation and PO. Furthermore, it is meant to provide a critical overview of the most significant epidemiological studies on the effects of dietary antioxidants on bone health and to devise a strategy to overcome the limitations emerged and controversial results.
Post-menopausal osteoporosis: From oestrogen decline to disease onset
To preserve structural integrity, the skeleton necessities to continuously remodel and repair the micro-cracks that take place in the two types of osseous tissue: the ‘spongy’ cancellous and the compact cortical bone. Bone remodelling cycle occurs through the balanced activities of its constituent cell types: bone-forming osteoblast, the bone-degrading osteoclast and the osteocyte, the primary trigger of the entire turnover process10.
Despite pivotal role of oestrogen on the bone, it is still difficult to identify the mechanism(s) by which these hormones modulate bone metabolism. The oestrogens are able to block bone resorption through two mechanisms: both by direct interaction with osteocytes and osteoclast and by regulation of T-cell and osteoblast formation and activity11,12. As described in some reviews11,13,14, the biological axis constituted by receptor activator of nuclear factor k-B (RANK), its natural ligand (RANKL) and decoy receptor for RANKL, osteoprotegerin (OPG), is the molecular link between oestrogen deficiency and bone loss15. Noteworthy, RANK/RANKL/OPG axis also appears to play a key role in mediating the widely hypothesized connection between immune system and bone, central concept of osteoimmunology field13,14.
Osteoclast precursors differentiate under the influence of macrophage colony-stimulating factor and RANKL13. The latter cytokine, expressed in both membrane-bound (in osteoblasts) and soluble forms, promotes differentiation of osteoclast precursors and activates mature osteoclast to reabsorb bone, through the interaction with RANK16. The osteoclastogenic effect can be prevented by OPG, which is produced by osteoblasts and stromal cells and competes with RANKL for RANK binding14. In addition, oestrogens can decrease osteoclast precursor responsiveness to RANKL15 by binding to nuclear factor-κβ (NF-κβ), which prevents the production of RANKL and promotes the release of OPG. Finally, oestrogens interfere with osteoclast signalling pathways downstream to RANK, thereby inhibiting osteoclast differentiation14. As a logical consequence, oestrogen withdrawal affects the ‘healthy’ balance between RANKL and OPG, with the result of prolonging osteoclast lifespan and accelerating bone resorption14.
Lymphocytes T and B may play an important role in the complicated network of biological interactions underlying oestrogen control of bone homeostasis13,14. In particular, T-cells have been deemed to be the master regulators of both life cycle and metabolism of osteoclasts/osteoblasts. These immune cells are capable of inducing bone loss through the production of several bone-reabsorbing cytokines including RANKL, interleukin (IL)-1, IL-6 and tumour necrosis factor-α (TNF-α). Moreover, oestrogen decline has a direct effect on the synthesis of cytokines in T-cells, as shown in experiments on ovariectomized (OvX) rodents14 and in a few human studies17,18. Our review does not aim at describing in detail the mechanisms implicated in the immune regulation of osteoporosis caused by oestrogen deficiency, which will not be dealt in-depth.
Pro-inflammatory cytokines are not the only factors that have been shown to be altered in a context of bone loss induced by oestrogen deficiency. Converging pre-clinical and epidemiological findings indicate that an excessive accumulation of oxidant substances, in particular, ROS, might contribute to the imbalance between bone resorption and formation that underlies PO development.
Reactive oxygen species (ROS) versus antioxidants: Perpetual struggle behind ageing processes and related diseases
ROS, the most abundant biological oxidants, can subtract electrons from all the biomolecules, including DNA, proteins, carbohydrates and lipids9. The modifications induced by these species are potentially toxic for cell biology because the induced damage easily spreads in the neighbouring cells if not adequately counteracted by the antioxidant system. A clear example of the contagious cytotoxicity of ROS is represented by the lipid peroxidation of cell membranes. Abstraction of one single electron from the polyunsaturated fatty acids incorporated in phospholipids triggers a chain reaction that diffuses throughout the lipid bilayer and finally leads to an irreversible structural and functional alteration of the plasmatic membranes9.
ROS are constantly produced in different cell sites: first, by mitochondria during the indispensable process of oxidative phosphorylation and, to a lesser extent, by cytosol, membrane, peroxisome and endoplasmic reticulum, and hence, their impact on cell health is not always deleterious. Accordingly, it is now well established that the dichotomy ROS-oxidation causes biological damage and, if perpetrated, disease, only upon exceeding a critical threshold9,19,20. A number of endogens (e.g. inflammation and dysmetabolic syndrome) and environmental factors (e.g. pollution, smoking and nutrition) can accelerate the production of ROS, thereby increasing the risk of pathological conditions9. Moreover, the severity of the effects does not only depend on the quantity but also on the quality of the oxidant species involved. In fact, ROS family includes various members ranging from highly unstable/reactive-free radicals (i.e. molecules lacking one or more electrons) to poorly reactive non-radical molecules, such as hydrogen peroxide (H2O2)9,20. The production of this compound depends on the enzyme superoxide dismutase (SOD). It catalyzes the dismutation of peroxide radical (O2−), which in turn is produced by different enzymes located in the mitochondria, cytosol or membrane9. In an hypothetical classification based on the antioxidant power, SOD and other enzymes are able to neutralize H2O2, i.e. catalase (CAT) and glutathione peroxidase (Gpx) most likely occupy the first place21. These three enzymes have been indicated as the first line of defence, thanks to their unique ability to directly scavenge ROS in vivo20,21. There are other endogen enzymatic and non-enzymatic molecules which effectively contrast such reactive species as glutathione (natural substrate of Gpx), thioredoxin, coenzyme Q and uric acid (UA, the most abundant antioxidant in plasma). The core of the antioxidant protective system belongs to a complex network of enzymes, signalling molecules and transcription factors that result from the evolutionary adaptation of cells to the risk of living in an oxygen-rich environment9.
Dietary antioxidants, such as vitamins E (also named α-tocopherol) and C, β-carotene and lycopene, are also important in this context. Even though almost all these molecules show a detectable antioxidant capacity in experimental conditions, only α-tocopherol seems to be really effective in vivo21. This property depends on its relatively rapid kinetics of reaction, as well as on its high bodily concentration, especially in high ROS-vulnerable cell membranes21. Growing pre-clinical evidence has highlighted the beneficial effects of a highly variegate category of food antioxidants: the polyphenols21. Several members of this family, in particular, curcumin (turmeric) and resveratrol (grapes), have the peculiar capacity to stimulate a general xenobiotic response in target cells. In fact, these activate multiple defensive genes, such as those encoding SOD and CAT9,21.
A central paradigm in the redox field is that ROS/RNS become pathological players only when they overwhelm the opposing forces of the innate (endogen) and acquired (dietary) antioxidants. The rupture of this redox homeostasis is the prelude to OxS, a condition that severely affects cell integrity and biology and predisposes to several diseases9,19.
Oestrogen deficiency: Starting point of oxidative stress-induced bone-degenerative processes heading to post-menopausal osteoporosis
OxS is considered the common pathogenic factor for the ‘menopausal syndrome’ that includes several disturbances (e.g. vasomotor complains, cognitive impairment and urogenital dystrophy) and diseases, such as PO. The oestrogen decline associated with menopausal transition is believed to be the ‘spark’ that triggers OxS.
Experimental and epidemiological evidence
Experiments on OvX animals provide the most convincing evidence in support of the potential antioxidant impact of 17 β-oestradiol (E2), i.e. the predominant type of oestrogen. A vast body of studies shows that bilateral oophorectomy induces a redox imbalance characterized by increased levels of lipoperoxidation markers and decreased activity of antioxidant enzymes22,23. Notably, it has also been reported that oestrogen replacement suppresses OxS in OvX rats24, thereby suggesting that the administration of exogenous hormones to the animals can reverse their pro-oxidative profile.
Despite the consistent experimental evidence, the concept of oestrogen as an antioxidant is not unanimously accepted mostly because of the contrasting results emerging from human epidemiological studies. Post-menopausal women showed higher serum levels of lipid peroxidation markers and lower levels of low molecular weight or enzymatic antioxidants compared to reproductive age women6,25. In contrast, data from other reports failed to prove the occurrence of OxS after menopause and questioned the hypothesis of an inverse correlation between E2 levels and peripheral markers of oxidative damage26,27.
The inconsistency of epidemiologic data on menopause-OxS relationship is due, at least in part, to the methodological limitations as well as to the differences in study design and in composition of population sample. Regardless of these drawbacks, there is still large consensus around the antioxidant potential of oestrogens. However, at present, the mechanisms by which these hormones provide tissue protection against oxidative challenge and the exact biochemical and cytological basis of the relation between OxS and osteoporosis are still unclear. Illustration of the most common hypotheses at this regard are briefed below.
Oestrogens as antioxidants: Possible mechanisms of action
Hypothesis 1: Oestrogens directly inhibit the peroxidative processes: The interest in the antioxidant proprieties of oestrogens was sparked by Sack et al28, showing that intra-arterial infusion of E2 in the post-menopausal women induced a decrease in the peroxidative damage of low-density lipoprotein (LDL) and that these beneficial effects were reversed after treatment interruption. However, the physiological levels of E2, even in the reproductive age women, are much lower than the threshold at which oestrogens play direct antioxidant effect in vitro6,27,29,30. Due to this evident constraint, along with subsequent negative results of the epidemiological studies, Sack's original hypothesis has been almost definitely refused by the experts in the field31,32.
Hypothesis 2: Oestrogens upregulate the expression of antioxidant enzymes: Viña and colleagues31,32 were the first to definitely prove that oestrogens modulated the molecular signalling pathways involved in cellular redox homeostasis. Along with other researchers, they have demonstrated that E2 stimulates the expression of SOD and Gpx32. The interaction between E2 and mitochondrial receptors sharply decreases the rate of ROS formation and simultaneously increases the membrane integrity of these organelles33. Accordingly, oestrogen deprivation in OvX animals has been repeatedly demonstrated to be associated with a downregulation of these defensive enzymes and with an increase of oxidative challenge32.
Hypothesis 3: Oestrogens prevent the disruption of redox balance by suppressing pro-oxidant sources: E2 may prevent redox balance impairment indirectly, i.e. by modulating and harmonizing the regional distribution of fat in female body. The level of oxidative damage is closely related with the amount, type and localization of body adiposity.
Menopause transition is associated with the preferential accumulation of fat in the abdominal region with a parallel increase in the visceral/subcutaneous fat ratio34,35. In fact, oestrogen is able to regulate regional lipid accumulation mostly through the interaction with oestrogen receptor α (ERα) that is more expressed in subcutaneous than in visceral adipocytes35. These receptors play a crucial role in the activity of adipocytes as well as in sexual dimorphism in fat distribution. The E2:ERα interaction appears to preserve and promote the typical gynoid (gluteofemoral) distribution, which is widely believed to be associated with a healthy metabolic profile36. In contrast, the menopause-related accumulation of intra-abdominal fat leads to the onset of a chronic ‘low-grade inflammation’, which, in turn, contributes to the development of metabolic diseases, such as type 2 diabetes mellitus and cardiovascular disease37.
The adverse effects of inflammation are further exacerbated by the concomitant increase in ROS production9,21. One of the prominent effects of a persistent inflammatory state is the generation of a pro-oxidative environment due to the production of high fluxes of reactive species38. For example, the exposure to inflammatory cytokines, such as IL-6, stimulates the generation of reactive species by phagocytic cells, such as macrophages and neutrophils39. In turn, OxS enhances the release of pro-inflammatory mediators from immunocompetent cells, thereby triggering a toxic self-perpetuating vicious cycle recently named oxinflammation19,40. This detrimental synergy is a latent feature of several diseases40 and may account for the close interplay between central adiposity and serum levels of oxidative damage markers in post-menopause, reported by various research groups including ours6,41,42,43,44.
Implication of oxidative stress in post-menopausal osteoporosis pathogenesis: Experimental and epidemiological evidence
In vitro and animal studies
Evidence from animal studies supports the detrimental effect of OxS on bone health45,46,47,48,49. Increase in the intracellular ROS has been shown to result from oophorectomy in animal models23,47. It has been observed that ROS-enriched bone environment stimulates osteoclastogenesis in two ways: primarily, by potentiating the responsiveness of osteoclast precursors to RANKL, and secondarily, by inducing additional osteoclastogenic cytokines (i.e. IL-1, IL-6 and IL-7)5,6. Furthermore, the treatment with H2O2 did not enhance the expression of OPG, but rather RANKL in human stromal cells9. Moreover, the induction of superoxide production was shown to increase both in vivo and in vitro the number and activity of osteoclasts44.
Cumulating evidence suggests that OxS may also influence the cell cycle of osteoblasts. Two separate studies showed that ROS decreased the life span of these cells in OvX or aged mice45. Overall, both endogen and dietary antioxidants appeared to mitigate and delay bone loss in a number of animal studies. Consistently, low bone mass was detected in transgenic mice lacking cytoplasmic SOD, created by the deletion of the corresponding gene49. Separate series of studies showed that various forms of vitamin E prevented the reduction in trabecular number and bone volume in OvX rats23,47. Deng et al47 reported that mice supplemented with gamma-trienol were significantly protected from ovariectomy-induced bone loss. Growing pre-clinical evidence also suggests that commonly consumed antioxidant-rich fruits have a pronounced effect on bone through the promotion of bone formation and the prevention of bone resorption48.
Human studies
The aforementioned compelling experimental evidence prompted a number of epidemiological studies on the topic. Multiple reports50,51,52,53,54,55,56,57,58(including two from ourselves)50,51 highlighted an inverse and positive association between peripheral markers of oxidative damage and antioxidant status, respectively, and bone mass density (BMD) at femoral neck, total hip or lumbar spine. Such findings led us to further explore the involvement of redox processes on bone loss, by focussing our attention on peripheral markers of bone remodelling in a cohort of post-menopausal women. We showed that higher serum concentration of OxS marker was correlated with an increased bone resorption rate51. In a more recent association study, our research group has provided further evidence in support of implication of OxS in the derangement of bone homeostasis58. We measured the serum levels of RANKL, OPG and 8-hydroxy-2-deoxyguanosine, a widely used marker of DNA oxidation, in a sample of normal, osteopenic and osteoporotic post-menopausal women (n=124), and found a positive and independent correlation between the OxS marker and RANKL/OPG ratio within the osteopenic subsample58.
Besides the evidence linking OxS to the pathogenesis of PO, some reports also addressed its relationship with the possible complications of such disease. In particular, a plasmatic indicator of OxS arose as a significant predictor for hip fracture in a prospective investigation on 996 women, followed up for up to 23 years54.
Among the several antioxidants with a hypothesized association with osteoporosis, UA was the one showing the most consistent and convincing results. A retrospective study on 615 Japanese women reported a positive and independent correlation between the levels of UA and lumbar spine BMD55. An Australian study involving 356 peri- and post-menopausal women sought to confirm the positive role of UA on bone turnover and assessed whether UA relates to changes in BMD longitudinally; multivariate analysis showed an association between UA levels and longitudinal change in BMD at several skeletal features56. The putative protecting role of UA on bone metabolism has been confirmed in a large study involving 7502 women57. Higher serum UA was associated with higher bone mass, lower bone turnover and lower prevalence of vertebral fracture. In vitro experiments showed that UA suppressed osteoclastogenesis in a dose-dependent manner and reduced the production of ROS in osteoclast precursors57.
Contrasting results are also worthy to be highlighted. Multiple studies failed to find a significant increase of OxS in osteoporotic as compared to healthy post-menopausal women51,52,58,59,60. In our view, these negative findings do not rule out the role of reactive species in PO development but define the limits of their involvement. As this bone disease is multifactorial, ROS can be reasonably considered just one of the multiple components of its complex and multifaceted pathogenic process. Moreover, detectable changes in the blood markers are expected to occur in more severe pathologic conditions characterized by intense tissue damage and mitochondrial impairment or in chronic metabolic disorders, such as diabetes and vascular chronic inflammation19. An additional point to consider is the intrinsic limitation of all the epidemiological studies dealing with OxS. The research has not yet validated a gold standard biomarker for the quantification of redox imbalance in biological fluids, and many of the indicators measured are scarcely specific and sensitive9,38. Finally, most of the reports have a cross-sectional design, and hence, they take a ‘static’ snapshot of a population at a specific point of time. In the specific case of the studies in the framework of interest, the picture essentially consists of the comparison between women affected by PO and healthy controls. Such an approach raises two main issues. First, it hinders any firm conclusion about the causal role of OxS in the disease occurrence. Second, it intrinsically misevaluates the influence of covariates (body fat parameters, lifestyle and ethnicity, concomitant diseases, therapies, age differences, alcohol abuse, physical activity, diet and smoking) on the relationship between the two variables. Longitudinal design is thus the most proper approach to establish the causality of the observed statistical associations, but these types of investigation are problematic mainly because of the high inter-individual variability in the duration and age of onset of menopause.
Anti-osteoporotic therapies targeting oxidative stress: Current state of the art
The questions that inevitably arise from the reported evidence are: how can we translate the research findings and acquired knowledge into clinical practice? And, in line with the aim of this review, is there any evidence that targeting OxS could be beneficial in terms of prevention and treatment of PO?
At present, there are various effective drug treatments for the management of PO and for the prevention of subsequent FFs61. Pharmacologic intervention is appropriate in case of previous fractures or osteoporosis at increased risk of fracture based on a fracture risk assessment tool, i.e. WHO FRAX (http:www.shef.ac.uk/FRAX)62. Whereas bisphosphonates, denosumab and teriparatide are used for the treatment of patients with a full-blown osteoporosis, selective oestrogen receptor modulators may be considered as the first-line treatment in preventing bone loss in women within 10 years of the menopause, side by side with the menopausal hormonal therapy (MHT) and the most recent combination of bazedoxifene and oestrogen63.
Convergent data from research studies and clinical trials indicate that MHT normalizes turnover and preserves BMD at all skeletal sites, thereby decreasing vertebral and non-vertebral fractures significantly64. Despite the initial concern with the safety of such treatment, recent studies reported encouraging results in this field. In particular, a 10 yr randomized trial completed in 200865 showed a significantly lower risk of mortality, heart failure and myocardial infarction without any apparent increase in risk of cancer in carefully selected women younger than 60 yr old receiving MHT early after menopause61,64. Epidemiologic evidence is consistent with the data from animal studies on OvX rats receiving oestrogen replacement therapy (ERT). López-Grueso et al66 showed that ERT could prevent redox imbalance and counteract the typical post-surgical dysmetabolic profile only when administered early after oophorectomy. Based on these results, the authors postulated that ERT could upregulate the antioxidant enzymes and hence promote longevity. Unfortunately, to the best of our knowledge, there is no longitudinal study supporting this hypothesis in humans.
Along with pharmacological interventions, a reduction in the risk of osteoporosis should be achieved through lifestyle interventions, including exercise, adequate intake of calcium and proteins and avoidance of risk factors, such as sedentary lifestyle, smoking and alcohol abuse67. According to wide-acknowledged guidelines, an adequate intake of calcium and vitamin D is mandatory to achieve peak bone mass and prevent post-menopausal bone loss68. Nutrition can also be the source of several antioxidants, with potential beneficial effects on bone health. However, the reliability of the studies on the possible benefit of dietary antioxidants on bone homeostasis, fracture risk and markers of bone turnover is hampered by several drawbacks. First, the long-term effects of phytochemicals have not been assessed in randomized controlled clinical trials on post-menopausal women. Second, there are only a few interventional studies, while observational research is highly heterogeneous in terms of study design and outcome measures. Finally, the studies published so far may produce biased results as these have mainly relied on dietary and food questionnaires and have not objectively assessed body stores through blood or urine analyses. Taking into account these multiple caveats, it is worth a brief overview of the reported findings.
The preventive effect of vitamin E on bone erosion observed in animal models prompted a number of large epidemiological surveys on the possible effect of its intake from diet and/or supplements46,69. In a study on 951 current-smoking post-menopausal women, high intake of vitamin E was associated with a significantly lower risk of hip fracture70. Accordingly, the supplementation with vitamin E was related to a reduced rate of fracture of any type in a large-scale epidemiological study (n=61,433 peri-menopausal women)71. In contrast with the aforementioned results, a longitudinal study conducted by MacDonald et al72 highlighted a direct association between increased vitamin E intake and greater femoral neck BMD loss. Finally, Wolf et al73 evaluated the effect of daily vitamin E intake (and serum level of the vitamin) in 11,068 elderly women and failed to show any significant association between vitamin E and BMD at any site in multivariate analysis. Besides vitamin E, many other studies evaluated the effect of other antioxidants on bone health. A large population-based case-control study showed that the intake of β-carotene and selenium, but not vitamin C, was related to a decreased rate of hip fracture48. Promising results also arose from the handful of small epidemiological studies on lycopene, one of the isomers of β-carotene, that is, especially present in tomato fruit. In particular, two studies indicated that daily consumption of this phytochemical may suppress bone resorption as suggested by the apparent inverse relation between lycopene intake and serum levels of resorption markers74,75. Unfortunately, the encouraging pre-clinical findings obtained with other bioactive compounds, especially present in fruits, such as flavonoids, resveratrol and pectin, have not been adequately replicated in humans yet69. On the contrary, robust epidemiological evidence gathered in the last two decades yields a wide consensus around the positive impact of dietary intake of vegetables and, in particular, fruits on bone health69,76.
Summary and novel roads ahead
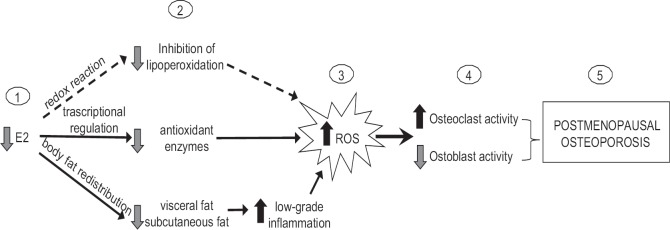
The body of evidence supporting the pathogenic role of OxS in PO is mostly from in vitro or animal studies. Ageing and oestrogen deficiency represent the noxious combination that ‘breaches’ the first line of defence (i.e. antioxidant enzymes) against the potentially cytotoxic reactive species. The erosive activity of ROS on the bone has been well characterized. These chemically unstable molecules can affect bone health at various levels, influencing both indirectly (by stimulating the release of pro-absorptive cytokines) and directly the life cycle of osteoblasts/osteoclasts (Figure).
Fig. 1.
Cause-effect relationship between oestrogen decline, oxidative stress and post-menopausal osteoporosis. The menopause-associated decline in oestrogens (E2) (1) results in a decrease of systemic and local (bone) protection against reactive oxygen species attack (2). This effect is due to the ability of 17β-oestradiol to act as a direct antioxidant and, most likely, to simultaneously upregulate the expression of the antioxidant enzymes and to contrast the accumulation of proinflammatory (and thus pro-oxidant) visceral fat. The uncontrolled increase of reactive oxygen species (ROS) leads to oxidative stress (3) which, in turn, alters the balance between bone formation and resorption (4), thereby increasing the latter activity and contributing to the onset of post-menopausal osteoporosis (5).
This putative connection between OxS and the clinical manifestations of the menopausal syndrome has sparked a constellation of observational studies exploring the beneficial effects of dietary antioxidants on the post-menopausal women. However, it is impossible to reach a firm conclusion as to the usefulness of phytochemicals in this context, due to the numerous methodological and design limitations as well as to the lack of randomized clinical trials. Nonetheless, taking into account also the safety of these natural compounds, we firmly believe, along with many others, that this could be the right road ahead.
The future interventional studies with antioxidants should address some criteria that have not been adequately considered so far. First, the modification of the usual diet (with increased intake of fruits and vegetables) or the use of antioxidant supplements might be suggested to accompany MHT to preserve correct redox homeostasis. It is now well accepted that no antioxidant is able to contrast oxidative challenge by itself. On the contrary, this task can be achieved only in concert with other antioxidants. A typical example in this field is α-tocopherol; after reacting with ROS, it becomes itself a free radical, which can be neutralized by the finely orchestrated synergic action of a network of endogen antioxidants, including glutathione and reduced glutathione77, and vitamin C. This consideration directly leads to the second benchmark to be addressed in the future trials: the interventions based on the administration of a single antioxidant are doomed to fail in the absence of an exhaustive picture of the individual redox profile. Such a tailored approach can be designed by measuring the peripheral levels of oxidative damage both overall and of the single antioxidants. Third and last points to take into account are that all types of antioxidant act as preventive, but not repairing agents. Translating these assumptions into practice: if the aim of a therapy is to effectively reduce the ‘weight’ of OxS contribution to PO development, then it must be prescribed to patients with normal or moderately low BMD, but not to patients with full-blown osteoporosis. The interventions aiming to reduce OxS may be accompanied with classical prevention strategies such as lifestyle modifications, avoidance of recognized risk factors and adequate intake of vitamin D, calcium and proteins.
Footnotes
Financial support & sponsorship: This work was supported by ‘Local Research Project’ grant from University of Ferrara, Italy
Conflicts of Interest: None.
References
- 1.Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, Gold DT, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2:16069. doi: 10.1038/nrdp.2016.69. [DOI] [PubMed] [Google Scholar]
- 2.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jane FM, Davis SR. A practitioner's toolkit for managing the menopause. Climacteric. 2014;17:564–79. doi: 10.3109/13697137.2014.929651. [DOI] [PubMed] [Google Scholar]
- 4.Mithal A, Bansal B, Kyer CS, Ebeling P. The Asia-Pacific Regional Audit-Epidemiology, costs, and burden of osteoporosis in India 2013: A report of International Osteoporosis Foundation. Indian J Endocrinol Metab. 2014;18:449–54. doi: 10.4103/2230-8210.137485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pansini F, Mollica G, Bergamini CM. Management of the menopausal disturbances and oxidative stress. Curr Pharm Des. 2005;11:2063–73. doi: 10.2174/1381612054065819. [DOI] [PubMed] [Google Scholar]
- 6.Cervellati C, Bergamini CM. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clin Chem Lab Med. 2016;54:739–53. doi: 10.1515/cclm-2015-0807. [DOI] [PubMed] [Google Scholar]
- 7.Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int. 2010;87:226–35. doi: 10.1007/s00223-010-9393-9. [DOI] [PubMed] [Google Scholar]
- 8.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–3. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies JMS, Cillard J, Friguet B, Cadenas E, Cadet J, Cayce R, et al. The Oxygen Paradox, the French Paradox, and age-related diseases. Geroscience. 2017;39:499–550. doi: 10.1007/s11357-017-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 11.Pacifici R. The immune system and bone. Arch Biochem Biophys. 2010;503:41–53. doi: 10.1016/j.abb.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23:576–81. doi: 10.1016/j.tem.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faienza MF, Ventura A, Marzano F, Cavallo L. Postmenopausal osteoporosis: The role of immune system cells. Clin Dev Immunol 2013. 2013 doi: 10.1155/2013/575936. 575936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest. 2006;116:1186–94. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–46. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofbauer LC, Kühne CA, Viereck V. The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact. 2004;4:268–75. [PubMed] [Google Scholar]
- 17.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL, et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–30. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: A key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Cervellati C, Wood PL, Romani A, Valacchi G, Squerzanti M, Sanz JM, et al. Oxidative challenge in Alzheimer's disease: State of knowledge and future needs. J Investig Med. 2016;64:21–32. doi: 10.1136/jim-2015-000017. [DOI] [PubMed] [Google Scholar]
- 20.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: The golden mean of healthy living. Redox Biol. 2016;8:205–15. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman HJ, Davies KJA, Ursini F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo . Free Radic Biol Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ha BJ. Oxidative stress in ovariectomy menopause and role of chondroitin sulfate. Arch Pharm Res. 2004;27:867–72. doi: 10.1007/BF02980181. [DOI] [PubMed] [Google Scholar]
- 23.Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, et al. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360:81–6. doi: 10.1016/j.cccn.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Hao F, Gu Y, Tan X, Deng Y, Wu ZT, Xu MJ, et al. Estrogen replacement reduces oxidative stress in the rostral ventrolateral medulla of ovariectomized rats. Oxid Med Cell Longev 2016. 2016 doi: 10.1155/2016/2158971. 2158971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolesnikova L, Semenova N, Madaeva I, Suturina L, Solodova E, Grebenkina L, et al. Antioxidant status in peri- and postmenopausal women. Maturitas. 2015;81:83–7. doi: 10.1016/j.maturitas.2015.02.264. [DOI] [PubMed] [Google Scholar]
- 26.Sowers MR, Randolph J, Jr, Jannausch M, Lasley B, Jackson E, McConnell D, et al. Levels of sex steroid and cardiovascular disease measures in premenopausal and hormone-treated women at midlife: Implications for the “timing hypothesis”. Arch Intern Med. 2008;168:2146–53. doi: 10.1001/archinte.168.19.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowers M, McConnell D, Jannausch ML, Randolph JF, Brook R, Gold EB, et al. Oestrogen metabolites in relation to isoprostanes as a measure of oxidative stress. Clin Endocrinol (Oxf) 2008;68:806–13. doi: 10.1111/j.1365-2265.2007.03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sack MN, Rader DJ, Cannon RO., 3rd Oestrogen and inhibition of oxidation of low-density lipoproteins in postmenopausal women. Lancet. 1994;343:269–70. doi: 10.1016/s0140-6736(94)91117-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, Bonet B, Gillenwater H, Knopp RH. Opposing effects of estrogen and progestins on LDL oxidation and vascular wall cytotoxicity: Implications for atherogenesis. Proc Soc Exp Biol Med. 1999;222:214–21. doi: 10.1046/j.1525-1373.1999.d01-138.x. [DOI] [PubMed] [Google Scholar]
- 30.Cervellati C, Pansini FS, Bonaccorsi G, Bergamini CM, Patella A, Casali F, et al. 17β-estradiol levels and oxidative balance in a population of pre-, peri-, and post-menopausal women. Gynecol Endocrinol. 2011;27:1028–32. doi: 10.3109/09513590.2011.579653. [DOI] [PubMed] [Google Scholar]
- 31.Viña J, Gambini J, García-García FJ, Rodriguez-Mañas L, Borrás C. Role of oestrogens on oxidative stress and inflammation in ageing. Horm Mol Biol Clin Investig. 2013;16:65–72. doi: 10.1515/hmbci-2013-0039. [DOI] [PubMed] [Google Scholar]
- 32.Borrás C, Sastre J, García-Sala D, Lloret A, Pallardó FV, Viña J, et al. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–52. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 33.Borrás C, Gambini J, López-Grueso R, Pallardó FV, Viña J. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim Biophys Acta. 2010;1802:205–11. doi: 10.1016/j.bbadis.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Cervellati C, Pansini FS, Bonaccorsi G, Pascale G, Bagni B, Castaldini C, et al. Body mass index is a major determinant of abdominal fat accumulation in pre-, peri- and post-menopausal women. Gynecol Endocrinol. 2009;25:413–7. doi: 10.1080/09513590902770123. [DOI] [PubMed] [Google Scholar]
- 35.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–4. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab. 2004;89:1869–78. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- 37.Cremonini E, Bonaccorsi G, Bergamini CM, Castaldini C, Ferrazzini S, Capatti A, et al. Metabolic transitions at menopause: In post-menopausal women the increase in serum uric acid correlates with abdominal adiposity as assessed by DXA. Maturitas. 2013;75:62–6. doi: 10.1016/j.maturitas.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10:1611–26. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- 39.Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, et al. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res. 2004;94:534–41. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 40.Valacchi G, Pecorelli A, Cervellati C, Hayek J. 4-hydroxynonenal protein adducts: Key mediator in Rett syndrome oxinflammation. Free Radic Biol Med. 2017;111:270–80. doi: 10.1016/j.freeradbiomed.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 41.Pansini F, Cervellati C, Guariento A, Stacchini MA, Castaldini C, Bernardi A, et al. Oxidative stress, body fat composition, and endocrine status in pre- and postmenopausal women. Menopause. 2008;15:112–8. doi: 10.1097/gme.0b013e318068b285. [DOI] [PubMed] [Google Scholar]
- 42.Uppoor RB, Rajesh A, Srinivasan MP, Unnikrishnan B, Holla R. Oxidative stress in obese postmenopausal women: An additive burden for atherosclerosis. J Clin Diagn Res. 2015;9:OC03–5. doi: 10.7860/JCDR/2015/16467.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mittal PC, Kant R. Correlation of increased oxidative stress to body weight in disease-free post menopausal women. Clin Biochem. 2009;42:1007–11. doi: 10.1016/j.clinbiochem.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR, et al. Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest. 1990;85:632–9. doi: 10.1172/JCI114485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–97. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guralp O. Effects of vitamin E on bone remodeling in perimenopausal women: Mini review. Maturitas. 2014;79:476–80. doi: 10.1016/j.maturitas.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Deng L, Ding Y, Peng Y, Wu Y, Fan J, Li W, et al. γ-Tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone. 2014;67:200–7. doi: 10.1016/j.bone.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD, et al. Antioxidant intake and risk of osteoporotic hip fracture in utah: An effect modified by smoking status. Am J Epidemiol. 2006;163:9–17. doi: 10.1093/aje/kwj005. [DOI] [PubMed] [Google Scholar]
- 49.Smietana MJ, Arruda EM, Faulkner JA, Brooks SV, Larkin LM. Reactive oxygen species on bone mineral density and mechanics in Cu, Zn superoxide dismutase (Sod1) knockout mice. Biochem Biophys Res Commun. 2010;403:149–53. doi: 10.1016/j.bbrc.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cervellati C, Bonaccorsi G, Cremonini E, Bergamini CM, Patella A, Castaldini C, et al. Bone mass density selectively correlates with serum markers of oxidative damage in post-menopausal women. Clin Chem Lab Med. 2013;51:333–8. doi: 10.1515/cclm-2012-0095. [DOI] [PubMed] [Google Scholar]
- 51.Cervellati C, Bonaccorsi G, Cremonini E, Romani A, Fila E, Castaldini MC, et al. Oxidative stress and bone resorption interplay as a possible trigger for postmenopausal osteoporosis. Biomed Res Int 2014. 2014 doi: 10.1155/2014/569563. 569563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sánchez-Rodríguez MA, Ruiz-Ramos M, Correa-Muñoz E, Mendoza-Núñez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord. 2007;8:124. doi: 10.1186/1471-2474-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sendur OF, Turan Y, Tastaban E, Serter M. Antioxidant status in patients with osteoporosis: A controlled study. Joint Bone Spine. 2009;76:514–8. doi: 10.1016/j.jbspin.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Yang S, Feskanich D, Willett WC, Eliassen AH, Wu T. Association between global biomarkers of oxidative stress and hip fracture in postmenopausal women: A prospective study. J Bone Miner Res. 2014;29:2577–83. doi: 10.1002/jbmr.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishii S, Miyao M, Mizuno Y, Tanaka-Ishikawa M, Akishita M, Ouchi Y, et al. Association between serum uric acid and lumbar spine bone mineral density in peri- and postmenopausal Japanese women. Osteoporos Int. 2014;25:1099–105. doi: 10.1007/s00198-013-2571-7. [DOI] [PubMed] [Google Scholar]
- 56.Makovey J, Macara M, Chen JS, Hayward CS, March L, Seibel MJ, et al. Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: A longitudinal study. Bone. 2013;52:400–6. doi: 10.1016/j.bone.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 57.Ahn SH, Lee SH, Kim BJ, Lim KH, Bae SJ, Kim EH, et al. Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos Int. 2013;24:2961–70. doi: 10.1007/s00198-013-2377-7. [DOI] [PubMed] [Google Scholar]
- 58.Cervellati C, Romani A, Cremonini E, Bergamini CM, Fila E, Squerzanti M, et al. Higher urinary levels of 8-hydroxy-2’-deoxyguanosine are associated with a worse RANKL/OPG ratio in postmenopausal women with osteopenia. Oxid Med Cell Longev 2016. 2016 doi: 10.1155/2016/6038798. 6038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–7. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 60.Cervellati C, Bonaccorsi G, Bergamini CM, Fila E, Greco P, Valacchi G, et al. Association between circulatory levels of adipokines and bone mineral density in postmenopausal women. Menopause. 2016;23:984–92. doi: 10.1097/GME.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 61.Adler RA. Osteoporosis treatment: Complexities and challenges. J Endocrinol Invest. 2016;39:719–20. doi: 10.1007/s40618-016-0437-5. [DOI] [PubMed] [Google Scholar]
- 62.Bonaccorsi G, Fila E, Cervellati C, Romani A, Giganti M, Rossini M, et al. Assessment of fracture risk in A population of postmenopausal Italian women: A comparison of two different tools. Calcif Tissue Int. 2015;97:50–7. doi: 10.1007/s00223-015-0009-2. [DOI] [PubMed] [Google Scholar]
- 63.Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: A combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548–61. doi: 10.1002/phar.1749. [DOI] [PubMed] [Google Scholar]
- 64.de Villiers TJ, Gass MLS, Haines CJ, Hall JE, Lobo RA, Pierroz DD, et al. Global consensus statement on menopausal hormone therapy. Climacteric. 2013;16:203–4. doi: 10.3109/13697137.2013.771520. [DOI] [PubMed] [Google Scholar]
- 65.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 66.López-Grueso R, Gambini J, Abdelaziz KM, Monleón D, Díaz A, El Alami M, et al. Early, but not late onset estrogen replacement therapy prevents oxidative stress and metabolic alterations caused by ovariectomy. Antioxid Redox Signal. 2014;20:236–46. doi: 10.1089/ars.2012.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–70. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dawson-Hughes B, Mithal A, Bonjour JP, Boonen S, Burckhardt P, Fuleihan GEH, et al. IOF position statement: Vitamin D recommendations for older adults. Osteoporos Int. 2010;21:1151–4. doi: 10.1007/s00198-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 69.Shen CL, von Bergen V, Chyu MC, Jenkins MR, Mo H, Chen CH, et al. Fruits and dietary phytochemicals in bone protection. Nutr Res. 2012;32:897–910. doi: 10.1016/j.nutres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 70.Melhus H, Michaëlsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14:129–35. doi: 10.1359/jbmr.1999.14.1.129. [DOI] [PubMed] [Google Scholar]
- 71.Michaëlsson K, Wolk A, Byberg L, črnlöv J, Melhus H. Intake and serum concentrations of α-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am J Clin Nutr. 2014;99:107–14. doi: 10.3945/ajcn.113.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacDonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: Evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. 2004;79:155–65. doi: 10.1093/ajcn/79.1.155. [DOI] [PubMed] [Google Scholar]
- 73.Wolf RL, Cauley JA, Pettinger M, Jackson R, Lacroix A, Leboff MS, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: Results from the Women's Health Initiative. Am J Clin Nutr. 2005;82:581–8. doi: 10.1093/ajcn.82.3.581. [DOI] [PubMed] [Google Scholar]
- 74.Mackinnon ES, Rao AV, Rao LG. Dietary restriction of lycopene for a period of one month resulted in significantly increased biomarkers of oxidative stress and bone resorption in postmenopausal women. J Nutr Health Aging. 2011;15:133–8. doi: 10.1007/s12603-011-0026-4. [DOI] [PubMed] [Google Scholar]
- 75.Rao LG, Mackinnon ES, Josse RG, Murray TM, Strauss A, Rao AV, et al. Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos Int. 2007;18:109–15. doi: 10.1007/s00198-006-0205-z. [DOI] [PubMed] [Google Scholar]
- 76.Zalloua PA, Hsu YH, Terwedow H, Zang T, Wu D, Tang G, et al. Impact of seafood and fruit consumption on bone mineral density. Maturitas. 2007;56:1–1. doi: 10.1016/j.maturitas.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Bando M, Inoue T, Oka M, Nakamura K, Kawai K, Obazawa H, et al. Isolation of ascorbate free radical reductase from rabbit lens soluble fraction. Exp Eye Res. 2004;79:869–73. doi: 10.1016/j.exer.2004.08.011. [DOI] [PubMed] [Google Scholar]