Abstract
This study evaluates the protective effect of corilagin against Parkinsonismin Japanese encephalitis virus (JEV) induced Parkinson’s disease. The JaGAr-01 strain of virus was used to induce JE. The virus was injected into the rats (13 days age) at the midpoint between the two ears. Adult rats, 12 week after the inoculation of virus, were used for the further study. Corilagin (20 mg/kg) and levodopa with dopa decarboxylase inhibitor (LEV, 10 mg/kg) were administered intraperitoneally for the duration of one week. Bradykinesia and the levels of dopamine in the brain were estimated at the end of protocol. There was a significant decrease inthe motor function in the corilagin, LEV and LEV + corilagin treated groupscompared to the negative control group. However treatment with corilagin, LEV and LEV + corilagin significantly increases the level of dopamine in the brain compared to the negative control group. This study concludes that corilagin ameliorates the Parkinsonismin JEV induced Parkinsonism. Moreover it shows a synergistic effect when treated with LEV. Data presented in the investigation supports that corilagin can be used clinically.
Keywords: Japanese encephalitis virus, Parkinsonism, Corilagin, Bradykinesia, Levodopa
Introduction
Japanese encephalitis (JE) commonly occursin children below the age of 6 years and the major factors that contribute to JE are neuronal plasticity, malnutrition of central nervous system and immunological factors [1]. Patients suffering from JEV infection show parkinsonism syndrome postencephalitis. The literature reveals that from the 200 acute cases of JE, 75% of patients show a mask like face symptom, more than 40% complain about muscular rigidity and 90% of people suffer from tremors [2].A report reveals that JE results in the development of postencephalitic Parkinsonism in Japanese encephalitis virus infected Fisher rats [3]. In the substantia nigra, the quantity of hydroxylase-positive cells is reducedin the rats suffering from JE [4]. JEV infection causes neuropathology such as the formation of plaques, shrinkage of neurons, neuronal degeneration, perivascular cuffing and cellular infiltration after 10 days of inoculation [5]. Moreover bradykinesia was also observed in JEV infected rats and treatment with levodopa improves the behavior of rats [6].
In the recent years alternative medicine such as herbs shows great potential against chronic disorders. Corilagin is an ellagitanninisolated from the Caesalpinia coriaria herb [7]. Previously reported studies suggested that corilagin is a potent antioxidant, hepatoprotective, anti-inflammatory, analgesic, antihypertensive, antitumor and has carbonic anhydrase inhibitor properties [8,9,10,11]. It is reported that corilagin possesses analgesic activity by altering the glutaminergic system and anti-inflammatory activity by decreasing the production of proinflammatory cytokines and mediators such as tumor necrosis factor- α (TNF-α), IL-1β, IL-6, NO (iNOS) and cycloxygenase-2 (COX-2) on both the protein and gene level by blocking NF-κB nuclear translocation [12, 13]. There are several drugs available for the management of Parkinsonism but neuroprotection is not achievable. However, corilagin is reported to posses neuroprotective effects by reducing inflammatory mediators and on the basis of its antioxidant activity. In addition, it alters the glutaminergic system that is responsible for the tremors.Thus, the present investigation evaluates the effect of corilagin againstParkinsonism.
Material and methods
Animals
Albino Wistar rats (13 days old) were procured from Shanghai medical college, China. All the animals were housed under controlled conditions as specified by the guidelines. All experimental procedures were approved by the institutional animal ethical committee ofHubei Provincial Hospital of Traditional Chinese Medicine (IAEC/HPHTCM/2016/08).
Induction of JE
The JaGAr-01 strain of virus was used to induce JE. The brain of the infected mouse was homogenated and the supernatant separated and diluted with Hemaccel(20%) in Eagle’s minimum essential medium. Later a specially designed two-step-thin 27-gauge needle was used to inoculate the virus intracerebrally. The virus was injected in the rats (13 days of age) at the midpoint between the two ears. Adult rats, 12 weeks after the inoculation of virus, were used for further study. However control group of rat receives only diluents. Thereafter JEV infected rats were further separated into four groups: negative control, corilagin (20 mg/kg, ip), levodopa with dopa decarboxylase inhibitor (10 mg/kg, ip) and corilagin+levodopa with dopa decarboxylase inhibitor for the duration of seven days.
Estimation of motor function
Bradykinesia was evaluated by estimating the motor activity through a pole test in rats. A pole test was done as per a previously reported study. In the pole test, the time taken by the rats of both control and JEV infected groups to fall down from the rough surfaced pole to the floor was estimated before and after the drug treatment.
Estimation of neurochemical levels
All the animals were sacrificed at the end of the protocol (i.e. immediately after the last dose of corilagin) and the brain was isolated from each animal. The isolated brain of each rat was dissected in to several parts as per a previously reported study. Each part of the brain was stored at – 80 °C in liquid nitrogen. Ethylenediaminetetraacetic acid (0.7 mM) and perchloric acid (0.1 M) was used to homogenizethe frozen brain tissues. High performance liquid chromatography (HPLC) was used to separate the catecholamines after purifying it with aluminum oxide. An electrochemical detector was used in the HPLC for the estimation of the catecholamine levels. We attached an ECD to the HPLC apparatus to make a system based on the principle of amperometry. Protein levels were determined as per a previously reported method, using bovine serum albumin as the standard.
Statistical Analysis
Statistical analysis and results are reported in the form of mean ± SD. One way analysis of variance (ANOVA) was performed for the comparison of results and P< 0.05 was considered a significant value. Graph Pad Prism version 5.0 for windows (San Diego, CA, USA) was used to analyze the results.
Result
Effect of corilagin on motor function
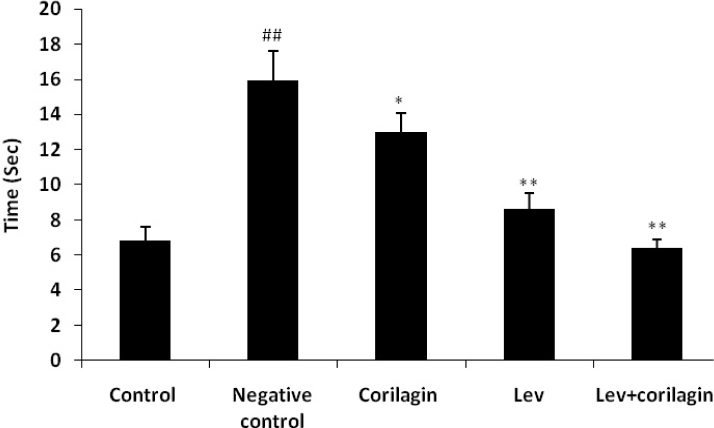
The effect of corilagin on motor function in JEV induced Parkinsonismis shown in Fig. 1. It was observed that JEV induced Parkinsonism results in an increase in the time tofall from the rod than control group. There was significant decrease in the time to fall down from the rod in corilagin, LEV and LEV+ corilagin treated group compared to thenegative control group. This data reveals that corilagin alone and in combination with LEV restores the motor function in JEV induced Parkinsonism rats.
Fig. 1.
Effect of corilagin on motor function in Japanese encephalitis virus induced Parkinsonism. Mean±SD (n=6); ##p<0.01 compared to the control group; *p<0.05; **p<0.01 compared to the negative control group
Effect of corilagin on the level of monoamines
The effect of corilagin on the concentration of dopamine in various part of the brain such as cerebral cortex, midbrain and hypothalamus isshown in Table 2. There was a significant decrease in the levels of dopamine in the hypothalamus and cerebral cortex of rat brainscompared to thenegative control group. However levels of dopamine was found to be attenuatedin corilagin, LEV and LEV+corilagin treated groups.
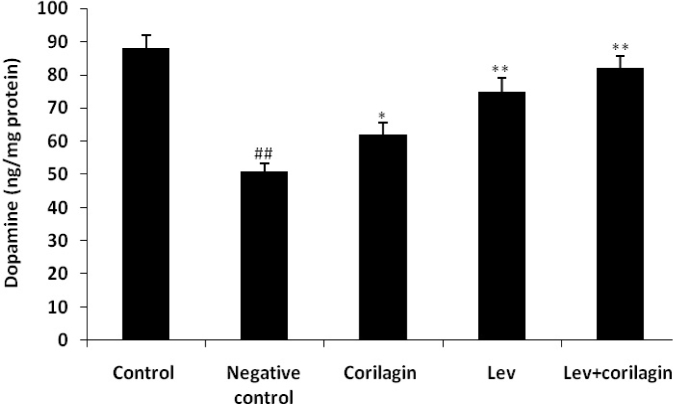
In addition, the effect of corilagin on the concentration of dopamine in the brain striatum is shown in Fig. 2. It was observed that treatment with corilagin and LEV alone and in combination significantly enhances the level of dopamine in the substantia nigra compared to thenegative control group. These result suggest that treatment with LEV+corilagin shows synergistic effects in the management of JEV induced Parkinsonism.
Fig. 2.
Effect of corilagin on the concentration of dopamine in the brain striatum. Mean±SD (n=6); ##p<0.01 compared to the control group; *p<0.05; **p<0.01 compared to the negative control group
Table 1.
Effect of corilagin on the concentration of dopamine in various parts of the brain
| Sr. No. | Group | Concentration of Dopamine (ng/mg protein) | ||
|---|---|---|---|---|
| Cerebral cortex | Midbrain | Hypothalamus | ||
| 1 | Control | 6.38±1.26 | 2.62±0.43 | 4.72±0.84 |
| 2 | Negative control | 2.17±0.83## | 3.45±0.53 | 2.94±0.32## |
| 3 | Corilgin | 3.24±0.62∗ | 3.29±0.42 | 3.67±0.72∗∗ |
| 4 | Lev | 4.16±0.92∗∗ | 3.11±0.59 | 2.96±0.53## |
| 5 | Lev+corilagin | 5.42±1.12∗∗ | 3.08±0.61 | 3.89±0.63∗∗ |
Mean±SD (n=6);
p<0.01 compared to control group
p<0.05
p<0.01 compared to negative control group
Discussion
A study reveals that Japanese encephalitis results in development of lesions in the substantia nigra, causingpost encephalitis Parkinsonism, and JEV infection causes neuropathology of the brain, confirmingthe parkinsonism in arat model too [14]. There are several factors that contributetothe pathogenesis of Parkinsonismsuch as infections andenvironmental and genetic factors [15]. Parkinsonisminduced by viralinfection is well provenin the literature [16]. Hence, our study usedthe JEV infection induced Parkinsonismin a rat model and evaluated the protective effect of corilagin by estimating the motor function and level of dopamine in the brain.
The literature suggested that the marked bradykinesia in JEV infected rats could be treatedwith LDOPA to attenuate these behavioral changes [17]. Our study reveals that treatment with corilagin significantly attenuates the motor dysfunction in rats with Parkinsonismand a synergistic effect was found in the rats treated with LEV+ corilagin.
Alterations in the levels of dopamine in brain is due to degeneration of dopaminergic neurons, developinginto Parkinsonism. The literature reveals that JEV infection leads to the developmentof postencephalitis Parkinsonism [3]. Moreover, alterations of motor function in Parkinsonism occurs due to a decrease in the level of dopamine in different areas of the brain. A reported study on JEV infected rats also reveals that the level of dopamine decreases in the hypothalamus and cerebral cortex of the brain [18]. Corilagin is reported to have a strong antioxidant and anti-inflammatory property on the basis of which it posseses neuroprotective effects and thus it could attenuate the parkinsonism by enhancing the level of dopamine in the brain. However, resultsfromthis study has shown that treatment with corilagin significantly improves the level of dopamine in the brain of rats compared to thenegative control group and also shows synergistic effects when treated with LEV.The present study has a limitation that this work needs to further study on the molecular and genetic levels for Abetter understanding of the mechanism of action of coraligin.
Conclusion
This study concludes that corilagin ameliorates the Parkinsonismin JEV induced Parkinsonism. Moreover corilaginshows a synergistic effect when treated with LEV. The data presented in the investigation supports that corilagin can be used clinically.
References
- [1].Kalita J., Misra U.K., Pandey S., Dhole T.N.. A comparison of clinical and radiological findings in adults and children with Japanese encephalitis. Arch. Neurol. 2003;60:1760–1764. doi: 10.1001/archneur.60.12.1760. [DOI] [PubMed] [Google Scholar]
- [2].Dickerson RB, Newton JR, Hansen JE. Diagnosis and immediate prognosis of Japanese B encephalitis. Am J Med. 1952;12:277–288. doi: 10.1016/0002-9343(52)90356-2. [DOI] [PubMed] [Google Scholar]
- [3].Ogata A., Hamaue N., Terado M., Minami M., Nagashima K., Tashiro K.. Isatin, an endogenous MAO inhibitor, improves bradykinesia and dopamine levels in a rat model of Parkinson’s disease induced by Japanese encephalitis virus. J. Neuro. Sci. 2003;206:79–83. doi: 10.1016/s0022-510x(02)00342-8. [DOI] [PubMed] [Google Scholar]
- [4].Ogata A., Tashiro K., Nukuzawa S., Nagashima K., Hall W.W.. A rat model of Parkinson’s disease induced by Japanese encephalitis virus. J. Neurovirol. 1997;3:141–147. doi: 10.3109/13550289709015803. [DOI] [PubMed] [Google Scholar]
- [5].Kumar S., Kalita J., Saxena V., Khan M.Y., Khanna V.K., Sharma S., Dhole T.N., Miara U.K.. Some observations on the tropism of Japanese encephalitis virus in rat brain. Brain Res. 2009;1268:135–141. doi: 10.1016/j.brainres.2009.02.051. [DOI] [PubMed] [Google Scholar]
- [6].Hamauea N, Ogatab A, Teradoc M, Tsuchidaa S, Yabeb I, Sasakib H, Hirafujic M, Togashic H, Aokia T. Entacapone, a catechol-O-methyltransferase inhibitor, improves the motor activity and dopamine content of basal ganglia in a rat model of Parkinson’s disease induced by Japanese encephalitis virus. Brain Research. 2010;1309:110–115. doi: 10.1016/j.brainres.2009.10.055. [DOI] [PubMed] [Google Scholar]
- [7].Sekhon L.H., Fehlings M.G.. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. 2001;26:S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- [8].Rios JL, Recio MC, Giner RM, Máñez S.. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–193. [Google Scholar]
- [9].Hosseinzadeh H, Talebzadeh F.. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- [10].Hosseinzadeh H. Karimi G andNiapoor M. Antidepressant effects of Crocus sativusstigma extracts and its constituents, crocin, and safranal, in mice. J Med Plants. 2004;3:48–58. [Google Scholar]
- [11].Nemati H. Boskabady MH and Ahmadzadef Vostakolaei H: Stimulatory effect of Crocus sativus (saffron) on beta2-adrenoceptors of guinea pig tracheal chains. Phytomedicine. 2008;15:1038–1045. doi: 10.1016/j.phymed.2008.07.008. [DOI] [PubMed] [Google Scholar]
- [12].Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- [13].Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativusL.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm PharmSci. 2005;8:387–393. [PubMed] [Google Scholar]
- [14].Hamaue N., Ogata A., Terado M., Ohno K., Kikuchi S., Sasaki H., Tashiro K., Hirafuji M., Minami M.. Brain catecholaminealteration and pathological features with aging in Parkinson’sdisease model rat induced by the Japanese encephalitis virus. Neurochem. Res. 2006;31:1451–1455. doi: 10.1007/s11064-006-9197-5. [DOI] [PubMed] [Google Scholar]
- [15].Hamaue N., Ogata A., Terado M., Endo T., Makiura T., Hirafuji M., Yasui K., Nagashima K., Tashiro K., Minami M., Parvez S.H.. Selegiline effects on bradykinesia anddopamine levels in a rat model of Parkinson’s disease inducedby the Japanese encephalitis virus. Biog. Amines 16. 2001:523–530. [Google Scholar]
- [16].Ogata A., Nagashima K., Hall W.W., Ichikawa M., Kimura-Kuroda J., Yasui K.. Japanese encephalitisvirus neurotropism is dependent on the degree of neuroralmaturity. J.Virol. 1991;65:880–886. doi: 10.1128/jvi.65.2.880-886.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaakkola S., Wurtman R.J.. Effects ofcatecholamine-O-methyltransferase inhibitors andL-3,4-dihydroxyphenylalanine with or without carbidopa onextracellular dopamine in rat striatum. J. Neurochem. 1993;60:137–144. doi: 10.1111/j.1471-4159.1993.tb05831.x. [DOI] [PubMed] [Google Scholar]
- [18].Kumar S., Kalita J., Saxena V., Khan M.Y., Khanna V.K., Sharma S., Dhole T.N., Miara U.K.. Some observations on thetropism of Japanese encephalitis virus in rat brain. Brain Res. 2009;1268:135–141. doi: 10.1016/j.brainres.2009.02.051. [DOI] [PubMed] [Google Scholar]