Abstract
Background
New-onset atrial fibrillation (AF) is common after atrial flutter (AFL) ablation, but it was unclear whether AF ablation could reduce the incidence of AF in AFL patients without AF history. The present meta-analysis was conducted to evaluate the benefit of prophylactic AF ablation in reducing the occurrence of AF in typical AFL patients.
Material/Methods
We systematically searched PubMed, EMBASE, and the Cochrane Library from inception to December 2017 for randomized controlled trials (RCTs) that assessed the efficacy of AF ablation in reducing the occurrence of AF in AFL patients without AF. Trial sequential analysis (TSA) was used to control random errors and calculate the required information size.
Results
Four trials (n=357 patients) met the inclusion criteria and were included in our meta-analysis. The incidence of AF after AFL ablation was 46.4%. We observed that prophylactic AF ablation reduced the AF incidence compared with simple AFL ablation (26.1% versus 46.4%, RR: 0.57, 95% CIs: 0.42–0.76, P=0.0002) with a prolonged procedure duration (P<0.00001) and fluoroscopy time (P=0.004). Further TSA indicated that more RCTs were needed to reach more conclusive results. There was no significant difference in clinical complications (P=0.33) between the 2 groups.
Conclusions
This meta-analysis provides evidence that prophylactic AF ablation may be more effective than simple AFL ablation in reducing AF incidence after AFL ablation. Large prospective RCTs are warranted to confirm the benefit of prophylactic AF ablation in AFL patients without AF history.
MeSH Keywords: Atrial Fibrillation, Atrial Flutter, Catheter Ablation
Background
Catheter ablation of the cavotricuspid isthmus (CTI) is regarded as a first-line therapy for typical atrial flutter (AFL), with a success rate of over 90% [1,2]. However, many patients remain at high risk of new-onset of atrial fibrillation (AF) during follow-up, the incidence of which depends on follow-up time. Previous studies reported rates of 25% to 82% after a follow-up time of 29 to 68 months [3–7].
Although the association between AFL and AF has been known for many years [8], the underlying mechanism for AF occurrence after AFL ablation has been unclear. Their coexistence or sequential occurrence in the same patient suggests they might share the same electrophysiologic triggers and/or atrial substrate [8–10]. Patients after successful AFL ablation still have an increased risk of stroke, mainly due to the high rate of AF development [11–13]. Ablation therapy to achieve pulmonary vein isolation (PVI) has been proven to be more effective to control symptomatic AF than pharmacologic agents. Thus, it appears reasonable to perform AF ablation simultaneously during CTI ablation for AFL. Several studies have investigated the efficacy of AF ablation combined with CTI ablation for AFL patients without documented AF [14,15]; some suggested a substantial reduction of new-onset AF, while other study showed a significant benefit only in relatively older patients [10]. Also, the sample sizes of the aforementioned studies were relatively small, which might influence their power to evaluate clinical outcomes. Therefore, we conducted the present meta-analysis to assess the efficacy of prophylactic AF ablation on AF occurrence after CTI ablation. Trial sequential analysis (TSA) was used to determine whether the currently available evidence was sufficient and conclusive.
Material and Methods
Literature search
We searched PubMed, EMBASE, and the Cochrane Library Register of Controlled Trials online databases, from inception to December 2, 2017. We used Medical Subject Heading (MeSH) terms in PubMed, EMTREE terms in EMBASE, and keyword search terms for “atrial fibrillation”, “atrial flutter”, “cavotricuspid isthmus”, “catheter ablation”, “ablation”, and “pulmonary vein isolation” in all 3 databases. We limited our search to articles published in English.
Study selection
Two investigators (Xie and Liu) independently screened all titles and abstracts to identify studies for further assessment. Studies fulfilling the following criteria were included: 1) randomized controlled trials (RCTs) published in peer-reviewed journals with full text available in English; 2) study population of typical AFL patients without prior documentation of AF; 3) comparison of CTI ablation alone with AF ablation ±CTI ablation; 4) reporting occurrence of AF/atrial arrhythmia as an outcome; and 5) follow-up time more than 12 months. Non-comparative trials, case reports, editorials, and reviews were excluded. Studies that did not adequately report outcomes of interest were also excluded.
Data collection and quality assessment
Two independent reviewers extracted data using a standardized data extraction form, with disagreements resolved by consensus or, when necessary, a third reviewer. For each included study, we extracted the following data: 1) study characteristics: year of publication, study design, inclusion and exclusion criteria, total number of randomized patients, median length of follow-up, and ablation strategy of each group; 2) patient characteristics: age, sex, clinical comorbidity, echocardiographic parameters, detailed information about AFL, AF, and atrial arrhythmia occurrence/recurrence; 3) complications related to interventional procedure; and 4) procedure duration and fluoroscopy duration. The quality of each study was assessed using the Jadad quality scale for RCTs [17].
Trial sequential analysis
Meta-analysis can result in type I errors (α) owing to repetitive testing of accumulated data, especially when the included studies have small sample sizes. Thus, we used TSA to examine the reliability and conclusiveness of our results. In the current meta-analysis, TSA was performed by maintaining 95% confidence intervals (CI), a 38% relative risk reduction (an estimate based on 2 studies with high Jadad quality scale included in this meta-analysis [10,15]), a 2-sided α=1% to minimize the possibility of type I error, and a statistical test power of 80%. TSA software version 0.9.5.10 Beta (http://www.ctu.dk/tsa) was used in this study. If the cumulative Z-curve crossed the trial sequential monitoring boundary or exceeded the required information size, a significant result had been reached and no further studies were needed. Otherwise, further studies were necessary to confirm the results [18].
Statistical analysis
Statistical analysis was performed using Cochrane RevMan version 5.0 software (the Cochrane Collaboration, UK). The results are reported as weighted mean differences and relative risk (RR) for continuous and dichotomous outcomes respectively, with 95% CI. The outcomes were pooled using the random-effects model when the heterogeneity was moderate or high (I2 >50%) and the fixed-effects model was used when the heterogeneity was low (I2 <50%). Publication bias was evaluated by means of funnel plots. Sensitivity analyses were undertaken by omitting 1 study at a time to examine the influence of that study on the overall summary estimate. All statistical testing was two-tailed with a statistical significance set at P<0.05.
Results
Search results
Our electronic search identified 503 potentially relevant publications (Figure 1). After excluding duplicates and screening titles/abstracts, we retrieved 5 publications for full-text review. One publication was further excluded because it was a conference abstract of an already included study. In the end, 4 RCTs fulfilled all the inclusion criteria [10,14–16]. Of these 4 studies, 3 [10,14,15] compared PVI plus CTI ablation with CTI ablation alone and 1 [16] assigned patients to 3 treatment groups: antiarrhythmic drugs, CTI ablation, and PVI. All studies used radiofrequency energy except for 1 [15] that used cryo-balloon ablation to achieve PVI.
Figure 1.
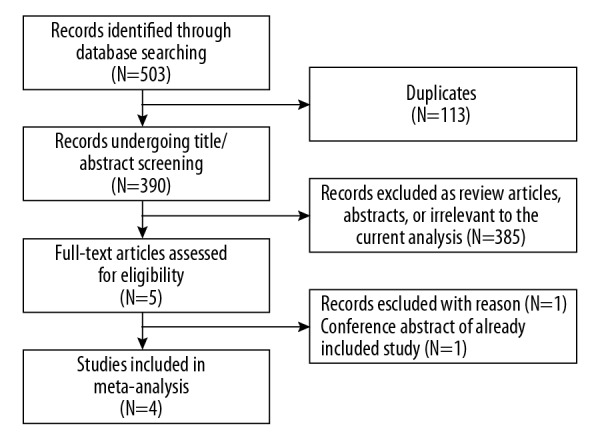
Flow diagram of the study selection process.
Publication bias
No significant publication bias was found for the primary outcome (freedom from AF at follow-up) as assessed by a funnel plot (Figure 2). This was verified by the Egger’s test (P=0.054).
Figure 2.
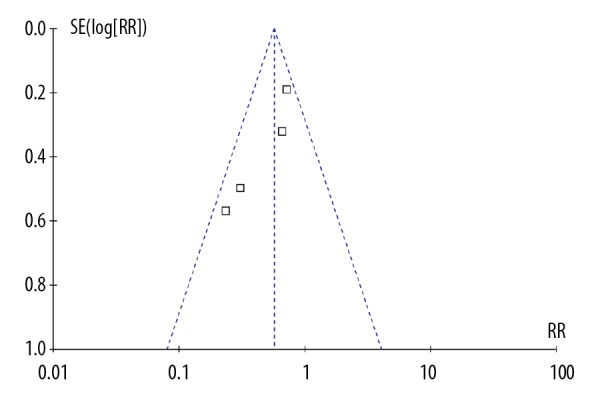
Funnel plot of meta-analysis.
Study and patients characteristics
Tables 1 and 2 summarize the characteristics of the included studies and patients. The 4 included RCTs randomized 357 patients to PVI ±CTI ablation (n=176) or CTI ablation alone (n=181). The sample sizes included in our analysis were not more than 50 patients in 3 RCTs [14–16]. One RCT [14] enrolled patients with persistent AFL, whereas 2 RCTs [15,16] recruited both paroxysmal and persistent AFL patients. The primary endpoint of each study was occurrence of AF or other atrial arrhythmia. The mean follow-up durations ranged from 1.0 to 2.0 years. One study [16] reported clinical outcomes both after a single and second ablation procedure, but we only extracted the data after a single PVI procedure to ensure results comparable among studies. Overall, there were no significant differences in mean age, sex ratio, left atrium diameter, mean LVEF, and proportion of hypertension between the 2 groups. The quality of the RCTs was relatively high (mean score, 4.5).
Table 1.
Characteristics of trials included in the meta-analysis.
| Study | Design | Subjects | Type of AFL Paroxysmal,% | Ablation strategy | Arrhythmia recurrence monitoring | Follow -up months | Quality assessment |
|---|---|---|---|---|---|---|---|
| Navarrete et al. 2011 | RCT | 48 | Persistent AFL | PVI+CTI ± stepwise ablation ± cardioversion versus CTI ablation | ECG; 48-hour Holter | 16 | 4 |
| Steinberg et al. 2014 | RCT | 50 | 56 | PVI (cryo-balloon ablation) + CTI versus CTI ablation | ICM | 12 | 6 |
| Mohanty et al. 2015 | Multicenter RCT | 216 | NR | PVI + CTI versus CTI ablation | ILR; Event recorders; 7-day Holter | 18 | 5 |
| Schneider et al. 2015 | RCT | 60* | 43** | PVI versus CTI ablation | ILR; 7-day Holter | 17 | 3 |
AFL – atrial flutter; CTI – cavotricuspid isthmus; ECG – electrocardiography; ILR – implantable loop recorders; ICM – implantable cardiac monitor; PVI – pulmonary vein isolation; RCT – randomized controlled trial; NR – not reported.
Included 17 patients assigned to antiarrhythmic drugs;
26 of 60 patients at the time of randomization.
Table 2.
Patient characteristics of the trials included in the meta-analysis.
| Navarrete et al., 2011 | Steinberg et al., 2014 | Mohanty et al., 2015 | Schneider et al., 2015 | |||||
|---|---|---|---|---|---|---|---|---|
| PVI+CTI | CTI only | PVI+CTI | CTI only | PVI+TI | CTI only | PVI | CTI only | |
| Subjects | 23 | 25 | 25 | 25 | 108 | 108 | 20 | 23 |
| Age, years | 56±6 | 55±5 | 57.3±9.0 | 56.7±10.0 | 62.4±9.3 | 61.2±9.7 | 61.1±10 | 63.9±7.9 |
| Male, % | NR | NR | 76 | 52 | 73 | 75 | 75 | 91.3 |
| Hypertension, % | 69 | 52 | 88 | 76 | 52 | 46 | 85 | 91.3 |
| Duration of AFL, months | 4.4±1.2 | 4.5±1.1 | 30.5±23.6 | 27.0±17.8 | 34.2±7.6 | 33.1±5.8 | NR | NR |
| LA diameter, cm | 4.2±0.1 | 4.1±0.1 | 51.9±2.7 | 51.1±3.2 | 44.7±6 | 45.5±8 | 46.1±7.0 | 43.2±6.7 |
| LVEF, % | 53±3 | 55±3 | 56.0±3.4 | 55.1±4.3 | 57±11 | 59±10 | 53.7±13.6 | 55.5±11.5 |
| AFL recurrence, % | NR | NR | 0 | 0 | 0 | 0 | 15%* | 8.7% |
| Freedom from arrhythmia, % | 87 | 44 | 88 | 48 | 71.3 | 60.2 | 60** | 39.1 |
AFL – atrial flutter; CTI – cavotricuspid isthmus; LA – left atrium; LVEF – left ventricular ejection fraction; PVI – pulmonary vein isolation; NR – not reported.
The data after single PVI procedure; the data were 5% after the second PVI procedure, and 0 after the third PVI procedure;
the data after the single PVI procedure; the data were 90% after mean 1.4 PVI procedures
Occurrence of AF after ablation
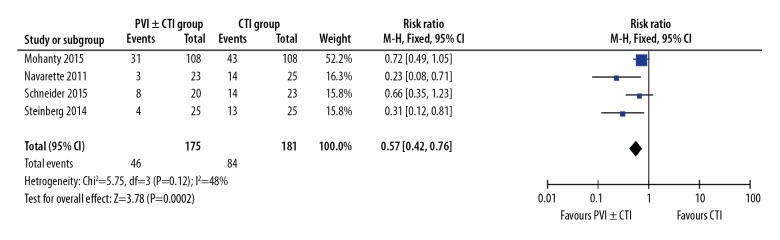
Though the overwhelming majority of atrial arrhythmias after ablation were AF, other atrial tachycardias, including AFL, were also included in this analysis. There were 130 events among the 357 participants (36.4%). The incidence of AF was 26.1% (46 of 176) in the PVI±CTI group and 46.4% (84 of 181) in the CTI group. Overall, we found a significantly lower occurrence of AF in the PVI±CTI group compared with the CTI group, with RR of 0.57, 95% CIs of 0.42 to 0.76, and P=0.0002 (Figure 3). Because no significant heterogeneity was noted among studies (P=0.12, I2=48%), we used the fixed-effects model instead of the random-effects model to complete the merge analyses.
Figure 3.
Forest plot of RR for AF occurrence after ablation using 4 studies.
Sensitivity analysis
We repeated our primary analysis with random-effects models and produced similar results to our primary analysis (RR, 0.52; 95% CI, 0.32–0.84, P=0.008). Additionally, we conducted an influence analysis to evaluate the influence of any individual trial on our overall results for the occurrence AF. This analysis also confirmed our primary results, with estimates ranging from an RR of 0.40 to 0.62 and 95% CIs of 0.20 to 0.96.
Trial sequential analysis
In the TSA, our calculations indicated that the required size needed to detect a difference in AF occurrence was 956 patients and the cumulative Z-curve did not cross the trial sequential monitoring boundary before exceeding the information size, which indicated the cumulative evidence was inconclusive regarding the efficacy of prophylactic PVI during AFL ablation (Figure 4).
Figure 4.
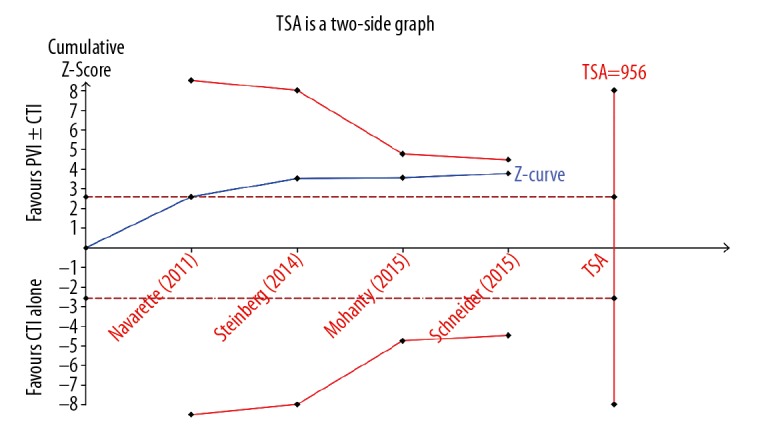
Trial sequential analysis evaluating the risk of AF occurrence after ablation.
Procedure duration and fluoroscopy time
All patients achieved bidirectional isthmus conduction block and PVI after AF and/or AFL ablation. Based on the data from 3 studies [10,14,15], additional AF ablation significantly prolonged the procedure duration (WMD=112.79, 95%CI: 65.05–160.53, P<0.00001) and fluoroscopy time (WMD=24.48, 95%CI: 7.60–41.35, P=0.004) compared with AFL ablation alone.
Complication
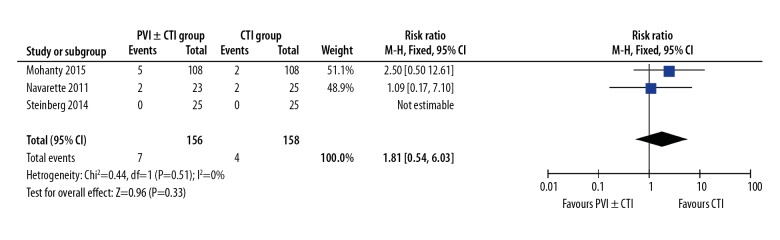
No deaths were reported in either group. There was no significant difference in clinical complications between the PVI ±CTI group and the CTI group (4.5% versus 2.5%, RR: 1.81, 95%CI: 0.54–6.03, P=0.33, Figure 5) [10,14,15]. Reported complications included groin hematoma, pseudoaneurysm, and pericardial effusion.
Figure 5.
Forest plot of RR for complications using 3 studies.
Discussion
To the best of our knowledge, this is the first meta-analysis to examine the efficacy of prophylactic AF ablation on AF occurrence compared with that of CTI ablation in AFL patients without prior AF history. Based on current clinical evidence, we demonstrated that the incidence of AF after AFL ablation was 46.4% after a mean follow-up of 1 year, and PVI ±CTI ablation resulted in a marked reduction of future AF occurrence with longer procedure duration and fluoroscopy time. No significant difference was found in the incidence of complications between the 2 groups. A total of 357 patients were randomized in the 4 trials and this number of patients was much smaller than our calculated optimal information size (956 patients) needed to reliably detect a plausible treatment effect of prophylactic PVI in AFL patients without AF. Thus, the current evidence for prophylactic PVI in isolated AFL patients is insufficient and inconclusive.
Occurrence of AF after AFL ablation
Despite the over 90% success rate of CTI ablation for AFL [1,19], new-onset of AF after AFL ablation was a common phenomenon with great clinical relevance and could lead to an increased risk of embolic complications, especially when patients discontinued anticoagulant drugs [11,20]. Furthermore, AF was an important cause of early readmission after AFL ablation [21]. The incidence of postablation AF varied among studies. Our study showed that the incidence of AF after AFL ablation was 46.4% after 1-year follow-up in patients with isolated AFL, which is consistent with previous studies [3,6]. Other researchers reported that over 80% of patients developed AF after successful ablation for AFL, even in the absence of prior AF history [5]. This discrepancy might be due to different study populations and/or follow-up times. The incidence of postablation AF was progressive and increased with follow-up duration, irrespective of the prior history of AF before ablation [1,4,22]. Differences in follow-up methods could also influence the detection rate of AF [23,24].
The current role of prophylactic PVI in isolated AFL patients
The incidence of AF after AFL ablation was high, with more than 40% of patients requiring additional PVI to control rhythm and symptoms [7]. These findings, together with our encouraging results showing that the risk of postablation AF was reduced by 43% after PVI±CTI ablation, indicated that prophylactic PVI at the time of CTI ablation might benefit AFL patients. Although these results were not confirmed by TSA, we still have reasons to believe that prophylactic PVI is an attractive and promising therapeutic means for AF prevention during AFL ablation, especially in patients with high risk of AF occurrence after AFL ablation, such as those age ≥55 years, as well as those with reduced LVEF, left atria enlargement, and induction of AF at electrophysiological testing [3,6,10,25]. Firstly, the follow-up times of these 4 studies were relatively short (mostly about 1 year). More obvious therapeutic effects would be anticipated after a longer follow-up time because of the progressive incidence of AF. Secondly, pulmonary vein trigger was an important initiator of AFL. In Schneider ‘s study [16], 95% of AFL patients were free of AFL after a mean of 1.4 PVI procedures without CTI ablation. Also, in patients with both AFL and AF, PVI alone might be sufficient to eliminate both arrhythmias [26]. In summary, CTI ablation appears to be sufficient for AFL but not for AFL patients, and the AFL triggers (especially from pulmonary veins) should receive the same attention as its maintenance substrate (CTI-dependent reentry).
Clinical implications for future clinical trials
Performing prophylactic PVI for all AFL patients would be excessive, but there must be some patients who would benefit from it, which should be better-defined in future trials. Several points should also be considered in the design of future clinical trials. Firstly, the sample size and follow-up time are 2 primary considerations. Our meta-analysis included 357 patients, which was 37% (357/956) of the calculated optimal information size. The optimal information size was calculated based on trials with about 1-year follow-up time. It is probable that a smaller optimal information size would be enough if the follow-up time is prolonged or if more high-risk patients, as mentioned above, are recruited. Secondly, additional PVI entailed increased hospital fees, although this might be offset by subsequent rehospitalization [21] or repeated PVI procedure [7]. The cost-effectiveness should also be evaluated in future trials. Thirdly, procedure safety was an inevitable issue. Procedure-related complications occurred in 7.8% of patients undergoing AF ablation, which was 3 times the incidence in patients undergoing AFL ablation [1,27]. Besides prolonged procedure duration and fluoroscopy time, more complications (4.5% versus 2.5%) were reported in the PVI±CTI group than in the CTI group, despite the difference was not statistically significant. Therefore, its safety should also be validated in large-scale clinical trials.
Limitations
Our study had several potential limitations. Firstly, the number of studies included in the analysis was small, and the total sample size was not sufficient, which could have an influence on the reliability and conclusiveness of analysis results despite the application of sensitivity analyses and TSA. Secondly, heterogeneity was present among the 4 RCTs with respect to procedure duration and fluoroscopy time, which might limit the reliability of our study, although the random-effects model was used. Thirdly, differences in operator experience, ablation protocol, and energy sources might also have affected the results of our analysis.
Conclusions
Our study suggested that PVI±CTI ablation was more efficacious than CTI ablation alone in reducing occurrence of AF; however, there was longer procedure duration and fluoroscopy time in the PVI±CTI ablation group. Due to limited clinical evidence, larger-scale RCTs with relatively longer follow-up time are needed to confirm these results and to identify the specific AFL patients most likely to benefit from PVI±CTI ablation.
Footnotes
Conflict of interest.
None.
Source of support: This work was supported by the Key Research and Development Project of Shandong Province (Grant No. 2016GSF201048) and Projects of the Medical and Health Technology Development Program of Shandong Province (Grant No. 2015WS0219)
References
- 1.Perez FJ, Schubert CM, Parvez B, et al. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: A meta-analysis. Circ Arrhythm Electrophysiol. 2009;2:393–401. doi: 10.1161/CIRCEP.109.871665. [DOI] [PubMed] [Google Scholar]
- 2.Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2016;13:e136–221. doi: 10.1016/j.hrthm.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Voight J, Akkaya M, Somasundaram P, et al. Risk of new-onset atrial fibrillation and stroke after radiofrequency ablation of isolated, typical atrial flutter. Heart Rhythm. 2014;11:1884–89. doi: 10.1016/j.hrthm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Chinitz JS, Gerstenfeld EP, Marchlinski FE, Callans DJ. Atrial fibrillation is common after ablation of isolated atrial flutter during long-term follow-up. Heart Rhythm. 2007;4:1029–33. doi: 10.1016/j.hrthm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: Left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799–802. doi: 10.1111/j.1540-8167.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Bai R, Deng W, et al. HATCH score in the prediction of new-onset atrial fibrillation after catheter ablation of typical atrial flutter. Heart Rhythm. 2015;12:1483–89. doi: 10.1016/j.hrthm.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 7.De Bortoli A, Shi LB, Ohm OJ, et al. Incidence and clinical predictors of subsequent atrial fibrillation requiring additional ablation after cavotricuspid isthmus ablation for typical atrial flutter. Scand Cardiovasc J. 2017;51:123–28. doi: 10.1080/14017431.2017.1304570. [DOI] [PubMed] [Google Scholar]
- 8.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–86. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 9.Wazni O, Marrouche NF, Martin DO, et al. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation. 2003;108(20):2479–83. doi: 10.1161/01.CIR.0000101684.88679.AB. [DOI] [PubMed] [Google Scholar]
- 10.Mohanty S, Mohanty P, Di Biase L, et al. Results from a single-blind, randomized study comparing the impact of different ablation approaches on long-term procedure outcome in coexistent atrial fibrillation and flutter (APPROVAL) Circulation. 2013;127:1853–60. doi: 10.1161/CIRCULATIONAHA.113.001855. [DOI] [PubMed] [Google Scholar]
- 11.Seara JG, Roubin SR, Gude Sampedro F, et al. Risk of atrial fibrillation, stroke, and death after radiofrequency catheter ablation of typical atrial flutter. Clin Res Cardiol. 2014;103:543–52. doi: 10.1007/s00392-014-0682-6. [DOI] [PubMed] [Google Scholar]
- 12.Tomson TT, Kapa S, Bala R, et al. Risk of stroke and atrial fibrillation after radiofrequency catheter ablation of typical atrial flutter. Heart rhythm. 2012;9:1779–84. doi: 10.1016/j.hrthm.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Exposito V, Rodriguez-Entem F, Gonzalez-Enriquez S, et al. Stroke and systemic embolism after successful ablation of typical atrial flutter. Clin Cardiol. 2016;39:347–51. doi: 10.1002/clc.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarrete A, Conte F, Moran M, et al. Ablation of atrial fibrillation at the time of cavotricuspid isthmus ablation in patients with atrial flutter without documented atrial fibrillation derives a better long-term benefit. J Cardiovas Electrophysiol. 2011;22:34–38. doi: 10.1111/j.1540-8167.2010.01845.x. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg JS, Romanov A, Musat D, et al. Prophylactic pulmonary vein isolation during isthmus ablation for atrial flutter: the PReVENT AF Study I. Heart Rhythm. 2014;11:1567–72. doi: 10.1016/j.hrthm.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Schneider R, Lauschke J, Tischer T, et al. Pulmonary vein triggers play an important role in the initiation of atrial flutter: Initial results from the prospective randomized Atrial Fibrillation Ablation in Atrial Flutter (Triple A) trial. Heart Rhythm. 2015;12:865–71. doi: 10.1016/j.hrthm.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative beta blockers in non-cardiac surgery? Systematic review and meta-analysis of randomised controlled trials. BMJ. 2005;331:313–21. doi: 10.1136/bmj.38503.623646.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spector P, Reynolds MR, Calkins H, et al. Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. Am J Cardiol. 2009;104:671–77. doi: 10.1016/j.amjcard.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 20.Attanasio P, Budde T, Lacour P, et al. Catheter ablation of atrial flutter: A survey focusing on post ablation oral anticoagulation management and ECG monitoring. Pacing Clin Electrophysiol. 2017;40:788–93. doi: 10.1111/pace.13122. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi B, Arora S, Mishra A, et al. Short-term outcomes of atrial flutter ablation. J Cardiovasc Electrophysiol. 2017;28:1275–84. doi: 10.1111/jce.13311. [DOI] [PubMed] [Google Scholar]
- 22.Gilligan DM, Zakaib JS, Fuller I, et al. Long-term outcome of patients after successful radiofrequency ablation for typical atrial flutter. Pacing Clin Electrophysiol. 2003;26:53–58. doi: 10.1046/j.1460-9592.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 23.Grond M, Jauss M, Hamann G, et al. Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke: A prospective multicenter cohort study. Stroke. 2013;44:3357–64. doi: 10.1161/STROKEAHA.113.001884. [DOI] [PubMed] [Google Scholar]
- 24.Verma A, Champagne J, Sapp J, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): A prospective, multicenter study. JAMA Intern Med. 2013;173:149–56. doi: 10.1001/jamainternmed.2013.1561. [DOI] [PubMed] [Google Scholar]
- 25.Romero J, Diaz JC, Di Biase L, et al. Atrial fibrillation inducibility during cavotricuspid isthmus-dependent atrial flutter ablation as a predictor of clinical atrial fibrillation. A meta-analysis. J Interv Card Electrophysiol. 2017;48(3):307–15. doi: 10.1007/s10840-016-0211-9. [DOI] [PubMed] [Google Scholar]
- 26.Wazni O, Marrouche NF, Martin DO, et al. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation. 2003;108:2479–83. doi: 10.1161/01.CIR.0000101684.88679.AB. [DOI] [PubMed] [Google Scholar]
- 27.Arbelo E, Brugada J, Blomstrom-Lundqvist C, et al. Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur Heart J. 2017;38:1303–16. doi: 10.1093/eurheartj/ehw564. [DOI] [PubMed] [Google Scholar]