Abstract
Background
The correlation between serum concentration of neuron specific enolase (NSE), S100B, and the prognosis of patients with acute spinal cord injury (ASCI) remains controversial.
Material/Methods
Sixty patients with confirmed diagnosis of ASCI were recruited for this study from February 2015 to January 2017. The serum level of NSE and S100B were dynamically measured: on the day of injury and for 2 weeks. The 60 cases were divided into Group A (1 or more than 1 ASIA grade improved at 6 months after the injury) and Group B (ASIA grades changed <1 at 6 months after the injury). The serum level of the 2 groups were compared at different time points. And the prognostic value of serum NSE and S100B as biomarkers in patients with ASCI were calculated by Bayes theorem.
Results
The serum levels of NSE in Groups A and B on the 2nd day of injury reached a peak at 66.80±13.76 g/L and 98.87±20.12 μg/L, respectively, and then declined gradually. On the 14th day of injury, the serum levels of NSE in both groups were 21.23±8.45 and 39.32±16.31 μg/L, respectively, which were much lower than those on the 2nd day (P<0.05). The serum levels of S100B in Groups A and B rose after the injury and reached a peak on the 4th day of injury. Then, the levels declined gradually to 1.14±0.64 and 1.97±0.98 μg/L, respectively, 2 weeks after the injury. Serum levels of NSE and S100B were good biomarkers for predicting the prognosis of ASCI patients with the sensitivity of 74.35% and 71.79%, the specificity of 71.43% and 66.67%. The cutoff value for serum NSE and S100B were 29.07 μg/L and 1.67 μg/L respectively. The AUCs were 0.78 (95% CI: 0.66–0.89) and 0.76 (95% CI: 0.63–0.89) respectively for serum NSE and S100B.
Conclusions
Serum levels of NSE and S100B protein can reflect the degree of spinal cord injury and could be potential biomarkers for the prognosis of acute spinal cord injury.
MeSH Keywords: Phosphopyruvate Hydratase, S100 Calcium Binding Protein beta Subunit, Spinal Cord Injuries
Background
Acute spinal cord injury (ASCI) is a serious disabling disease that usually leads to sensory disturbance and paresis [1]. ASCI patients often require lifelong care and rehabilitation treatment, which becomes a great burden to the patients themselves, their family, and even society [2–4]. Epidemiological data has shown that in the United States, 30 to 40 new cases of ASCI emerge per million people every year and may reach up to 60 cases in some areas [5].
China still lacks nationwide epidemiological data on ASCI incidence. The number of ASCI patients is estimated to have exceeded 1 000 000 and increase at a rate of 120 000 yearly [6,7]. For a long time, most scholars believed that ASCI patients do not recover or only have a small chance to recuperate when the nerve cells show no sign of improvement at the early period after ASCI. Clinically, the severity and prognostic evaluation of ASCI mostly depends on computed tomography (CT), magnetic resonance imaging (MRI), or other morphologic imaging examinations, as well as the physical examination of neurological functions. However, few studies have employed objective serological indicators to evaluate the prognosis of ASCI patients. Clinically, NSE and S100B proteins are usually used for assessing the severity and prognosis of brain injury [11–13]. Moreover, the correlation of ASCI patients’ serum levels of NSE and S100B proteins with their prognosis and the recovery of their neurological function has also been evaluated according to the previously published studies [14,15]. However, the predicting performance as serum biomarkers was not exactly the same.
Material and Methods
Patients inclusion
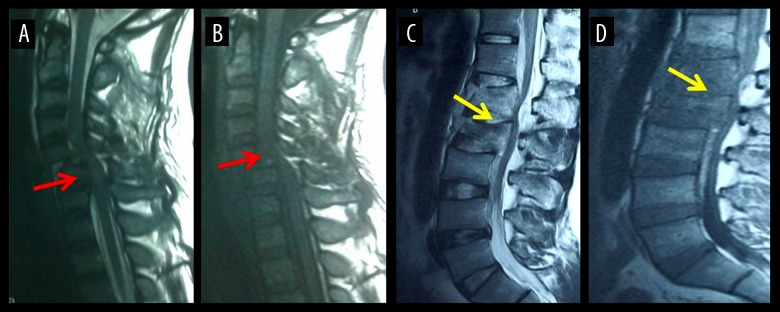
Sixty ASCI patients were recruited in this study from February 2015 to January 2017 in the Department of Spine Center of Tianjin Union Medicine Center. The inclusion criteria were: 1) age from 18 to 65; 2) admission time less than 24 hours after spinal cord injury; 3) injury site included cervical spinal cord, thoracic spinal cord, and lumbar spinal cord (Figure 1); 4) must have evidence of history of trauma; and 5) this work has been complied with all the relevant national regulations, institutional policies, and in accordance with the tenets of the Helsinki Declaration, and was approved by the institutional review board of Tianjin Union Medicine Center. Exclusion criteria: 1) injury of the caudaequina or caudaequina root; 2) combined injury of the brain demonstrated by cranial magnetic resonance imaging; 3) concurrent infection in the injury site of the spinal cord or other sites; 4) partially or completely disrupted ASCI; 5) pure spinal shock, spinal concussion, or other functional impairment; and 6) other concurrent malignant tumors.
Figure 1.
Magnetic resonance imaging of spinal cord injury: (A, B) cervical spinal cord injury (red arrow); (C, D) lumbar spinal cord injury (yellow arrow).
Severity injury evaluation and grouping
The International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) of American Spinal Injury Association (ASIA) was used to classify the severity of patients’ functional injuries. The sensory score (SS) and motor score (MS) based on the ASIA classification were adopted to quantify ASCI severity. Evaluation was conducted on the day the patients were admitted to the hospital and in the 6th month after injury. The samples were divided into the following 2 groups based on the patients’ feeling and motor recovery situation 6 months after the injury: well-recovered group (Group A: 1 or more than 1 ASIA grade improved 6 months after the injury) and modestly recovered group (Group B: ASIA grades slightly changed (<1 ASIA) 6 months after the injury).
Peripheral blood collection
Peripheral venous blood (10 mL) was sampled from the patients definitively diagnosed with ASCI on the injured day and on the 2nd, 4th, 6th, 8th, 10th, 12th, and 14th day after injury. The sampled blood was allowed to stand for 10 min at 25°C for coagulation and then centrifuged at 3000 r/min for 20 min. The supernatant was obtained and then kept in cold storage at −80°C for further testing.
Serum NSE and S100B examination
Enzyme-linked immunosorbent assay was used to detect the NSE and S100B proteins serum levels. The NSE kit used in this study was purchased from Xiamen Huijia Biotechnology Co., Ltd., whereas the S100B kit was purchased from Shanghai Xinran Industrial Co., Ltd. The NSE and S100B assays strictly followed the instructional manuals. The sample of each case obtained from each time point was measured twice, and the mean value was calculated and used. The test was carried out with the kits of the same batch and on the same instrument.
Statistical analysis
The data was calculated by SPSS 17.0 software (IBM, Armonk, NY, USA). Measurement data was demonstrated by χ̄±s. Data from multiple time points were processed by analysis of variance for repeated measurements. Prognosis prediction sensitivity and specificity was calculated by the equation of sensitivity=true positive/(true positive+ false negative), specificity=true negative/(true negative+ false positive). The cutoff value of serum NSE and S100B as biomarker for ASCI patient prognosis were calculated according to the Youden’s index. A 2-tail P<0.05 was considered statistically significant.
Results
The general character of ASCI patients
The general characteristics of the 2 groups are shown in Table 1. There was no statistical difference between Group A and Group B in the aspects of age, gender, and injury site (P>0.05). However, the ASIA sensation and motor scores for the Group A were much higher than those of Group B (P<0.001).
Table 1.
The general characteristics of group A and group B.
| Factors | Group A (n=21) | Group B (n=39) | χ2/t | P |
|---|---|---|---|---|
| Age (year) | 40.6±11.2 | 42.6±13.4 | 0.58 | 0.56 |
| Gender [n, (%)] | 0.33 | 0.56 | ||
| Male | 17 (80.9%) | 29 (74.4%) | ||
| Female | 4 (19.1%) | 10 (25.6%) | ||
| Sampling time from injury (h) | 3.6±2.3 | 3.4±2.0 | 0.35 | 0.73 |
| ASIA sensation score | 114.2±16.2 | 94.7±18.6 | 4.05 | <0.001 |
| ASIA motor score | 67.6±16.5 | 50.2±17.4 | 3.80 | <0.001 |
| Injury site | ||||
| Cervical vertebra | 6 (28.6%) | 9 (23.1%) | 0.27 | 0.88 |
| Thoracic vertebra | 5 (23.8%) | 11 (28.2%) | ||
| Lumbar vertebra | 10 (47.6%) | 19 (48.7%) | ||
Serum NSE and S100B levels
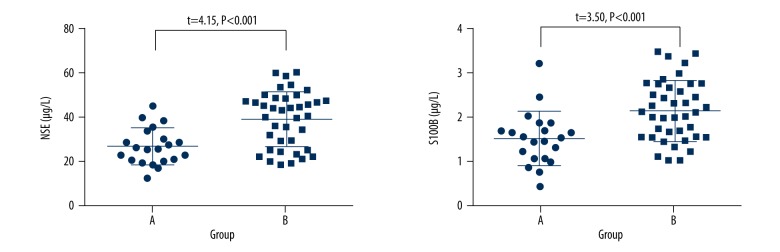
The serum level of NSE and S100B were 26.69±8.21 μg/L, 1.52±0.60 μg/L for Group B and 39.24±12.47 μg/L for the 2.14±0.68 μg/L for Group A, respectively. The serum level of NSE and S100B in Group B were significant higher than those of Group A (P<0.05), (Figure 2).
Figure 2.
Scatter plot of serum level of NSE and S100B in the 2 groups.
Dynamic changes of serum NSE and S100B
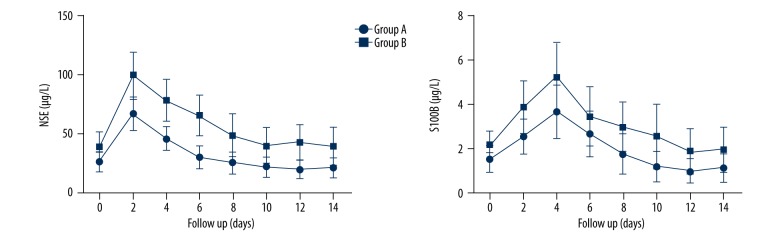
The serum level of NSE in Groups A and B on the 2nd day of injury reached the peak at 66.80±13.76 g/L and 98.87±20.12 μg/L, respectively, and then declined gradually. On the 1th day of injury, the serum concentration of NSE in both groups were 21.23±8.45 and 39.32±16.31 μg/L, respectively, which were much lower than those on the 2nd day (P<0.05). The serum level of S100B in Groups A and B rose after the injury reached the peak on the 4th day of injury. Then, the levels declined gradually to 1.14±0.64 and 1.97±0.98 μg/L, respectively, 2 weeks after the injury (Figure 3).
Figure 3.
Dynamic changes of serum NSE and S100B in ASCI patients.
Prognostic value of serum NSE and S100B
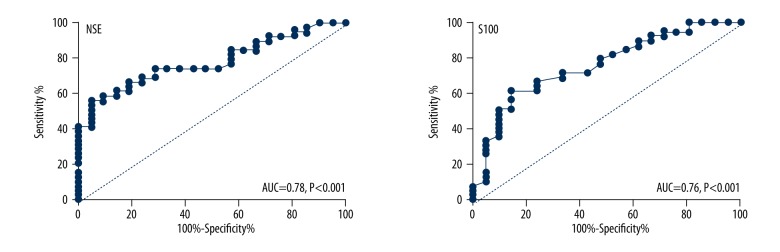
Serum level of NSE and S100B were good biomarkers for predicting the prognosis of ASCI patients with sensitivity of 74.35% and 71.79%, the specificity of 71.43% and 66.67% respectively (Table 2). The cutoff value for serum NSE and S100B were 29.07 μg/L and 1.67 μg/L respectively. The AUCs were 0.78 (95% CI: 0.66–0.89) and 0.76 (95% CI: 0.63–0.89) respectively for serum NSE and S100B (Figure 4).
Table 2.
The prognostic value of serum NSE and S100B.
| Markers | NSE | S100B | ||
|---|---|---|---|---|
| Point estimate | 95% CI | Point estimate | 95% CI | |
| Sensitivity (%) | 74.35 | 57.87–86.96 | 71.79 | 55.13–85.00 |
| Specificity (%) | 71.43 | 47.82–88.72 | 66.67 | 43.03–85.41 |
| Cut off value (μg/L) | 29.07 | NA | 1.67 | NA |
| AUC | 0.78 | 0.66–0.89 | 0.76 | 0.63–0.89 |
| Likelihood ratio | 2.60 | NA | 2.15 | NA |
Figure 4.
The ROC curve of serum NSE and S100B as biomarkers for ASCI patient prognosis predication.
Discussion
Neurological, imaging, and electrophysiological examinations are commonly used to evaluate ASCI severity but fail to quantify the damage extent. Therefore, a quantitative index for ASCI severity must be established to allow the objective evaluation of ASCI severity and prognosis. NSE is mainly located in central neurons but not in gliocytes or other nervous tissues; it is a specific enzyme to the central neurons. By contrast, the S100 calcium binding protein (S100B) is mainly found in the gliocytes of the nervus centralis and is a specific protein in neurogliocytes [16]. At present, NSE and S100B are usually adopted for evaluating the conditions and prognosis of brain injury and stroke patients in clinical studies. However, few studies have explored their role in assessing ASCI severity in patients with spine trauma. NSE and S100B are distributed variably in the nervous centralis. Besides neurons, gliocytes and other nervous tissues may be damaged during ASCI [17]. A previous animal experiment [18] showed obvious increases in the serum levels of NSE and S100B in ASCI rats. Furthermore, ASCI severity has been correlated with the serum levels of NSE and S100B proteins, indicating that the serum levels of these proteins could be used as potential markers of ASCI severity, which is in accordance with the Marquardt et al. study [19]. Peng et al. dynamically monitored the serum level of NSE and S100B in 27 acute ASCI patients. They found that the serum levels of NSE and S100B in the patients rose rapidly within a short time and then declined gradually and reached the normal level around the 10th day of injury. The serum levels of NSE and S100B were significantly higher in severe ASCI patients with improved neurological function than in those with substantially recovered neurological function. Pouw et al. [20] explored the expression of NSE and S100B in 16 patients who suffered from acute thoracic ASCI. They found significant increases in the serum levels of NSE and S100B in acute ASCI patients. Another study evaluated S100B for individual prediction of functional outcome in spinal epidural empyema and found that S100B was a promising serum marker with prognostic significance in the event of spinal cord compression resulting from epidural empyema. The aforementioned published studies showed that serum NSE and S100B were useful serological markers for predicting the prognosis of patients with ASCI [21].
Conclusions
In our present study, we included 60 patients with ASCI and divided these patients into well-recovered and modest-recovered groups. The serum NSE and S100B level were dynamitic tested and applied as biomarkers for the evaluation of the prognosis of patients with ASCI. We found that the serum levels of NSE and S100B increased obviously early in acute ASCI patients and declined gradually. This indicated that after injury, elevated NSE and S100B can be quickly detected in patients with ASCI. We also found that the prognosis predicting performance of serum NSE and S100B as biomarkers was good for the evaluation of neurologic functional recovery with the sensitivity of 74.35% for NSE and 71.79% for S100B, and specificity of 71.43% for NSE and 66.67% for S100B. And the cutoff value for serum NSE and S100B were 29.07 μg/L and 1.67 μg/L respectively. The cutoff value indicates that patients with more than the cutoff value for NSE and S100B had increased risk of developing poor prognosis. The serum levels were low in the well-recovered group but very high in the modest-recovered group. Patient recovery was mainly associated with the extent of ASCI damage at the time of injury. The serum levels of NSE and S100B were successfully adopted as serologic markers to evaluate acute ASCI, and high serum levels of NSE and S100B indicated high ASCI severity.
Footnotes
Conflict of interest
None.
Source of support: Foundation of Tianjin Union Medical Center(No. 2017YJ007)
References
- 1.Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.Kim YH, Ha KY, Kim SI. Spinal cord injury and related clinical trials. Clin Orthop Surg. 2017;9:1–9. doi: 10.4055/cios.2017.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein DM, Sheth KN. Management of acute spinal cord injury. Continuum. 2015;21:159–87. doi: 10.1212/01.CON.0000461091.09736.0c. [DOI] [PubMed] [Google Scholar]
- 4.Stahel PF, VanderHeiden T, Finn MA. Management strategies for acute spinal cord injury: Current options and future perspectives. Curr Opin Crit Care. 2012;18:651–60. doi: 10.1097/MCC.0b013e32835a0e54. [DOI] [PubMed] [Google Scholar]
- 5.Vitale MG, Goss JM, Matsumoto H, et al. Epidemiology of pediatric spinal cord injury in the United States: years 1997 and 2000. J Pediatr Orthop. 2006;26:745–49. doi: 10.1097/01.bpo.0000235400.49536.83. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Xiang Q, Li C, et al. Epidemiology of traumatic cervical spinal fractures and risk factors for traumatic cervical spinal cord injury in China. J Spinal Disord Tech. 2013;26:E306–13. doi: 10.1097/BSD.0b013e3182886db9. [DOI] [PubMed] [Google Scholar]
- 7.Wu Q, Li YL, Ning GZ, et al. Epidemiology of traumatic cervical spinal cord injury in Tianjin, China. Spinal Cord. 2012;50:740–44. doi: 10.1038/sc.2012.42. [DOI] [PubMed] [Google Scholar]
- 8.Kan EM, Ling EA, Lu J. Stem cell therapy for spinal cord injury. Curr Med Chem. 2010;17:4492–510. doi: 10.2174/092986710794182971. [DOI] [PubMed] [Google Scholar]
- 9.Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012;122:3824–34. doi: 10.1172/JCI64124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muheremu A, Peng J, Ao Q. Stem cell based therapies for spinal cord injury. Tissue Cell. 2016;48:328–33. doi: 10.1016/j.tice.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Kessler FH, Woody G, Portela LV, et al. Brain injury markers (S100B and NSE) in chronic cocaine dependents. Rev Bras Psiquiatr. 2007;29:134–39. doi: 10.1590/s1516-44462006005000029. [DOI] [PubMed] [Google Scholar]
- 12.Ondruschka B, Pohlers D, Sommer G, et al. S100B and NSE as useful postmortem biochemical markers of traumatic brain injury in autopsy cases. J Neurotrauma. 2013;30:1862–71. doi: 10.1089/neu.2013.2895. [DOI] [PubMed] [Google Scholar]
- 13.Zurek J, Fedora M. The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta Neurochir (Wien) 2012;154:93–103. doi: 10.1007/s00701-011-1175-2. discussion 103. [DOI] [PubMed] [Google Scholar]
- 14.Marquardt G, Setzer M, Szelenyi A, et al. Prognostic relevance of serial S100b and NSE serum measurements in patients with spinal intradural lesions. Neurol Res. 2009;31:265–69. doi: 10.1179/174313209X382287. [DOI] [PubMed] [Google Scholar]
- 15.Szelényi A, Heukamp C, Seifert V, Marquardt G. S100B, intraoperative neuromonitoring findings and their relation to clinical outcome in surgically treated intradural spinal lesions. Acta Neurochir (Wien) 2014;156:733–39. doi: 10.1007/s00701-013-1969-5. [DOI] [PubMed] [Google Scholar]
- 16.Michetti F, Corvino V, Geloso MC, et al. The S100B protein in biological fluids: More than a lifelong biomarker of brain distress. J Neurochem. 2012;120:644–59. doi: 10.1111/j.1471-4159.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- 17.Patil S, Raza WA, Jamil F, et al. Functional electrical stimulation for the upper limb in tetraplegic spinal cord injury: A systematic review. J Med Eng Technol. 2014;39:419–23. doi: 10.3109/03091902.2015.1088095. [DOI] [PubMed] [Google Scholar]
- 18.Cao F, Yang XF, Liu WG, et al. Elevation of neuron-specific enolase and S-100beta protein level in experimental acute spinal cord injury. J Clin Neurosci. 2008;15:541–44. doi: 10.1016/j.jocn.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Marquardt G, Setzer M, Seifert V. Serum biomarkers for experimental acute spinal cord injury: Rapid elevation of neuron-specific enolase and S-100 beta. Neurosurgery. 2006;58:E590. doi: 10.1227/01.NEU.0000207967.91316.8D. author reply E590. [DOI] [PubMed] [Google Scholar]
- 20.Pouw MH, Kwon BK, Verbeek MM, et al. Structural biomarkers in the cerebrospinal fluid within 24 h after a traumatic spinal cord injury: A descriptive analysis of 16 subjects. Spinal Cord. 2014;52:428–33. doi: 10.1038/sc.2014.26. [DOI] [PubMed] [Google Scholar]
- 21.Marquardt G, Setzer M, Seifert V. Protein S-100b for individual prediction of functional outcome in spinal epidural empyema. Spine (Phila Pa 1976) 2004;29:59–62. doi: 10.1097/01.BRS.0000103661.78939.02. [DOI] [PubMed] [Google Scholar]