Abstract
Skeletal muscle can be ultrastructurally damaged by eccentric exercise, and the damage causes metabolic disruption in muscle. This study aimed to determine changes in the metabolomic patterns in urine and metabolomic markers in muscle damage after eccentric exercise. Five men and 6 women aged 19~23 years performed 30 min of the bench step exercise at 70 steps per min at a determined step height of 110% of the lower leg length, and stepping frequency at 15 cycles per min. 1H NMR spectral analysis was performed in urine collected from all participants before and after eccentric exercise-induced muscle damage conventionally determined using a visual analogue scale (VAS) and maximal voluntary contraction (MVC). Urinary metabolic profiles were built by multivariate analysis of principal component analysis (PCA) and orthogonal partial least square-discriminant analysis (OPLS-DA) using SIMCA-P. From the OPLS-DA, men and women were separated 2 hr after the eccentric exercise and the separated patterns were maintained or clarified until 96 hr after the eccentric exercise. Subsequently, urinary metabolic profiles showed distinct trajectory patterns between men and women. Finally, we found increased urinary metabolites (men: alanine, asparagine, citrate, creatine phosphate, ethanol, formate, glucose, glycine, histidine, and lactate; women: adenine) after the eccentric exercise. These results could contribute to understanding metabolic responses following eccentric exercise-induced muscle damage in humans.
Keywords: Metabolomic analysis, Skeletal muscle, Eccentric exercise, Sex-dependent patterns
INTRODUCTION
Exercise-induced muscle damage (EIMD) occurs by unaccustomed physical activity or rapid and excessive activity (1). EIMD is more commonly associated with eccentric exercise that lengthens the muscle under tension rather than concentric exercise that shortens it (2). In the previous studies, eccentric contractions have shown to generate more powers (3) and decrease recruitment of motor units (4) than concentric contraction. In addition, it has suggested that eccentric action decreases attached cross-bridges as it increases the muscle fibre length (5). Therefore, eccentric contraction can more mechanically stress out the muscle than concentric contraction and the mechanical stress can contribute to inducing failure of febres (6).
EIMD physically causes stiffness, swelling, decreased force of muscular contraction, and delayed-onset muscular soreness (7), and it physiologically increases muscle blood flow, creatine kinase (CK) activity, lactate dehydrogenase, aldolase, aspartate transferase, and the circulating concentration of muscular cells such as skeletal troponin I, myoglobin and myosin in the blood plasma or serum (8–19). Unlike blood plasma or serum, there is no urinary CK standard because of the limitation of available information for exercise-induced CK increases and renal function (20). Instead, indexes of pigmenturia such as hematuria, hemoglobinuria and myoglobinuria are commonly applied to evaluate rhabdomyolysis in urine from EIMD. Clinically, these change appear from 24 to 48 hr after exercise, and usually disappear after 72 hr of rest (21). Additionally, EIMD based on urine could be evaluated by creatine/creatinine ratio, collagen constituents including hydroxyproline and hydroxylysine, or uric acid (22). However, the urinary markers for EIMD are still limited because these indexes cover the phenotypes that include muscle diseases and renal failure (23).
EIMD-induced muscle is known to result in the release of cellular components through serial processes, beginning with depleting ATP, causing the leakage of extracellular calcium ions into intracellular space due to both Na-K-ATPase and Ca2+-ATPase pump dysfunction. The leakage of the contents of muscular cells into the circulation can increase intracellular proteolytic enzyme activity and promote muscle protein degradation and augmented cell permeability (24,25). Especially, EIMD can be triggered by the loss of Ca2+ homeostasis and the initiation of the Ca2+ overload because of decreased action of Ca2+-adenosine triphosphatase (ATPase) during physical activity (26,27). The disruption of cellular Ca2+ balance activates Ca2+-dependent proteolytic and phospholipolytic pathways which are related with the removal and repair of the damaged muscle (28,29). These cellular responses are regarded as a series of complex events involving increased oxidative stress and inflammatory and immune responses (6,30). However, the mechanisms and metabolic processes of EIMD are not entirely understood.
The omics is a systemic biology to understand the biological process for genomes, transcriptomes, proteomes, and metabolomes in organisms. Metabolomics of the omics is defined as the scientific study of biochemical processes involving endogenous metabolites and informs us about instantaneous and final responses to stresses in various organisms including humans (31,32). Recently, these metabolomic approaches have been extended to sports science to identify biomarkers related to changes in physical performance, muscle fatigue, and muscle damage caused by exercise (33).
In this study, we tried to evaluate EIMD and trace its recovery pattern after eccentric exercise using urinary metabolites. Our results could contribute to understanding changes in the metabolomic patterns in urine and to identifying metabolomic markers in muscle damage after eccentric exercise.
MATERIALS AND METHODS
Subjects
Five male subjects (mean ± standard deviation, age: 21.20 ± 2.05 years; weight: 73.38 ± 7.52 kg; height: 173.40 ± 4.04 cm; muscle: 34.86 ± 1.90 kg; body fat: 14.64 ± 4.41 kg; body mass index: 24.18 ± 1.57) and 6 female subjects (mean ± standard deviation; age: 20.50 ± 0.55 years; weight: 55.87 ± 2.83 kg; height: 162.17 ± 5.31 cm; muscle: 23.07 ± 1.02 kg; body fat: 22.50 ± 3.71; body mass index: 21.28 ± 1.73) were included in the study. Individual information is shown in Table 1. All participants self-reported as being recreationally active (undertaking no more than 1 hr of “moderate” physical activity per week) and did not take part in any structured resistance training. None of the female participants had ever used any form of estrogen-based contraception. All women reported regular menstrual cycles, documenting an average cycle length of 28 ± 1 days. Females were tested on the 14th day (self-reported) of the menstrual cycle to measure estrogen levels at ovulation (34). Exclusion criteria included any resistance training in the last six months, occupation or lifestyle that required regular heavy lifting or carrying, any known muscle disorder, the use of dietary supplements (i.e., vitamin E), and any musculoskeletal injury in the last three months. All inclusion and exclusion criteria were determined through participant questionnaire prior to inclusion within this study. This study was approved by the Ethics Committee of Dankook University, in accordance with the ethical standards of the Declaration of Helsinki (DKU 2015-10-005).
Table 1.
Physical properties including sex, age, height, body weight, muscle, body fat, and body mass index (BMI) of the individual subjects were recorded before the eccentric exercise
| ID | Sex | Age (year) | Height (cm) | Body weight (kg) | Muscle (kg) | Body fat (kg) | Body mass index (BMI) |
|---|---|---|---|---|---|---|---|
| Subject1 | Male | 22.00 | 168.00 | 67.20 | 34.30 | 11.50 | 23.00 |
| Subject2 | Male | 23.00 | 173.00 | 70.90 | 33.50 | 11.90 | 23.70 |
| Subject3 | Male | 19.00 | 172.00 | 67.20 | 33.10 | 13.50 | 22.70 |
| Subject4 | Male | 19.00 | 175.00 | 76.70 | 35.60 | 14.00 | 25.00 |
| Subject5 | Male | 23.00 | 179.00 | 84.90 | 37.80 | 22.30 | 26.50 |
| Mean | 21.20 | 173.40 | 73.38 | 34.86 | 14.64 | 24.18 | |
| SD | 2.05 | 4.04 | 7.52 | 1.90 | 4.41 | 1.57 | |
| Subject6 | Female | 21.00 | 170.00 | 56.60 | 24.70 | 19.80 | 19.60 |
| Subject7 | Female | 20.00 | 158.00 | 53.00 | 23.00 | 21.00 | 21.20 |
| Subject8 | Female | 21.00 | 164.00 | 53.60 | 22.90 | 22.40 | 19.90 |
| Subject9 | Female | 20.00 | 166.00 | 56.80 | 23.00 | 25.40 | 20.60 |
| Subject10 | Female | 20.00 | 158.00 | 60.70 | 23.30 | 18.20 | 24.30 |
| Subject11 | Female | 21.00 | 157.00 | 54.50 | 21.50 | 28.20 | 22.10 |
| Mean | 20.50 | 162.17 | 55.87 | 23.07 | 22.50 | 21.28 | |
| SD | 0.55 | 5.31 | 2.83 | 1.02 | 3.71 | 1.73 |
SD, standard deviation.
Eccentric exercise
To induce muscle damage by eccentric exercise, the subjects performed a single bout of 30 min of bench-stepping at 60 steps per min at a predetermined step height of 110% of the lower leg length (35). Using the present exercise protocol, the exercise work rate can be determined using the mass lifted (body mass or body mass + 10 kg) the step height (110% of the lower leg length, on average 58 cm), the gravitational constant and the stepping frequency (15 cycles per min) (36).
Measuring muscle soreness
A visual analogue scale (VAS) was used to assess the volunteers’ muscle soreness. In each evaluation, the same researcher instructed the subjects in a standardized manner to perform a sub-maximal voluntary isometric knee extensor contraction, marking a vertical line at the scale point that best reflected their muscle soreness. The 100-mm horizontal line of the VAS had no marks or numbers, only indications of no soreness at the beginning or extreme soreness at the end of the exercise line. The soreness was quantified using the distance between the initial point line (0 mm) and the point marked by the subject (37).
Measurement of muscle strength
Subjects were properly positioned with the dominant lower limb on the Isokinetic Dynamometer Biodex System 3 Pro (Biodex Medical System, Shirley, NY, USA), following the manufacturer’s recommendations for evaluating knee flexion-extension movements. Before each evaluation, a warm-up was performed of 10 concentric knee flexion-extension repetitions at 180° seg−1 and maximal range of motion. Muscle strength was assessed through the highest torque value obtained among three 5-s maximal voluntary contraction (MVC) at 60° of knee flexion (0° = full knee extension). Participants received a two-min rest between each MVC to minimize any possible fatigue effects. Volunteers were previously instructed to perform maximal force and verbal encouragement was given by researchers in each MVC (38).
Urine collection
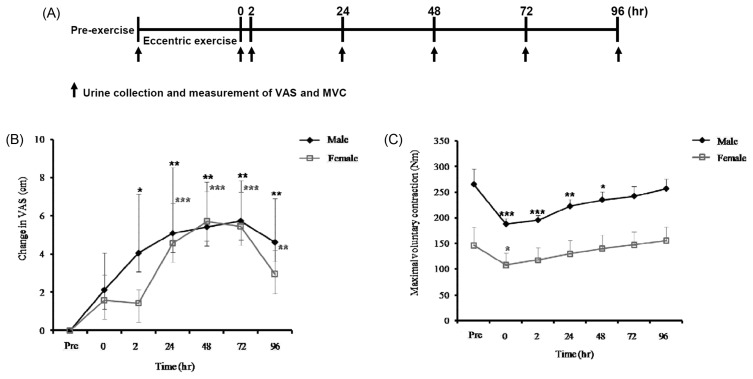
Urine was collected from the subjects before the exercise and again immediately, 2 hr, 24 hr, 48 hr, 72 hr, and 96 hr after the exercise (Fig. 1A). The urine samples were stored at −70°C until analysis.
Fig. 1.
(A) Schematic diagram of the experimental schedule in the study and (B) changes in visual analogue scale (VAS) and (C) maximal voluntary contraction (MVC) before the eccentric exercise (Pre) and according to the elapsed time (at 0, 2, 24, 48, 72, and 96 hr) after the eccentric exercise. Values are mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
1H NMR spectroscopic analysis
After the urine samples were thawed at 4°C, they were centrifuged to remove solids. A 600 μL aliquot of the supernatant was added to a microcentrifuge tube containing 70 μL of D2O solution with 5 mM DSS and 10 mM imidazole. DSS was used as the qualitative standard for the chemical shift scale. In addition, 30 μL of 0.42% sodium azide was added. After vortexing, this solution was adjusted to pH 6.8, and the urine sample was analyzed with a nuclear magnetic resonance (NMR) spectrometer within 48 hr. All spectra were determined using a Varian Unity Inova 600 MHz spectrometer at Pusan National University (Busan, Korea) operating at 26°C. One-dimensional NMR spectra were acquired with the following acquisition parameters: spectral width 24038.5 Hz, 12.53 min acquisition time, and 128 nt. Additional conditions were set of a relaxation delay time of 1 s and saturation power of 4 to suppress massive water peaks. The NMR spectra were reduced to data using the Chenomx NMR Suit program (ver. 4.6, Chenomx Inc., Edmonton, Alberta, Canada). The δ0.0~10.0 spectral region was segmented into regions of 0.04 ppm width to provide 250 integrated regions in each NMR spectrum. This binning process endowed each segment with an integral value that provided an intensity distribution of the whole spectrum with 250 variables prior to the pattern recognition analysis. The spectrum region of water (δ4.5~5.0) was removed from the analysis to prevent variation in water suppression efficiency. We also identified and quantified the spectra using the Chenomx NMR Suit Professional software package ver. 4.6 (Chenomx Inc.). DSS was used as the concentration reference at a concentration of 0.5 mM, and 2D NMR analysis was also performed to validate the identification of endogenous metabolites. Metabolite concentrations were expressed as relative ratio values normalized to creatinine concentration, assuming a constant rate of creatinine excretion in every urine sample.
Principal component analysis (PCA) and orthogonal partial least square-discriminant analysis (OPLS-DA)
All data were converted from the NMR software format into Microsoft Excel (Microsoft, Seattle, WA, USA). One-dimensional NMR spectra data were imported into SIMCA-P (version 12.0, Umetrics Inc., Kinnelon, NJ, USA) for multivariate statistical analysis to examine intrinsic variations in the data set. These data were scaled using centered scaling prior to the PCA and OPLS-DA. For the scaling process, the average value of each variable was calculated and then subtracted from the data. PCA and OPLS-DA score plots were used to interpret intrinsic variation in the data.
Statistical analyses
The changes in the muscle soreness and MVC over time after the eccentric exercise were analyzed by a one-way repeated measures ANOVA, followed by a Tukey’s post hoc test when a significant time effect was found to locate the time points that were different from the baseline values. All analyses were conducted with SPSS software (version 20.0, SPSS Inc., Chicago, IL, USA), and a p < 0.05 was considered statistically significant. Means and standard deviations of the metabolites were also calculated using Microsoft Excel. The statistical significance (p < 0.05, p < 0.01, or p < 0.001) of apparent differences in metabolite concentrations between before and after the eccentric exercise were assessed using analysis of variance, followed by Bartlett’s test (Prism 5.01, GraphPad, San Diego, CA, USA).
RESULTS
Muscle soreness developed after the eccentric exercise, peaking at 48 to 72 hr later in both male and female subjects (Fig. 1B). The MVC torque decreased at 0 to 48 hr after the eccentric exercise in the male subjects and 2 hr later among the female subjects (Fig. 1C). However, the MVC torque had returned to baseline by 96 hr after the eccentric exercise in both male and female subjects (Fig. 1C).
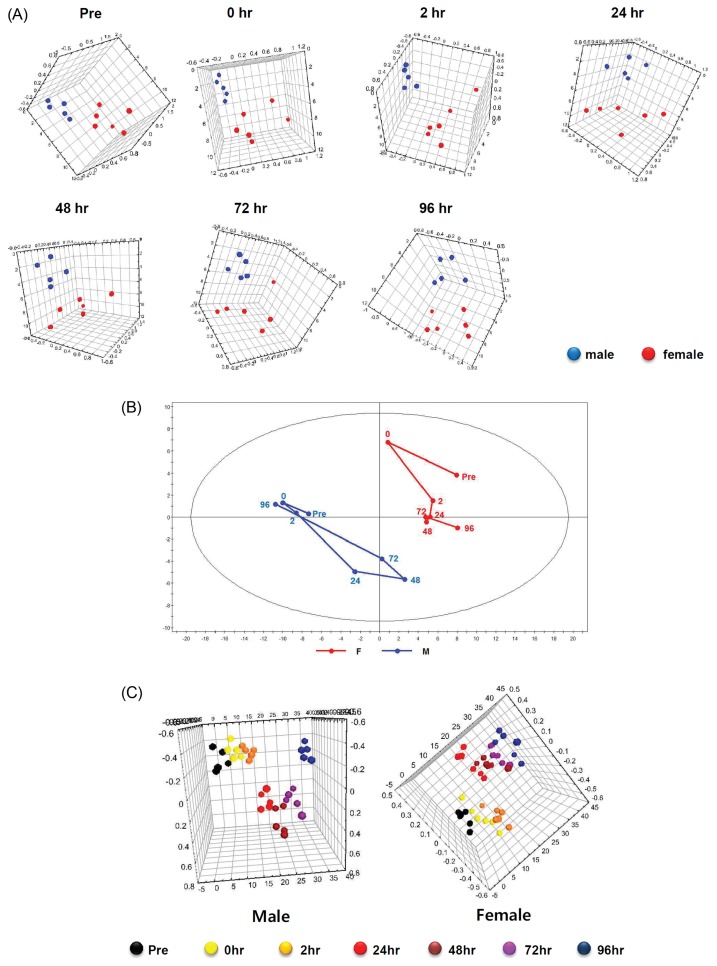
Through the metabolite analysis for the urine samples, the PCA showed clear separation of the metabolic patterns between male and female subjects at all time points (Fig. 2A). Subsequently, the trajectory analysis of PCA score plots for the metabolic patterns between male and female subjects can really reflect the sexual differences from muscle damages to its recovery. From the trajectory analysis, the metabolomic patterns of male subjects had changed rapidly from 24 to 72 hr after the eccentric exercise and then gradually restored themselves by 96 hr. However, the metabolomic patterns of female subjects had changed conspicuously at only 0 hr after the eccentric exercise and did not show the restoration that was seen among the males (Fig. 2B). When OPLS-DA was performed on the metabolites, metabolomic patterns in the male and female subjects were clearly separated at all time points (Fig. 2C).
Fig. 2.
(A) Comparisons of metabolic patterns between male and female subjects according to the elapsed time after the eccentric exercise. Principal Components Analysis (PCA) before the eccentric exercise (Pre) (R2X: 0.653; Q2: 0.0614) and PCA at 0 hr (immediately after the eccentric exercise) (R2X: 0.608; Q2: 0.107), at 2 hr (R2X: 0.61; Q2: −0.0429), 24 hr (R2X: 0.58; Q2: 0.0539), 48 hr (R2X: 0.563; Q2: −0.141), 72 hr (R2X: 0.506; Q2: −0.21), and 96 hr (R2X: 0.665; Q2: 0.188) after the eccentric exercise, compared between males and females, respectively. (B) Trajectory analysis using the principal components analysis (PCA) (R2X: 0.681; Q2: 0.547) based on mean data for 1H NMR of urine samples of the subjects according to elapsed time (at 0, 2, 24, 48, 72, and 96 hr) after the eccentric exercise. (C) The score plots showing that the clustering are completely separated in male (R2X: 0.551; R2Y: 0.191; Q2: −0.0411) and female (R2X: 0.483; R2Y: 0.158; Q2: −0.192) using the Orthogonal Partial Least Square-Discriminant Analysis (OPLS-DA).
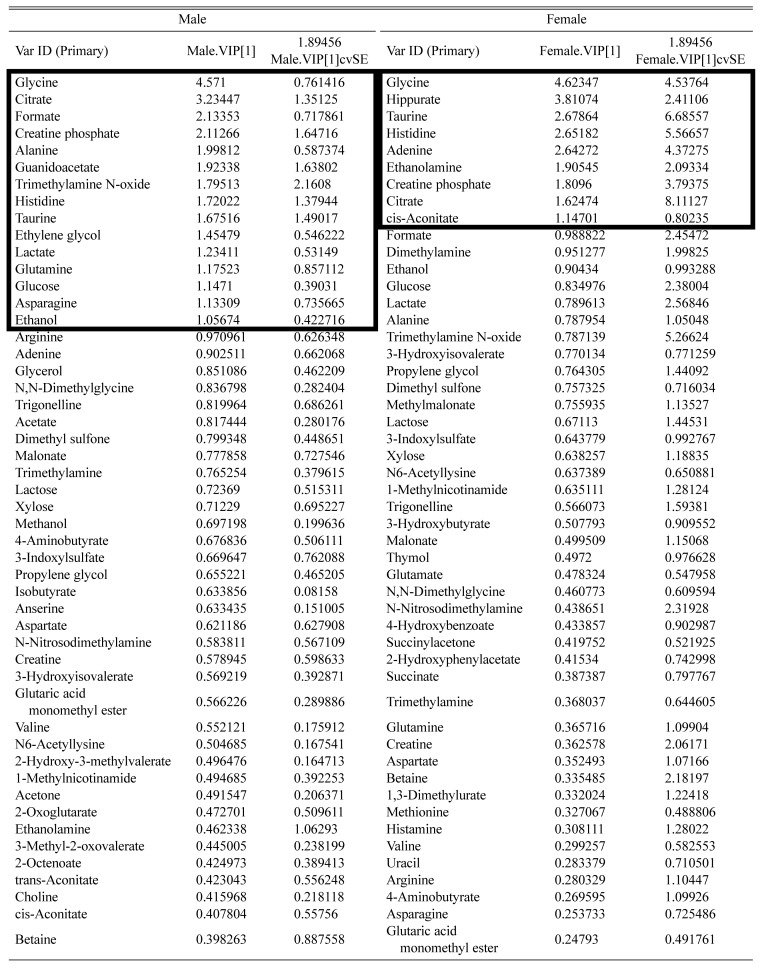
To evaluate metabolites which contribute to metabolomic patterns for each group, variable importance plots (VIPs) were derived from the OPLS-DA between male and female subjects. Total 82 metabolites were scored to reflect their importance for the metabolomic patterns between male and female subjects (Table 2). When VIP scores were confined to > 1 from the VIPs to select more convincing metabolites, 10 and 4 metabolites were found in males and females and 5 metabolites were commonly found in both males and females (Table 3).
Table 2.
VIP scores showed that list of metabolites which contributed to separation of the clustering in male (R2X: 0.551; R2Y: 0.191; Q2: −0.0411) and female (R2X: 0.483; R2Y: 0.158; Q2: −0.192) by OPLS-DA
VIP, variable important plots.
Bold box indicates major metabolites which have more than VIP score 1 by the OPLS-DA.
Table 3.
List of sex-specific and common metabolites from the major metabolites (VIP scores > 1) in male and female
| Clustering | Total | Urinary metabolites |
|---|---|---|
| Male only | 10 | Alanine, asparagine, ethanol, ethylene glycol, formate, glucose, glutamine, guanidoacetate, lactate, trimethylamine n-oxide |
| Female only | 4 | Adenine, cis-aconitate, ethanolamine, hippurate |
| Overlap between male and female | 5 | Citrate, creatine phosphate, glycine, histidine, taurine |
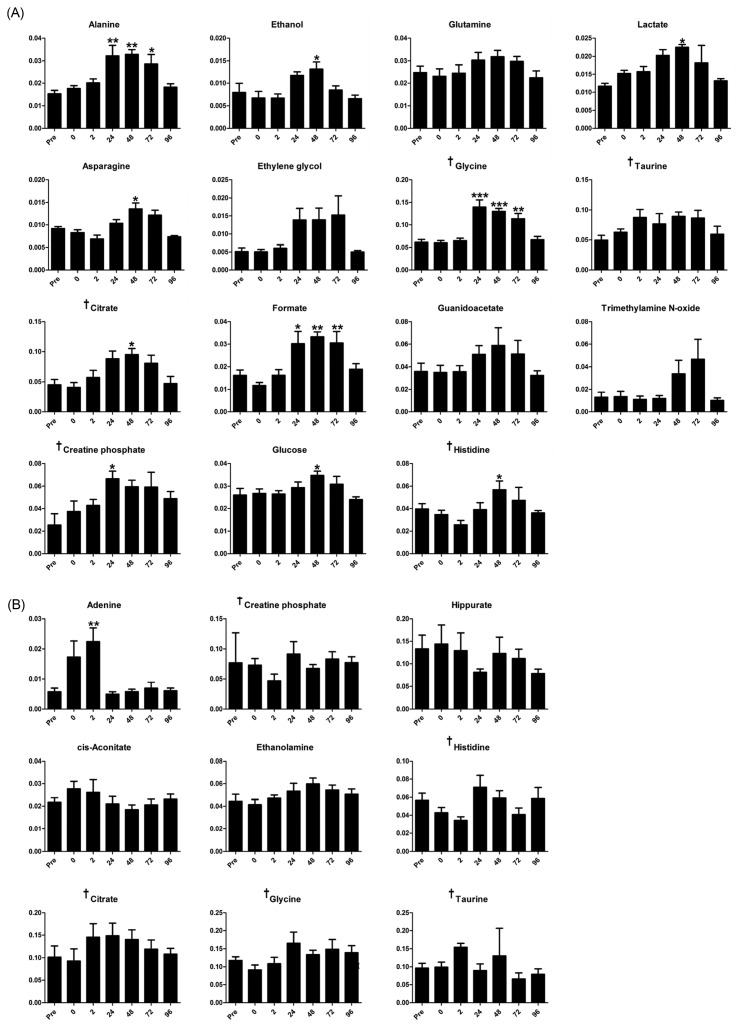
From the statistical analysis, 10 of the selected 15 metabolites in male subjects had increased significantly after the eccentric exercise, most between 24 and 72 hr later. Alanine, formate, and glycine had commonly increased between 24 hr and 72 hr, whereas asparagine, citrate, ethanol, glucose, histidine, and lactate only increased 48 hr later. In addition, creatine phosphate increased 24 hr later (Fig. 3A). In contrast, among the females, only adenine from the selected 9 metabolites had increased significantly 2 hr after the eccentric exercise (Fig. 3B).
Fig. 3.
(A) From the male VIP scores, concentration of the major metabolites (VIP > 1) in urine samples of the subjects before the eccentric exercise (Pre) and according to the elapsed time (at 0, 2, 24, 48, 72, and 96 hr) after the eccentric exercise. (B) From the female VIP scores, concentration of the major metabolites (VIP > 1) in urine samples of the subjects before the eccentric exercise (Pre) and according to the elapsed time (at 0, 2, 24, 48, 72, and 96 hr) after the eccentric exercise. Error bars are expressed as SD. *p < 0.05; **p<0.01; ***p < 0.001. †Indicates overlapped metabolites among the major metabolites of male and female.
DISCUSSION
Exercise-induced muscle damage that is closely related with eccentric exercise leads to increased intramuscular proteins in the blood (e.g., creatine kinase) (39) and the delayed onset of muscle soreness (40) but decreased muscle strength and range of motion (41). Researchers in previous studies have measured VAS and MVC to assess muscle soreness and muscle strength, respectively (38,42). In the current study, muscle soreness in both males and females increased after the eccentric exercise and distinctly decreased beginning 72 hr later, and the patterns were similar between the men and the women (Fig. 1B). In addition, male MVC torque decreased rapidly at 0 hr after the eccentric exercise and steadily recovered from 2 to 96 hr later, and female MVC torque decreased only at 0 hr after the exercise (p < 0.05); however the recovery patterns were similar in both groups (Fig. 1C). These results suggested that the subjects responded to EIMD in a sex-dependent manner.
Notably, PCA metabolomic pattern results also differed between males and females without the eccentric exercise (Fig. 2A). A number of studies have shown sex differences in muscle damage and repair processes (30). Other studies, however, have reported that these sex-different responses to muscle-damaging exercise are related to poor study design and that there may be little or no difference between males and females in the responses under elaborated conditions (43,44). Furthermore, the previous metabolomic research has shown distinct baseline metabolomic patterns between males and females (45,46), and the hormone fluctuations related to menstruation could directly or indirectly affect responses to exercise-induced muscle disruption (47). In our results, the metabolomic patterns among the females were more dispersed than were the metabolomic patterns in the men (Fig. 2A). Collectively, the differences in the metabolomic patterns between the male and female subjects suggest origins in innate sex differences rather than effects from muscle damage.
In the metabolomic trajectory pattern using PCA, the mean pre-exercise values among the men shifted rapidly at 24 hr and reached their maximums at 48 hr after the eccentric exercise, appearing to then return to the pre-exercise values by 96 hr later. In contrast, the shift in the female mean reached its maximum during pre-exercise, following which the women’s shift patterns were more erratic than those of the men (Fig. 2B). From these results, we suggest that metabolic changes in men following muscle damage reach their maximum at 48 hr after eccentric exercise and that these changes stabilize to their pre-exercise values more slowly than do conventional VAS and MVC.
In metabolomic approaches, creatine kinase and the related metabolites such as creatine and creatine phosphate have been widely considered biomarkers for muscle damage (30). The creatine phosphate circuit, which shows the rephosphorylation of creatine in mitochondria using ATP, together form the core of an energy network in both the cytosol and mitochondria of tissues that demand high energy such as the muscles and the brain (48). In addition, muscles take in glucose from the circulating blood to produce energy, and the glucose in the muscles degrades to pyruvate through glycolysis. Subsequently, pyruvate generates acetyl-CoA to form citrate through the tricarboxylic acid cycle or is converted to alanine or lactate through serial processes. Recently, citrate synthase activity was validated as a biomarker for mitochondrial density in skeletal muscle (49). Meanwhile, the transmitted alanine and lactate are transferred from muscles to liver by blood circulation to resynthesize glucose. From the energy metabolism processes, alanine and lactate increase in the body’s circulation system (50). Thus, alanine, citrate, creatine phosphate, glucose, and lactate could be evaluated as markers that are correlated with energy production after energy consumption by exercise.
Intensive and prolonged exercise has also been correlated with the generation of free radicals and with oxidative damage to cellular constituents (51,52). One study showed that histidine in microbes could be utilized as a source of antioxidant to cells (53). This result implies that histidine in the body could contribute to relieving oxidative stress from exercise. Muscle damage also involves inflammatory responses in the body (54). Previously, glycine infusion has been shown to have a cytoprotective effect against ischemia-reperfusion injury (55,56), and recent studies have indicated that glycine could be an effective anti-inflammatory agent that preserves muscle function (57,58). Therefore, histidine and glycine could be considered indirect EIMD markers.
Asparagine, ethanol, and formate were also increased after the eccentric exercise. Formate and ethanol in microbes have in particular been reported for anaerobic energy production, including glucose metabolism under anaerobic conditions or fermentation after pyruvate formation (59–61). However, the roles of these metabolites after EIMD are still unclear.
In conclusion, we found changes in urinary metabolomic patterns after participation in eccentric exercise and increased levels of specific metabolites from 24 to 72 hr, which suggested involvement in muscle damage or recovery. These results also suggested that the endogenous metabolites increased more among men following EIMD and that further study is needed among females in order to understand possible interferential effects of female hormones such as estrogen.
ACKNOWLEDGMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant (NRF-2011-0013659 and NRF-2017R1A2B4004758) funded by the Korea government (MEST). No conflicts of interests, financial, or otherwise are declared by the authors.
REFERENCES
- 1.Stauber WT. Eccentric action of muscles: physiology, injury, and adaptation. Exerc Sport Sci Rev. 1989;17:157–185. [PubMed] [Google Scholar]
- 2.Brown S, Day S, Donnelly A. Indirect evidence of human skeletal muscle damage and collagen breakdown after eccentric muscle actions. J Sports Sci. 1999;17:397–402. doi: 10.1080/026404199365911. [DOI] [PubMed] [Google Scholar]
- 3.Woledge RC, Curtin NA, Homsher E. Energetic aspects of muscle contraction. Monogr Physiol Soc. 1985;41:1–357. [PubMed] [Google Scholar]
- 4.Bigland-Ritchie B, Woods JJ. Integrated EMG and oxygen uptake during dynamic contractions of human muscles. J Appl Physiol. 1974;36:475–479. doi: 10.1152/jappl.1974.36.4.475. [DOI] [PubMed] [Google Scholar]
- 5.McCully KK, Faulkner JA. Characteristics of lengthening contractions associated with injury to skeletal muscle fibers. J Appl Physiol. 1986;61:293–299. doi: 10.1152/jappl.1986.61.1.293. [DOI] [PubMed] [Google Scholar]
- 6.Tee JC, Bosch AN, Lambert MI. Metabolic consequences of exercise-induced muscle damage. Sports Med. 2007;37:827–836. doi: 10.2165/00007256-200737100-00001. [DOI] [PubMed] [Google Scholar]
- 7.Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34:49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Asp S, Daugaard JR, Richter EA. Eccentric exercise decreases glucose transporter GLUT4 protein in human skeletal muscle. J Physiol. 1995;482:705–712. doi: 10.1113/jphysiol.1995.sp020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asp S, Rohde T, Richter EA. Impaired muscle glycogen resynthesis after a marathon is not caused by decreased muscle GLUT-4 content. J Appl Physiol. 1997;83:1482–1485. doi: 10.1152/jappl.1997.83.5.1482. [DOI] [PubMed] [Google Scholar]
- 10.Asp S, Daugaard JR, Kristiansen S, Kiens B, Richter EA. Exercise metabolism in human skeletal muscle exposed to prior eccentric exercise. J Physiol. 1998;509:305–313. doi: 10.1111/j.1469-7793.1998.305bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asp S, Daugaard JR, Rohde T, Adamo K, Graham T. Muscle glycogen accumulation after a marathon: roles of fiber type and pro- and macroglycogen. J Appl Physiol. 1999;86:474–478. doi: 10.1152/jappl.1999.86.2.474. [DOI] [PubMed] [Google Scholar]
- 12.Costill DL, Pascoe DD, Fink WJ, Robergs RA, Barr SI, Pearson D. Impaired muscle glycogen resynthesis after eccentric exercise. J Appl Physiol. 1990;69:46–50. doi: 10.1152/jappl.1990.69.1.46. [DOI] [PubMed] [Google Scholar]
- 13.Evans WJ, Meredith CN, Cannon JG, Dinarello CA, Frontera WR, Hughes VA, Jones BH, Knuttgen HG. Metabolic changes following eccentric exercise in trained and untrained men. J Appl Physiol. 1986;61:1864–1868. doi: 10.1152/jappl.1986.61.5.1864. [DOI] [PubMed] [Google Scholar]
- 14.Kirwan JP, Hickner RC, Yarasheski KE, Kohrt WM, Wiethop BV, Holloszy JO. Eccentric exercise induces transient insulin resistance in healthy individuals. J Appl Physiol. 1992;72:2197–2202. doi: 10.1152/jappl.1992.72.6.2197. [DOI] [PubMed] [Google Scholar]
- 15.Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc. 1995;27:1263–1269. doi: 10.1249/00005768-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Selkow NM, Day C, Liu Z, Hart JM, Hertel J, Saliba SA. Microvascular perfusion and intramuscular temperature of the calf during cooling. Med Sci Sports Exerc. 2012;44:850–856. doi: 10.1249/MSS.0b013e31823bced9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semark A, Noakes TD, St Clair GA, Lambert MI. The effect of a prophylactic dose of flurbiprofen on muscle soreness and sprinting performance in trained subjects. J Sports Sci. 1999;17:197–203. doi: 10.1080/026404199366091. [DOI] [PubMed] [Google Scholar]
- 18.Sorichter S, Puschendorf B, Mair J. Skeletal muscle injury induced by eccentric muscle action: muscle proteins as markers of muscle fiber injury. Exerc Immunol Rev. 1999;5:5–21. [PubMed] [Google Scholar]
- 19.Tuominen JA, Ebeling P, Bourey R, Koranyi L, Lamminen A, Rapola J, Sane T, Vuorinen-Markkola H, Koivisto VA. Postmarathon paradox: insulin resistance in the face of glycogen depletion. Am J Physiol. 1996;270:E336–E343. doi: 10.1152/ajpendo.1996.270.2.E336. [DOI] [PubMed] [Google Scholar]
- 20.Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757–767. doi: 10.1515/CCLM.2010.179. [DOI] [PubMed] [Google Scholar]
- 21.Miles MP, Andring JM, Pearson SD, Gordon LK, Kasper C, Depner CM, Kidd JR. Diurnal variation, response to eccentric exercise, and association of inflammatory mediators with muscle damage variables. J Appl Physiol. 2008;104:451–458. doi: 10.1152/japplphysiol.00572.2007. [DOI] [PubMed] [Google Scholar]
- 22.Oosterom DL, Betjes MG. Exertion-related abnormalities in the urine. Ned Tijdschr Geneeskd. 2006;150:606–610. [PubMed] [Google Scholar]
- 23.Chung YL, Rider LG, Bell JD, Summers RM, Zemel LS, Rennebohm RM, Passo MH, Hicks J, Miller FW, Scott DL. Juvenile dermatomyositis disease activity collaborative study G. Muscle metabolites, detected in urine by proton spectroscopy, correlate with disease damage in juvenile idiopathic inflammatory myopathies. Arthritis Rheum. 2005;53:565–570. doi: 10.1002/art.21331. [DOI] [PubMed] [Google Scholar]
- 24.Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis -- an overview for clinicians. Crit Care. 2005;9:158–169. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67:272–283. [PubMed] [Google Scholar]
- 26.Duchen MR, Valdeolmillos M, O’Neill SC, Eisner DA. Effects of metabolic blockade on the regulation of intracellular calcium in dissociated mouse sensory neurones. J Physiol. 1990;424:411–426. doi: 10.1113/jphysiol.1990.sp018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan CJ. Role of calcium in triggering rapid ultrastructural damage in muscle: a study with chemically skinned fibres. J Cell Sci. 1987;87:581–594. doi: 10.1242/jcs.87.4.581. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- 29.Busch WA, Stromer MH, Goll DE, Suzuki A. Ca2+-specific removal of Z lines from rabbit skeletal muscle. J Cell Biol. 1972;52:367–381. doi: 10.1083/jcb.52.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baird MF, Graham SM, Baker JS, Bickerstaff GF. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J Nutr Metab. 2012;2012:960363. doi: 10.1155/2012/960363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barding GA, Jr, Salditos R, Larive CK. Quantitative NMR for bioanalysis and metabolomics. Anal Bioanal Chem. 2012;404:1165–1179. doi: 10.1007/s00216-012-6188-z. [DOI] [PubMed] [Google Scholar]
- 32.Jordan KW, Nordenstam J, Lauwers GY, Rothenberger DA, Alavi K, Garwood M, Cheng LL. Metabolomic characterization of human rectal adenocarcinoma with intact tissue magnetic resonance spectroscopy. Dis Colon Rectum. 2009;52:520–525. doi: 10.1007/DCR.0b013e31819c9a2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ra SG, Maeda S, Higashino R, Imai T, Miyakawa S. Metabolomics of salivary fatigue markers in soccer players after consecutive games. Appl Physiol Nutr Metab. 2014;39:1120–1126. doi: 10.1139/apnm-2013-0546. [DOI] [PubMed] [Google Scholar]
- 34.Hicks KM, Onambele GL, Winwood K, Morse CI. Muscle damage following maximal eccentric knee extensions in males and females. PLoS ONE. 2016;11:e0150848. doi: 10.1371/journal.pone.0150848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newham DJ, Jones DA, Edwards RH. Large delayed plasma creatine kinase changes after stepping exercise. Muscle Nerve. 1983;6:380–385. doi: 10.1002/mus.880060507. [DOI] [PubMed] [Google Scholar]
- 36.Vissing K, Overgaard K, Nedergaard A, Fredsted A, Schjerling P. Effects of concentric and repeated eccentric exercise on muscle damage and calpain-calpastatin gene expression in human skeletal muscle. Eur J Appl Physiol. 2008;103:323–332. doi: 10.1007/s00421-008-0709-7. [DOI] [PubMed] [Google Scholar]
- 37.Wilson JM, Kim JS, Lee SR, Rathmacher JA, Dalmau B, Kingsley JD, Koch H, Manninen AH, Saadat R, Panton LB. Acute and timing effects of beta-hydroxy-beta-methylbutyrate (HMB) on indirect markers of skeletal muscle damage. Nutr Metab (Lond) 2009;6:6. doi: 10.1186/1743-7075-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baroni BM, Leal EC, Junior, De Marchi T, Lopes AL, Salvador M, Vaz MA. Low level laser therapy before eccentric exercise reduces muscle damage markers in humans. Eur J Appl Physiol. 2010;110:789–796. doi: 10.1007/s00421-010-1562-z. [DOI] [PubMed] [Google Scholar]
- 39.Selkow NM, Herman DC, Liu Z, Hertel J, Hart JM, Saliba SA. Blood flow after exercise-induced muscle damage. J Athl Train. 2015;50:400–406. doi: 10.4085/1062-6050-49.6.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twist C, Eston RG. The effect of exercise-induced muscle damage on perceived exertion and cycling endurance performance. Eur J Appl Physiol. 2009;105:559–567. doi: 10.1007/s00421-008-0935-z. [DOI] [PubMed] [Google Scholar]
- 41.Chen TC, Lin KY, Chen HL, Lin MJ, Nosaka K. Comparison in eccentric exercise-induced muscle damage among four limb muscles. Eur J Appl Physiol. 2011;111:211–223. doi: 10.1007/s00421-010-1648-7. [DOI] [PubMed] [Google Scholar]
- 42.Penailillo L, Blazevich A, Numazawa H, Nosaka K. Rate of force development as a measure of muscle damage. Scand J Med Sci Sports. 2015;25:417–427. doi: 10.1111/sms.12241. [DOI] [PubMed] [Google Scholar]
- 43.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 44.Rinard J, Clarkson PM, Smith LL, Grossman M. Response of males and females to high-force eccentric exercise. J Sports Sci. 2000;18:229–236. doi: 10.1080/026404100364965. [DOI] [PubMed] [Google Scholar]
- 45.Ruoppolo M, Scolamiero E, Caterino M, Mirisola V, Franconi F, Campesi I. Female and male human babies have distinct blood metabolomic patterns. Mol Biosyst. 2015;11:2483–2492. doi: 10.1039/C5MB00297D. [DOI] [PubMed] [Google Scholar]
- 46.Tso VK, Sydora BC, Foshaug RR, Churchill TA, Doyle J, Slupsky CM, Fedorak RN. Metabolomic profiles are gender, disease and time specific in the interleukin-10 gene-deficient mouse model of inflammatory bowel disease. PLoS ONE. 2013;8:e67654. doi: 10.1371/journal.pone.0067654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dieli-Conwright CM, Spektor TM, Rice JC, Sattler FR, Schroeder ET. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J Appl Physiol. 2009;107:853–858. doi: 10.1152/japplphysiol.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saks V. The phosphocreatine-creatine kinase system helps to shape muscle cells and keep them healthy and alive. J Physiol. 2008;586:2817–2818. doi: 10.1113/jphysiol.2008.155358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vigelso A, Andersen NB, Dela F. The relationship between skeletal muscle mitochondrial citrate synthase activity and whole body oxygen uptake adaptations in response to exercise training. Int J Physiol Pathophysiol Pharmacol. 2014;6:84–101. [PMC free article] [PubMed] [Google Scholar]
- 50.Koopman R, Ly CH, Ryall JG. A metabolic link to skeletal muscle wasting and regeneration. Front Physiol. 2014;5:32. doi: 10.3389/fphys.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- 53.Son DO, Satsu H, Shimizu M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005;579:4671–4677. doi: 10.1016/j.febslet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 54.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 55.Mangino MJ, Murphy MK, Grabau GG, Anderson CB. Protective effects of glycine during hypothermic renal ischemia-reperfusion injury. Am J Physiol. 1991;261:F841–F848. doi: 10.1152/ajprenal.1991.261.5.F841. [DOI] [PubMed] [Google Scholar]
- 56.Rush GF, Ponsler GD. Cephaloridine-induced biochemical changes and cytotoxicity in suspensions of rabbit isolated proximal tubules. Toxicol Appl Pharmacol. 1991;109:314–326. doi: 10.1016/0041-008X(91)90178-H. [DOI] [PubMed] [Google Scholar]
- 57.Ascher E, Hanson JN, Cheng W, Hingorani A, Scheinman M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery. 2001;129:231–235. doi: 10.1067/msy.2001.112594. [DOI] [PubMed] [Google Scholar]
- 58.Ham DJ, Murphy KT, Chee A, Lynch GS, Koopman R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin Nutr. 2014;33:448–458. doi: 10.1016/j.clnu.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Becker A, Fritz-Wolf K, Kabsch W, Knappe J, Schultz S, Volker Wagner AF. Structure and mechanism of the glycyl radical enzyme pyruvate formate-lyase. Nat Struct Biol. 1999;6:969–975. doi: 10.1038/13341. [DOI] [PubMed] [Google Scholar]
- 60.Dashko S, Zhou N, Compagno C, Piskur J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014;14:826–832. doi: 10.1111/1567-1364.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doi Y, Ikegami Y. Pyruvate formate-lyase is essential for fumarate-independent anaerobic glycerol utilization in the Enterococcus faecalis strain W11. J Bacteriol. 2014;196:2472–2480. doi: 10.1128/JB.01512-14. [DOI] [PMC free article] [PubMed] [Google Scholar]