Abstract
Lactobacillus (LAB) have been reported to exert both harmful and beneficial effects on human and animal health. Recently, it has been reported that dysbiosis and bacterial translocation contribute to liver fibrosis. However, the role of Gram-positive LAB in the situation of chronic liver diseases has not been yet elucidated. Liver injury was induced by bile duct ligation (BDL) in LAB or control-administered mice. Liver fibrosis was enhanced in LAB-administered mice compared with control-treated mice as demonstrated by quantification of Sirius-red positive area, hydroxyproline contents and fibrosis-related genes (Col1α1, Acta2, Timp1, Tgfb1). Moreover, LAB-administered mice were more susceptible to BDL-induced liver injury as shown by increased ALT and AST level of LAB group compared with control group at 5 days post BDL. Consistent with serum level, inflammatory cytokines (TNF-α, IL-6 and IL-1β) were also significantly increased in LAB-treated mice. Of note, LAB-treated liver showed increased lipoteichoic acid (LTA) expression compared with control-treated liver, indicating that LAB-derived LTA may translocate from intestine to liver via portal vein. Indeed, responsible receptor or inflammatory factor (PAFR and iNOS) for LTA were upregulated in LAB-administered group. The present findings demonstrate that administration of LAB increases LTA translocation to liver and induces profibrogenic inflammatory milieu, leading to aggravation of liver fibrosis. The current study provides new cautious information of LAB for liver fibrosis patients to prevent the detrimental effect of LAB supplements.
Keywords: LAB, Liver fibrosis, LTA
INTRODUCTION
Lactobacillus (LAB) have been used widely in foods and dairy products for over a hundred years. Recently, there has been increasing interest in their use to prevent or treat various diseases. Recently, the Agency for Healthcare Research and Quality (AHRQ) concluded that, although the existing LAB clinical trials reveal no evidence of increased risk, “the current literature is not well equipped to answer questions on the safety of LAB in intervention studies with confidence (1).” Because LAB have been shown to affect both the innate and adaptive immune systems, including effects on cytokine production and immune cell function (2–5), concern has been raised about the potential to overly stimulate the immune response in some individuals, possibly leading to excessive inflammation or autoimmune phenomena. Furthermore, this concern has not been reported in any liver diseases.
Liver fibrosis is a consequence of the chronic wound healing response to continuous hepatocellular damage. Such injury results in a strong inflammatory response within the liver, activating hepatic stellate cells to produce large amounts of extracellular matrix (6). Liver cirrhosis, the end stage of liver fibrosis is associated with development of various complications such as ascites, renal failure, hepatic encephalopathy, and portal hypertension, and may progress to hepatocellular carcinoma (7). Thus, investigating the pathogenesis of liver fibrosis is the key step in understanding a number of life-threatening complications in chronic liver disease.
LAB has been proposed to modulate various inflammatory conditions in body (8,9). Especially, LAB-derived bacterial products, like lipoteichoicacid (LTA), translocate to the liver via portal vein and can mediate several immune responses with participation of inflammatory cytokines. LTA can bind to Toll-like receptor 2 (TLR2) or platelet activating factor receptor (PAFR) present in hepatic cells, which triggers the release of TNFα, IL-6, IL1β, and other inflammatory cytokines (10). In addition, This signaling promote the production of collagen by activated-HSC (2, 11). Because of the importance of gut-liver axis in fibrosis progression, it has been hypothesized that the change of intestinal microbiota using LAB could modulate the gut barrier and inflammatory and fibrogenic response in liver disease. To examine the our hypothesis, we used murine liver fibrosis model induced by common bile duct ligation (BDL) surgery.
The present study examined the in vivo role of LAB by assessing liver fibrosis induced by BDL in mice with oral administration of LAB. Our results demonstrated that LAB aggravates liver fibrosis by activation of TLR2 signaling.
MATERIALS AND METHODS
Strain and culture conditions
Lactobacillus sakei was kindly provided by Dr. Suk-heung Oh, the department of Food and Biotechnology, Woosuk University (Wanju, Korea). These LAB were precultured in Lactobacilli MRS broth from BD Difco TM (CA, USA) at 37°C for 24 hr. Cultured cells were collected and washed two times with PBS by centrifugation at 3,000 g for 5 min. For oral administration, mice received 109 CFU of LAB resuspended in PBS or PBS only (200 μL/mouse) for either 5 or 21 consecutive days.
Mice and liver injury model
C57BL/6 mice were maintained in a standard condition (24 ± 2°C, 50 ± 5% humidity), pathogen-free environment and fed a sterile standard rodent chow diet and water ad libitum. Experimental and animal management procedures were undertaken in accordance with the requirements of the Animal Care and Ethics Committees of Chonbuk National University.
For BDL procedures, mice were anesthetized Zoletil (Virbac, 1.6 μL/g) by intramuscular injection. Sham-operated wt mice, used as controls, underwent laparotomy with exposure but no ligation of the common bile duct. The fascia and skin of the midline abdominal incision were closed with sterile surgical 6-0 sutures (Dafilon; B.Braun/Aesculap AG, Tuttlingen, Germany). C57BL/6 mice underwent BDL. After either 5 days (BDL, n = 8 per group; Sham, n = 4 per group) or 21 days (BDL, n = 8 per group; Sham, n = 4 per group) of surgery, mice were reanesthetized, and blood was obtained directly from the heart for serum hepatic enzyme determinations.
Determination of liver fibrosis by Sirius red staining
Liver fibrosis was quantified at 21 days post BDL using Sirius red staining as described by Kim et al (12). Direct red 80 was obtained from Sigma-Aldrich Diagnostics. Liver sections of were stained, and red-stained collagen fibers were quantified by the percentage of positive area per total liver section. Total liver section images were analyzed in individual animals using a light microscope (BX-51, Olympus Corp., Tokyo, Japan) and digital image software (analySIS TS, Olympus Corp., Tokyo, Japan). Data were expressed as the percentage of Sirius red-positive area per field.
Measurement of hepatic hydroxyproline content
Hydroxyproline content was measured as previously described (13). Liver specimens were weighed and 20 mg of freeze-dried sample was hydrolyzed in 6 N HCl at 120°C for 12 hr. Fifty μL of each hydrolyzed sample was transferred to a 96-well plate and evaporated to dryness under vacuum. The hydrolysate was evaporated under vacuum and the sediment was re-dissolved in 1 mL distilled water. Samples were incubated with chloramine-T solution for 20 min at room temperature, followed by incubation in Ehrlich’s solution at 65°C for 20 min. After cooling, the absorbance was read at 561 nm. Hydroxyproline concentration was calculated from a standard curve prepared with high purity hydroxyproline. The results were expressed as μg hydroxyproline per 10 mg of liver protein.
Quantitative real-time polymerase chain reaction (qPCR)
We extracted total RNA from liver tissue using the Easy-Spin Total RNA extraction kit (iNtRon Biotech, Seoul, Korea). Following incubation with RNase-free DNase I (Promega, Madison, WI, USA), reverse transcription was performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. cDNA was subjected to real-time PCR on a CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, CA, USA) using SYBR Green I as a double-strand DNA-specific binding dye. After the reaction was completed, we verified specificity by melting curve analysis. Quantification was performed by comparing Ct values of each sample after normalization to GAPDH. Sequences of primers were summarized in Supplementary Table 1.
Serum liver enzyme quantification
We measured serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphate (ALP) using the Roche Diagnostics COBAS INTEGRA 400 Plus (Indianapolis, IN, USA).
Immunoblot analysis
Liver tissue was directly lysed with an extraction buffer (T-PER, Thermo Fisher Scientific Inc., Rockford, lL, USA) for 30 min on ice. After centrifugation at 13,000 g for 15min at 4°C, protein concentration in the supernatant was measured using Bradford’s reagent (Thermo Fisher Scientific Inc.). Protein (30 μg) was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a gradient gel and then transferred onto PVDF membranes. Blocking was carried out using blocking buffer [5% nonfat dairy milk in Tris-buffered saline (20 mM Tris, 150 mM NaCl, pH 7.4) containing 0.05% Tween-20] for 1 hr at room temperature. Primary antibodies were diluted 1:1,000 in a blocking buffer and incubated at 4°C overnight. The following antibodies were used: anti-LTA (mouse antibody, Invitrogen, Waltham, MA, USA). To detect antigen antibody complexes, anti-rabbit or anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, TX, USA) were diluted 1:3000 in blocking buffer and incubated at room temperature for 45 min. Immune complexes were visualized using chemiluminescent substrate (Millipore, Burlington, MA, USA) according to the manufacturer’s instructions.
Statistical analysis
All data were expressed as mean ± standard error. Differences between two groups were compared using a two-tailed Student’s t-test. A value of p < 0.05 was considered statistically significant.
RESULTS
Administration of LAB exacerbates BDL-induced liver fibrosis
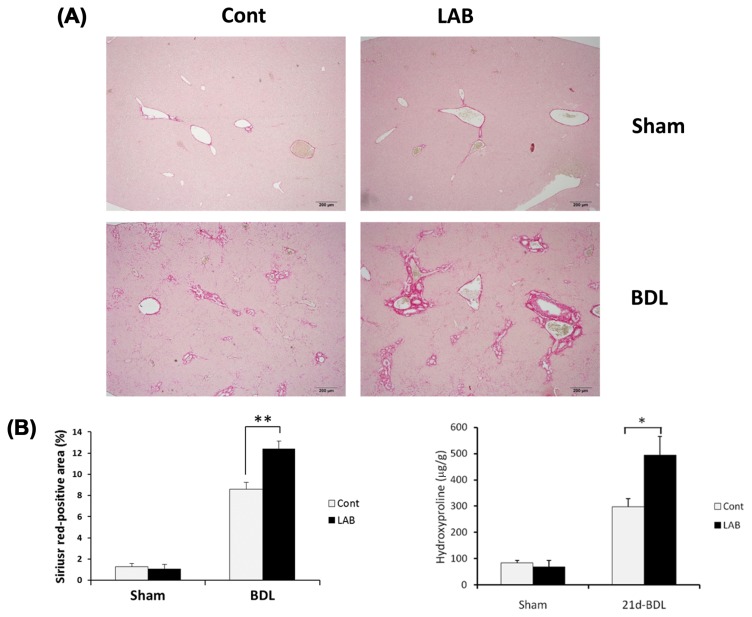
To investigate the effect of LAB in liver fibrosis, LAB or control vehicle-treated WT mice were subjected to BDL. At 21 days after BDL, LAB-administered mice displayed significantly increased liver fibrosis compared with control-treated mice as determined by quantification of Sirius red-positive area, which is specific for collagen deposition. Furthermore, hydroxyproline content was also significantly increased in LAB-administered mice (Fig. 1A, 1B). Therefore, these results suggest that LAB administration enhances liver fibrosis induced by BDL.
Fig. 1.
Administration of LAB exacerbates BDL-induced liver fibrosis. (A–B) Control or LAB-administered mice underwent sham operation (n = 4 per group) or BDL for 21 days (n = 8 per group). Fibrillar collagen deposition was determined by quantification of the Sirius red-positive area and hydroxyproline contents. LAB administration induced significant increase of Sirius red-positive area and hydroxyproline contents compared with control group. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *p < 0.05, **p < 0.01. Original magnification, ×200 (Sirius-Red).
LAB-administered mice are more susceptible to BDL-induced liver injury
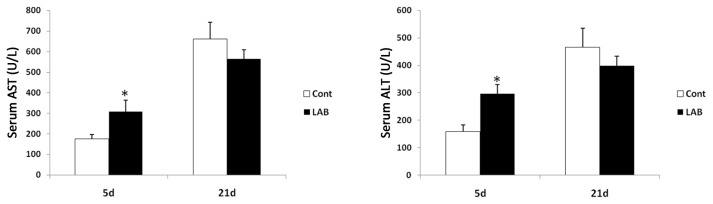
Next, we investigated the effect of LAB on liver injury induced by BDL. Consistent with results of Fig. 1, increased liver injury in LAB-treated group was confirmed by increased serum ALT and AST levels (Fig. 2) at 5 days after BDL. Thus, LAB may increase the susceptibility of cholestasis-induced liver injury.
Fig. 2.
LAB-administered mice are more susceptible to BDL-induced liver injury. (A) ALT level of LAB group were significantly higher than control group at 5 days post BDL. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *p < 0.05.
LAB administration favors progression of liver fibrosis
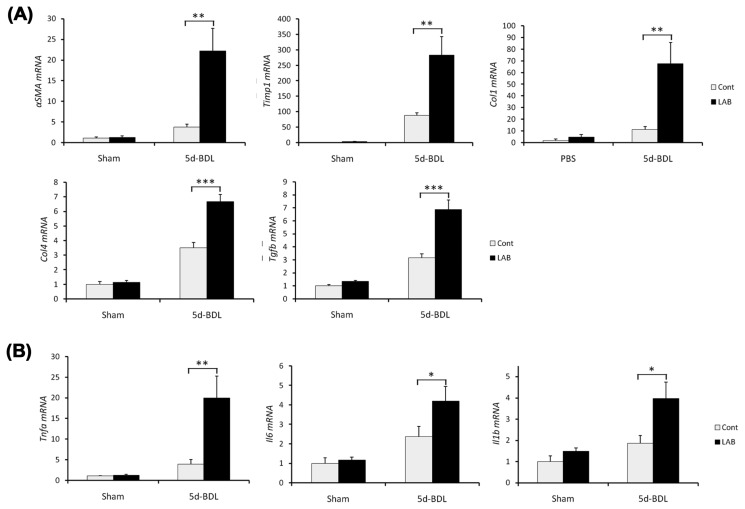
The exacerbation of liver fibrosis was also confirmed by qRT-PCR analysis of hepatic expression of fibrogenic genes (Fig. 3). The mRNA levels of Col1a1, Col4, Acta2, Timp1, and Tgfb1 were significantly elevated in LAB-treated group compared with control group (Fig. 3A). Furthermore, inflammatory responses of LAB-administered mice were significantly higher than those of control-treated mice as demonstrated by hepatic expression of proinflammatory cytokines including IL-1β, TNF-α and IL-6 (Fig. 3B). These results suggest that LAB promotes profibrogenic milieu of cholestasis of liver injury.
Fig. 3.
LAB administration favors progression of liver fibrosis. (A–B) Control or LAB-administered mice underwent sham operation (n = 4 per group) or BDL for 5 days (n = 6 per group). Expression of genes were determined by quantitative real time PCR. (A) Fibrosis related genes in liver were increased in LAB-administered group compared with control-treated group. (B) Various proinflammatory genes were also significantly increased in LAB-treated group. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *p<0.05, **p < 0.01, ***p< 0.001.
LAB-treated mice show increased hepatic LTA expression and related responses
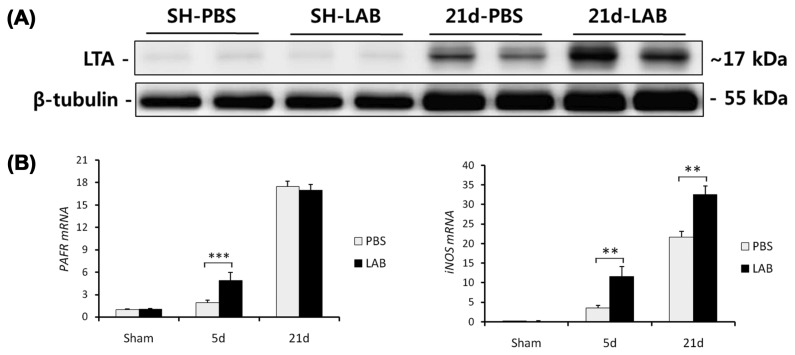
Above results (Fig. 1–3) prompted us to test whether oral administration LAB affects the translocation of lactobacillus LTA to liver. Indeed, LAB-treated liver showed increased LTA expression compared with control-treated liver, indicating that LAB-originated LTA may translocate from intestine to liver via portal vein (Fig. 4A). Moreover, LTA-related receptor or inflammatory factor (PAFR and iNOS) were significantly upregulated in LAB-administered group compared with control group. Collectively, these results indicate that oral administration of LAB increases the LTA translocation to liver and inflammatory signaling.
Fig. 4.
LAB-treated mice shows increased hepatic LTA expression and related responses. (A–B) Hepatic expression of LTA were measured by western blot. (A) LTA expression in LAB group were much higher than control group. (B) LTA-related receptor or inflammatory factor (PAFR and iNOS) were also upregulated in LAB-administered group. Two-tailed Student’s t-test, **p < 0.01, ***p< 0.001.
DISCUSSION
We demonstrate herein that LAB administration exacerbates liver fibrosis in mice. LAB increased the expressions of profibrogenic and proinflammatory cytokines and exhibited increased the susceptibility to BDL-induced liver injury. Because LAB-originated LTA could translocates to activate the hepatic inflammation, their administration leads to increased inflammation and progression of liver fibrosis.
Previously, many studies have been investigated the beneficial effects of LAB on various diseases. However, little is known about the side effect of exposure to LAB in human and animals. Specifically, it has been reported that LAB are closely related with inflammation of gut-liver axis (9,14). Therefore, we used the BDL-induced liver fibrosis model to study LAB-mediated inflammation and subsequent liver fibrosis.
Unexpectedly, we found that LAB aggravated liver injury and fibrosis. The translocation of intestinal bacteria to liver have been suggested to be a key step in the pathogenesis of liver fibrosis (14). In current study, LAB-originated LTA was translocated to liver. It has been reported that LTA from gram-positive bacteria induces nitric oxide production using a PAFR signaling pathway to activate STAT1 via Jak2 (15). Consistently, our data showed LAB increased the expression of PAFR and NO. Therefore, LAB could be a detrimental factor for liver disease patients through excessive stimulation of liver immune system.
The exact mechanisms responsible for exacerbation of liver fibrosis in LAB-administered mice remain unclear, but the following data are relevant. First, LAB-treated mice generated high levels of inflammatory cytokines (TNF-α, IL-6 and IL-1β) and nitric oxide, which are potent activators of the immune system in the liver (16). Second, we showed that administration of LAB enhances susceptibility to liver injury (ALT and AST), which is critical event in the course of liver fibrosis (17). Third, we observed that livers of LAB treated mice have increased translocation of bacterial LTA, which is believed to directly promote fibrosis (18). Accumulating evidence suggests that LTA is a ligand for PAFR as well as TLR2 signaling. Furthermore these receptors have been reported to express on hepatic stellate cells (HSCs) and Kupffer cells (KCs), which are responsible cell type for liver fibrognenesis (11,19).
In conclusion, LAB induce the profibrogenic milieu in response to BDL via induction of LTA translocation to liver and may stimulate the PAFR-mediated signaling in order to activate HSCs and KCs in liver fibrosis. Thus, the present study provides additional information of detrimental effect about LAB to pave the way for the development and evaluation of dietary constituents as health supplemental agents.
Supplementary Information
ACKNOWLEDGMENTS
This research was supported by a grant (NRF-2017R1D1A3B03030521) of the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Republic of Korea. It was also supported by the grant (NRF-2017R1C1B2004423) funded by the Ministry of Science and ICT, Republic of Korea, and by the research grant of the Chungbuk National University in 2016.
Footnotes
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to disclose.
REFERENCES
- 1.Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, Johnsen B, Shanman R, Slusser W, Fu N, Smith A, Roth B, Polak J, Motala A, Perry T, Shekelle PG. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep) 2011;200:1–645. [PMC free article] [PubMed] [Google Scholar]
- 2.Vaarala O. Immunological effects of probiotics with special reference to lactobacilli. Clin Exp Allergy. 2003;33:1634–1640. doi: 10.1111/j.1365-2222.2003.01835.x. [DOI] [PubMed] [Google Scholar]
- 3.Veckman V, Miettinen M, Pirhonen J, Siren J, Matikainen S, Julkunen I. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1-type cytokines and chemokines in human monocyte-derived dendritic cells. J Leukoc Biol. 2004;75:764–771. doi: 10.1189/jlb.1003461. [DOI] [PubMed] [Google Scholar]
- 4.Braat H, de Jong EC, van den Brande JM, Kapsenberg ML, Peppelenbosch MP, van Tol EA, van Deventer SJ. Dichotomy between Lactobacillus rhamnosus and Klebsiella pneumoniae on dendritic cell phenotype and function. J Mol Med (Berl) 2004;82:197–205. doi: 10.1007/s00109-003-0509-9. [DOI] [PubMed] [Google Scholar]
- 5.Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299–3309. doi: 10.1128/IAI.72.6.3299-3309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi: 10.1016/S0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 7.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang SS, Ryu YH, Baik JE, Yun CH, Lee K, Chung DK, Han SH. Lipoteichoic acid from Lactobacillus plantarum induces nitric oxide production in the presence of interferon-gamma in murine macrophages. Mol Immunol. 2011;48:2170–2177. doi: 10.1016/j.molimm.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Vizoso Pinto MG, Rodriguez Gomez M, Seifert S, Watzl B, Holzapfel WH, Franz CM. Lactobacilli stimulate the innate immune response and modulate the TLR expression of HT29 intestinal epithelial cells in vitro. Int J Food Microbiol. 2009;133:86–93. doi: 10.1016/j.ijfoodmicro.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Chao W, Liu HL, Zhou WG, Hanahan DJ, Olson MS. Regulation of platelet-activating factor receptor and platelet-activating factor receptor-mediated biological responses by cAMP in rat Kupffer cells. J Biol Chem. 1990;265:17576–17583. [PubMed] [Google Scholar]
- 11.Chen Y, Wang CP, Lu YY, Zhou L, Su SH, Jia HJ, Feng YY, Yang YP. Hepatic stellate cells may be potential effectors of platelet activating factor induced portal hypertension. World J Gastroenterol. 2008;14:218–223. doi: 10.3748/wjg.14.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JW, Yun H, Choi SJ, Lee SH, Park S, Lim CW, Lee K, Kim B. Evaluating the influence of side stream cigarette smoke at an early stage of non-alcoholic steatohepatitis progression in mice. Toxicol Res. 2017;33:31–41. doi: 10.5487/TR.2017.33.1.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung YR, Lee YJ, Lee NJ, Lin CM, Moon JH, Chai HY, Kang JK. Inhibitory effect of 1-O-hexyl-2,3,5-trimethylhydroquinone on dimethylnitrosamine-induced liver fibrosis in male SD rats. Toxicol Res. 2010;26:193–201. doi: 10.5487/TR.2010.26.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han SH, Kim JH, Seo HS, Martin MH, Chung GH, Michalek SM, Nahm MH. Lipoteichoic acid-induced nitric oxide production depends on the activation of platelet-activating factor receptor and Jak2. J Immunol. 2006;176:573–579. doi: 10.4049/jimmunol.176.1.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, Bar-Sagi D, Frey AB, Miller G. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu R, Zhang Z, Wang FS. Liver fibrosis: mechanisms of immune-mediated liver injury. Cell Mol Immunol. 2012;9:296–301. doi: 10.1038/cmi.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330–1340.e1. doi: 10.1053/j.gastro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.