Abstract
Introduction
Although ART has improved the outcome of people living with HIV/AIDS, still some patients develop TB while receiving ART. The literature on the magnitude of this problem is still scarce in our setting especially northwestern Tanzania. This study was designed to determine the prevalence of active TB among HIV patients on ART and assess its potential risk factors.
Methods
A retrospective cohort study was done among adult HIV-positive patients initiated on ART at Bugando Medical Centre. Patients who were TB positive before ART initiation were excluded. Data regarding demographic, clinical, and laboratory information, TB status on receipt of ART, and time on ART were collected and analyzed using STATA 11 to determine the prevalence of TB and its associated factors.
Results
In total, 391 patients were enrolled in this study. The median age was 39 (32–46) years, and a total of 129 (32.99%) participants had CD4 counts <200 cells/µl and 179 (45.78%) had WHO stage 3 and 4 illnesses. A total of 43 (11.0%) participants developed TB while receiving ART which was independently associated with male gender (OR = 2.9; p=0.007), WHO clinical stage 3 and 4 (OR = 1.4; p=0.029), baseline CD4 count <200 cells/µl (OR = 9.1; p < 0.001), and having not used IPT (OR = 3.1; p=0.05).
Conclusions
Active TB is prevalent among HIV patients while receiving ART in northwestern Tanzania which is independently associated with male gender, advanced HIV disease, and nonuse of IPT. Universal HIV testing could reduce late HIV diagnosis and hence reduce the risk of developing TB while receiving ART in our setting. Also IPT should be widely used for those who are negative for TB on screening.
1. Background
HIV/AIDS is a continuing health problem globally that causes considerably high morbidity and mortality especially in resource-limited countries. It has so far caused more than 35 million deaths since its discovery, and as of 2015, there were about 37 million people who were living with HIV/AIDS [1]. Sub-Saharan Africa (SSA) is the most struck region of the world which harbors about 71% of the world's burden of HIV/AIDS [2] of whom more than 1.4 million people are living in Tanzania, representing 4% of all people living with HIV/AIDS globally [3].
The HIV virus infects CD4-positive cells as its host cells in which it replicates causing progressive lysis and reduction of the number and quality of functional immune cells [4–6]. With time, the body fails to control the viral replication and immune paresis sets in, being marked by low CD4 counts with increased morbidity and mortality from opportunistic infections [7] with tuberculosis being the most common opportunistic presentation at HIV diagnosis [1].
TB/HIV is the most common coinfection which still carries high mortality and morbidity worldwide. The 2016 WHO report indicates that, in 2015, there were 10.4 million new TB cases worldwide with 11% of these cases being HIV coinfected. Additionally, there were 1.8 million deaths worldwide with 0.4 million occurring among HIV-positive patients [8]. Tuberculosis occurs as the first manifestation of HIV/AIDS in more than 50% of HIV-positive patients [9], and deaths that are linked to TB are significantly high especially in sub-Saharan Africa, where in some countries, this rate is reported to be in excess of 50% [10].
The advent of ART has generally improved the prognosis of PLHA [11, 12], as reflected by an overall reduction of HIV/AIDS-related morbidity and mortality and improved survival among PLHA [13, 14]. With use of ART, occurrence of TB has been reduced by 67–80% in most study settings [15, 16]. Even with these advantages of ART, still a significant proportion of patients on ART develop active TB with a varying prevalence rate of 2.5–30.1% in most studies [17, 18]. In our setting, the literature on the magnitude of this problem is still scarce especially in the northwestern part of Tanzania. So, the aim of this study was to determine the prevalence of active TB among HIV-positive patients while receiving ART at Bugando Medical Centre in the northwestern part of Tanzania.
2. Materials and Methods
This was a retrospective cohort hospital-based study which was conducted at Bugando Medical Centre (BMC), HIV Care and Treatment Centre (CTC), between August 2016 and May 2017. Bugando is a tertiary and teaching university hospital for the lake and Western zone of the United Republic of Tanzania. It is located along the shores of Lake Victoria in Mwanza city. It has a catchment population of more than 16 million people from 8 catchment regions, namely, Mara, Mwanza, Geita, Shinyanga, Simiyu, Tabora, Katavi, and Kigoma. It has a bed capacity of about 1000 and more than 1000 employees, running both inpatients' and outpatients' services. CTC activities are part and parcel of routine outpatients' activities as a referral centre. HIV patients are routinely enrolled for care and treatment services from within the hospital and some from nearby facilities. At diagnosis of HIV, patients are usually screened for TB which is done using a symptom-based tool as per national TB and leprosy (NTL) guidelines [19], which includes presence of cough ≥2 weeks, hemoptysis ≥2 weeks, excessive night sweats ≥2 weeks, fever ≥2 weeks, and noticeable weight of ≥3 kilogram over 4 weeks. Patients with one or more of these symptoms undergo further testing including sputum smear and chest radiography, and those diagnosed to have TB get started on standard treatment for TB and HIV. Currently, patients who test negative for TB screen are started on isoniazid preventive therapy (IPT) which has recently been adopted in Tanzania. On subsequent follow-up, patients are also routinely screened for TB to be able to diagnose them as early as possible to improve their outcome while receiving ART. About 400–600 patients are diagnosed with TB every year.
This study included all HIV patients who were diagnosed and initiated on ART at BMC. Patients who were diagnosed to have TB before initiation of ART were excluded from the study. A minimum sample size of 384 patients was estimated using the Kish Lisle formula (1965) for cross-sectional studies assuming 10% of HIV patients developed TB while receiving ART [20] with an allowable error of 0.03 at 95% confidence interval (CI).
A CTC database was reviewed to identify all patients who were TB negative at initiation of ART. Patients' file numbers were used to retrieve the files. From the files, data of research interest were retrieved and recorded, including age, gender, address, occupation and marital status, TB status as YES or NO, and if yes, then timing of TB in months while on ART use, WHO clinical stage, on diagnosis opportunistic infection, baseline CD4, current CD4, full blood picture, and ART regimen.
The data were double entered and cleaned using Epi Info, and data analysis was done using STATA 11 software. All continuous variables were summarized as medians with interquartile range while the categorical variables were expressed as proportions with percentages. The proportion of patients who developed TB while receiving ART was calculated and expressed as percentage, and the logistic regression model was used to determine the odds ratios and 95% CI to find out the degree of association between the outcome of interest and the potential predictors of TB while using ART. In all our calculations, factors were said to have a significant statistical association with the outcome of interest if p < 0.05.
2.1. Ethical Consideration
Ethical clearance was sought from the Faculty of Medicine and from the joint Bugando Medical Centre (BMC) and CUHAS research and ethical committee. To maintain the confidentiality, the files of the patients were handled by researchers alone, and patients' identifiers were not used in analysis.
3. Results
3.1. General Characteristics of the Study Population
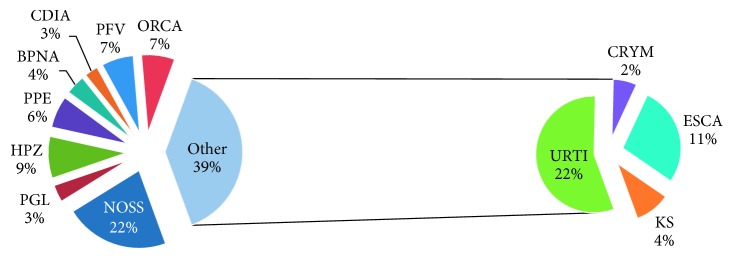
A total of 391 patients were enrolled in this study. The median age was 39 (32–46) years, and most patients, 295 (75.45%), were females. Of the study participants, 164 (41.94%) were married and about a third, 128 (32.74%), were doing small business. Of the studied patients, 129 (32.99%) presented with severe immune suppression and 179 (45.78%) had WHO clinical stage 3 and 4 AIDS-defining illnesses on diagnosis of HIV (Table 1) with distribution of opportunistic conditions as summarized in Figure 1. Furthermore, the majority of the studied patients, 375 (95.90%), were on tenofovir- (TDF-) based regimens, while the rest were on zidovudine- (ZVD-) based regimens (Figure 2).
Table 1.
Distribution of demographic, clinical, and laboratory characteristics among 391 adult HIV-positive study participants.
| Variables | Frequency | Percentage or median (IQR) |
|---|---|---|
| Age in years | 391 | 39 (32–46) |
| Gender | ||
| Male | 096 | 24.55 |
| Female | 295 | 75.45 |
| Marital status | ||
| Single | 054 | 13.81 |
| Married | 164 | 41.94 |
| Widow | 061 | 15.60 |
| Divorce | 112 | 28.64 |
| Occupation | ||
| Formal employment | 025 | 06.39 |
| Peasant | 102 | 26.09 |
| Small business | 128 | 32.74 |
| Driver | 012 | 03.07 |
| Student | 009 | 02.30 |
| Housewife | 041 | 10.49 |
| Others | 074 | 18.93 |
| BMI categories | ||
| Under WT | 062 | 15.86 |
| Normal WT | 225 | 57.54 |
| Over WT | 070 | 17.90 |
| Obese | 034 | 8.70 |
| WHO stage | ||
| 3 and 4 | 179 | 45.78 |
| 1 and 2 | 212 | 54.22 |
| Baseline CD4 in cells/µl | 289 (125–509) | |
| <200 | 129 | 32.99 |
| ≥200 | 262 | 67.01 |
| HB in g/dL | 11 (9.6–12.6) | |
| Anemia | ||
| Yes | 189 | 48.34 |
| No | 202 | 51.66 |
| Duration on ART (mo) | 15 (11–21) | |
| IPT use status | ||
| No | 103 | 26.34 |
| Yes | 288 | 73.66 |
| TB while on ART | ||
| Yes | 043 | 11.00 |
| No | 348 | 89.00 |
ART: antiretroviral therapy; HB: hemoglobin; CD4: cluster of differentiation 4; IPT: isoniazid preventive therapy; IQR: interquartile range; mo: months; TB: tuberculosis; WHO: World Health Organization; WT: weight.
Figure 1.
The distributions of opportunistic infection at diagnosis of HIV. BPNA: recurrent bacterial pneumonia; CDIA: chronic diarrhoea; CRYM: cryptococcal meningitis; ESCA: esophageal candidiasis; HPZ: Herpes zoster; KS: Kaposi sarcoma; NOSS: asymptomatic; ORCA: oral candidiasis; PFV: persistent fever; PGL: persistent generalized lymphadenopathy; PPE: pruritic.
Figure 2.
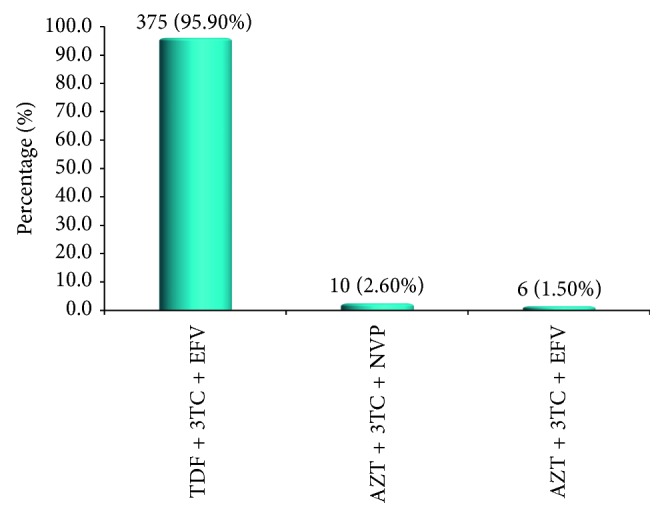
The distribution of ART regimens among 391 study participants. AZT: zidovudine; EFV: efavirenz; 3TC: lamivudine; NVP: nevirapine; TDF: tenofovir.
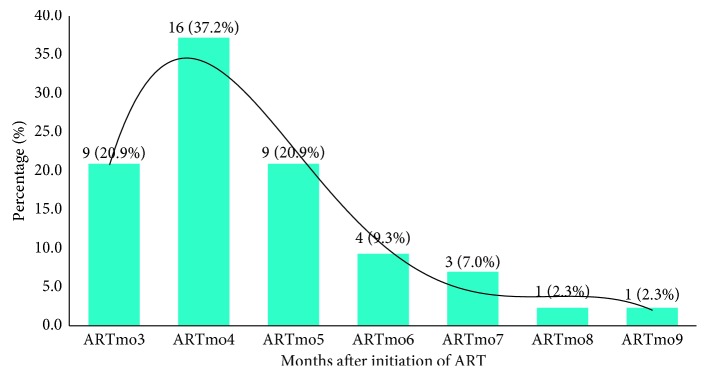
Of the studied patients, 43 (11.0%) developed TB while receiving ART (Table 1), whereby more than 88% of this TB occurred within the first six months of ART, as summarized in Figure 3. The odds of developing active TB while receiving ART were independently associated with male gender (OR = 2.9; p=0.007), having WHO clinical stage 3 and 4 AIDS-defining illness at baseline (OR = 1.4; p=0.029), lower CD4 count than 200 cells/µl (OR = 9.1; p < 0.001), and having not used IPT (OR = 3.1; p=0.05). The difference in distribution of other factors was not statistically significant (Table 2).
Figure 3.
The distribution of TB occurrence by time on ART.
Table 2.
Univariate and multivariate analysis for factors associated with development of active TB while receiving ART.
| Variables | TB while receiving ART | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|
| Yes (N=43) | No (N=348) | OR (95% CI) | p value | OR (95% CI) | p value | |
| Gender | ||||||
| Male | 24 (55.81) | 072 (20.69) | ||||
| Female | 19 (44.19) | 276 (79.31) | 4.8 (2.5–9.3) | <0.001 | 2.9 (1.3–6.2) | 0.007 |
| Age group | ||||||
| ≥50 years | 07 (16.28) | 005 (16.67) | ||||
| <50 years | 36 (83.72) | 290 (83.33) | 1.0 (0.4–2.2) | 0.949 | ||
| Marital status | ||||||
| Single | 04 (09.30) | 050 (14.37) | 0.6 (0.2–1.7) | 0.368 | ||
| Married | 21 (48.84) | 143 (41.09) | 1.4 (0.7–2.5) | 0.333 | ||
| Divorced | 17 (39.53) | 095 (27.30) | 1.7 (0.9–3.3) | 0.166 | ||
| Widowed | 01 (02.33) | 060 (17.24) | 0.1 (0.0–0.8) | 0.034 | ||
| Occupation | ||||||
| Formal | 02 (04.65) | 023 (06.61) | 0.7 (0.2–3.0) | 0.622 | ||
| Peasant | 06 (13.95) | 096 (27.59) | 0.4 (0.2–1.0) | 0.061 | ||
| Petty business | 18 (41.86) | 110 (31.61) | 1.5 (0.8–2.9) | 0.179 | ||
| Driver | 04 (09.30) | 008 (02.30) | 4.3 (1.2–15.0) | 0.027 | 2.5 (0.4–13.3) | 0.294 |
| Student | 02 (04.65) | 007 (02.01) | 2.3 (0.4–11.8) | 0.290 | ||
| H/wife | 05 (11.63) | 036 (10.34) | 1.1 (0.4–3.0) | 0.796 | ||
| Others | 06 (13.95) | 058 (19.54) | 0.7 (0.2–1.6) | 0.380 | ||
| Nutrition status | ||||||
| Under WT | 14 (32.56) | 048 (13.79) | 3.0 (1.4–6.1) | 0.002 | 1.4 (0.6–3.2) | 0.463 |
| Normal WT | 21 (48.84) | 204 (58.62) | 0.7 (0.3–1.2) | 0.223 | ||
| Over WT | 06 (13.95) | 064 (18.39) | 0.7 (0.3–1.8) | 0.476 | ||
| Obese | 02 (03.23) | 053 (08.76) | 0.4 (0.1–2.0) | 0.329 | ||
| WHO stage | ||||||
| 3 and 4 | 31 (72.09) | 148 (42.53) | ||||
| 1 and 2 | 12 (27.91) | 200 (57.47) | 3.5 (1.7–7.0) | <0.001 | 2.4 (1.0–5.2) | 0.029 |
| Baseline CD4 | ||||||
| <200 cells/µl | 35 (81.40) | 094 (27.01) | ||||
| ≥200 cells/µl | 08 (18.60) | 254 (72.99) | 11.8 (5.2–26.4) | <0.001 | 9.1 (3.9–21.0) | <0.001 |
| Anemia | ||||||
| Yes | 22 (51.16) | 167 (47.99) | ||||
| No | 21 (48.84) | 181 (52.01) | 1.1 (0.6–2.1) | 0.694 | ||
| IPT use | ||||||
| No | 39 (90.70) | 249 (71.55) | ||||
| Yes | 04 (09.30) | 099 (28.45) | 3.8 (1.3–11.1) | 0.012 | 3.1 (1.0–9.4) | 0.05 |
| ART regimen | ||||||
| TDF/3TC/EFV | 39 (90.70) | 336 (96.55) | 0.3 (0.1–1.1) | 0.080 | ||
| AZT/3TC/EFV | 02 (04.65) | 004 (01.15) | 4.1 (0.7–23.6) | 0.104 | ||
| AZT/3TC/NVP | 02 (04.65) | 008 (02.30) | 2.0 (0.4–10.1) | 0.367 | ||
ART: antiretroviral therapy; AZT: zidovudine; CI: confidence interval; CD4: cluster of differentiation 4; EFV: efavirenz; 3TC: lamivudine; H/wife: housewife; IPT: isoniazid preventive therapy; NVP: nevirapine; TDF: tenofovir; WT: weight.
4. Discussion
The objective of this study was to determine the prevalence of TB among adult HIV-positive patients while receiving ART and assess the associated risk factors at Bugando HIV Care and Treatment Centre. Of the 391 studied patients, 43 (11.0%) were found to develop TB while receiving ART, and the risk of developing TB was independently increased among male patients, those with WHO clinical stage 3 and 4 AIDS illnesses and CD4 <200 cells/µl, and those who did not use IPT.
The prevalence rate of TB while receiving ART in the index study is similar to a previous prevalence rate of 10% reported from South Africa in 2011 [20], and it is also similar to a rate reported in 2015 from Muhimbili, where a total of 67686 were enrolled and 7602 (11.2%) were found to develop active TB while on ART follow-up program [21]. However, a much lower rate of TB was reported in 2014 from a study involving 1824 ART experienced patients in Mexico where only 45 (2.47%) developed active TB [17]. Another smaller TB rate of 5.6% was reported from the Netherlands in 2010 [22]. On the other hand, a much higher prevalence of TB was reported from a recent study in South Africa by Gupta involving 1544 patients on ART where 424 (30.1%) developed active TB in a course of 5 years [18]. The difference in TB rates could partly be explained by the overall prevalence of TB and HIV which is lower in most of the developed countries as compared to resource-restricted countries. However, even with these differences, it is important to note that though ART has significant effect on the overall occurrence of TB among HIV patients [16, 23], the occurrence of TB while receiving ART is still higher as compared to the general population as reported previously [24, 25], and it has been noted to be associated with increased mortality rate in this subgroup of patients [26, 27].
A number of factors were investigated for their potential association with the occurrence of TB while receiving ART. In the index study, male patients were found to have an increased risk of developing TB. Several studies have had similar findings including a study from South Africa by Gupta and colleagues [28], a study from Dar es Salaam Tanzania by Liu et al. [21], and also a study from Mexico by Martin-Echevarria et al. [17]. The social behavior and hormonal-related differences between male and female susceptibility to TB have been suggested as potential explanation of TB predominance among adult male patients [29, 30] as also supported by fewer studies demonstrating high prevalence of TB among female patients [31, 32]. On the other hand, gender difference in susceptibility to TB is clinically important since it has also been shown that male patients have higher risk of mortality. For instance, in a study assessing mortality in Uganda where male patients were found to be more severely ill with most of WHO clinical stage 3&4 AIDS-defining illnesses (36% versus 33%; p < 0.0001), they were also demonstrated to have 37% higher risk of death than female patients on receipt of ART (OR = 1.37; p < 0.001) [33].
Patients who had advanced HIV disease were also found to have an increased risk of developing TB while receiving ART in the index study. In Burkina Faso, patients who had lower CD4 count at diagnosis were also shown to have an increased risk of developing TB [34]. Another study from Nigeria reported similar findings that patients who had lower baseline CD4 counts <200 cells/µl and those with prior history of TB had increased risk of developing TB while receiving ART [35]. Reporting 8% prevalence rate of TB while receiving ART in addition to lower CD4 counts, patients who developed TB on ART were also most likely to have a positive tuberculin skin test and prior history of admission [36]. Patients who start ART at the baseline CD4 counts <200 cells/µl have frequently been demonstrated to have a subsequent poor immune recovery on receipt of ART [37, 38], taking a much long time before gaining an adequate immunity against most opportunistic infections (OIs) including TB [25]. They have also been shown to have an increased risk of both AIDS and non-AIDS-related morbidity and mortality [39, 40].
In the current study, it was also observed that 35 (81.40%) and 31 (72.09%) of those who developed TB had severe immune suppression and stage 3 and 4 AIDS-defining illness at the baseline, respectively. This is in agreement with several other studies. For instance, in 2005, Lawn and colleagues from South Africa indicated that patients who had WHO clinical stage 3 and 4 defining illnesses at the baseline were 3.6 times more likely to have TB on receipt of ART (AOR = 3.60; p=0.01) [24]. Also, in another study from Ethiopia by Melkamu et al., it was found that development of active TB while receiving ART was significantly higher among those patients who were in WHO clinical stage 3 and 4 as compared to those in WHO clinical stage 1 and 2 (AOR = 2.29; p=0.003) [41]. These findings suggest that strategies for early HIV diagnosis to increase timely diagnosis of HIV before it is adversely advanced could potentially reduce the occurrence of TB while receiving ART in our setting.
In this study also, patients who did not receive IPT while on ART were likely to develop TB as compared to those who received IPT. In 2015, one study from Ethiopia had a similar observation to our finding [42]. In this study, it was demonstrated that occurrence of TB while receiving ART was more common among those who did not use IPT as compared to those who used IPT (AHR = 2.41; p < 0.05). Similarly, a prior study in Dar es Salaam, Tanzania, by Liu et al. had indicated that patients who did not use IPT had increased risk of developing active TB while receiving ART (AHR = 2.25; p < 0.001) as compared to those who were on IPT [21]. These findings suggest that occurrence of TB while receiving ART is still a common problem in Tanzania as well and IPT could potentially reduce the TB-related morbidity and mortality in these patients. The use of IPT alone has been shown to reduce the risk of active TB by 32% among those living with HIV [43], whereas ART alone has been shown elsewhere to reduce the risk of active TB by up to 67% [15] and risk of mortality by 64–95% [44] especially when initiated timely. Concomitant use of ART and IPT was reported previously to have a much greater effect on incidence of TB of up to 80% [45].
In conclusion, these findings suggest that active TB is still a common problem among patients receiving ART in our settings. Patients who are at increased risk of developing active TB while receiving ART include male patients, those who are diagnosed with advanced HIV disease, and those who do not receive IPT. A timely diagnosis and treatment of HIV could potentially reduce the incidence of TB while receiving ART. These results also support the use of IPT among patients who are negative for TB to reduce the magnitude of this problem while receiving ART.
Acknowledgments
The authors would like to acknowledge the support given by all staff members at Bugando CTC during the study.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Daniel W. Gunda designed the study, acquired and analyzed the data, interpreted the data, and drafted the manuscript. Simon C. Maganga assisted in designing the study and acquiring the data and critically reviewed the manuscript for its intellectual content. Igembe Nkandala, Semvua B. Kilonzo, Bonaventura C. Mpondo, and Elichilia R. Shao assisted with data analysis and critically reviewed the manuscript for its intellectual content.
References
- 1.UNAIDS. HIV Fact Sheet 2016, Global Statistics. Geneva, Switzerland: UNAIDS; 2016. [Google Scholar]
- 2.WHO. Global Health Observatory (GHO) Data. Geneva, Switzerland: WHO/HIV-AIDS; 2015. [Google Scholar]
- 3.MOHSW. Global AIDS Response Country Progress Report. Dar es salaam, Tanzania: MOHSW; 2014. [Google Scholar]
- 4.Martin N., Sattentau Q. Cell-to-cell HIV-1 spread and its implications for immune evasion. Current Opinion in HIV and AIDS. 2009;4(2):143–149. doi: 10.1097/coh.0b013e328322f94a. [DOI] [PubMed] [Google Scholar]
- 5.Alexaki A., Liu Y., Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Current HIV Research. 2008;6(5):388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford E. S., Puronen C. E., Sereti I. Immunopathogenesis of asymptomatic chronic HIV Infection: the calm before the storm. Current Opinion in HIV and AIDS. 2009;4(3):206–214. doi: 10.1097/coh.0b013e328329c68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks J. T., Kaplan J. E., Holmes K. K., Benson C., Pau A., Masur H. HIV-associated opportunistic infections–going, going, but not gone: the continued need for prevention and treatment guidelines. Clinical Infectious Diseases. 2009;48(5):609–611. doi: 10.1086/596756. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Global Tuberculosis Report 2016. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 9.WHO. Interim Policy on Collaborative TB/HIV Activities. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 10.WHO. Global tuberculosis control 2011. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 11.Palella F. J., Jr., Delaney K. M., Moorman A. C., et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. New England Journal of Medicine. 1998;338(13):853–860. doi: 10.1056/nejm199803263381301. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi M. K. What changes are needed to the current direction and interpretation of clinical cancer research to meet the needs of the 21st century? Medical Journal of Australia. 2009;190(8):p. 461. doi: 10.5694/j.1326-5377.2009.tb02508.x. [DOI] [PubMed] [Google Scholar]
- 13.Reniers G., Slaymaker E., Nakiyingi-Miiro J., et al. Mortality trends in the era of antiretroviral therapy: evidence from the Network for Analysing Longitudinal Population based HIV/AIDS data on Africa (ALPHA) AIDS. 2014;28(4):S533–S542. doi: 10.1097/qad.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M., May M., Chêne G., et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. The Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 15.Lawn S. D., Wood R., De Cock K. M., Kranzer K., Lewis J. J., Churchyard G. J. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. The Lancet Infectious Diseases. 2010;10(7):489–498. doi: 10.1016/s1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 16.Badri M., Wilson D., Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. The Lancet. 2002;359(9323):2059–2064. doi: 10.1016/s0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Echevarria E., Serrano-Villar S., Sainz T., et al. Development of tuberculosis in human immunodeficiency virus infected patients receiving antiretroviral therapy. International Journal of Tuberculosis and Lung Disease. 2014;18(9):1080–1084. doi: 10.5588/ijtld.13.0757. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A., Wood R., Kaplan R., Bekker L.-G., Lawn S. D. Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055824.e55824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MOHSW. Manual of the National Tuberculosis and Leprosy programme in Tanzania. 5th. Dar es Salaam, Tanzania: Ministry of Health and Social Welfaire; 2006. [Google Scholar]
- 20.Van Rie A., Westreich D., Sanne I. Tuberculosis in patients receiving antiretroviral treatment: incidence, risk factors, and prevention strategies. Journal of Acquired Immune Deficiency Syndromes. 2011;56(4):349–355. doi: 10.1097/qai.0b013e3181f9fb39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu E., Makubi A., Drain P., et al. Tuberculosis incidence rate and risk factors among HIV-infected adults with access to antiretroviral therapy. AIDS. 2015;29(11):1391–1399. doi: 10.1097/qad.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermans S. M., Kiragga A. N., Schaefer P., Kambugu A., Hoepelman A. I. M., Manabe Y. C. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010527.e10527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girardi E., Antonucci G., Vanacore P., et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14(13):1985–1991. doi: 10.1097/00002030-200009080-00015. [DOI] [PubMed] [Google Scholar]
- 24.Lawn S. D., Badri M., Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19(18):2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 25.Lawn S. D., Myer L., Edwards D., Bekker L.-G., Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23(13):1717–1725. doi: 10.1097/qad.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habte E., Yami A., Alemseged F., et al. Predictors of HIV serodiscordance among couples in Southwestern Ethiopia. Journal of the International Association of Providers of AIDS Care. 2015;14(3):234–240. doi: 10.1177/2325957413488177. [DOI] [PubMed] [Google Scholar]
- 27.Moore D. J., Kim J. I., Sonawane S., et al. Progress toward antibody-induced transplantation tolerance. Critical Reviews™ in Immunology. 2007;27(2):167–218. doi: 10.1615/critrevimmunol.v27.i2.40. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A., Wood R., Kaplan R., Bekker L.-G., Lawn S. D. Tuberculosis incidence rates during 8 years of follow-up of an antiretroviral treatment cohort in South Africa: comparison with rates in the community. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0034156.e34156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neyrolles O., Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Medicine. 2009;6(12) doi: 10.1371/journal.pmed.1000199.e1000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uplekar M. W., Rangan S., Weiss M. G., Ogden J., Borgdorff M. W., Hudelson P. Attention to gender issues in tuberculosis control. International Journal of Tuberculosis and Lung Disease. 2001;5(3):220–224. [PubMed] [Google Scholar]
- 31.Codlin A. J., Qadeer E., Ara I., et al. Gender differences in tuberculosis notification in Pakistan. American Journal of Tropical Medicine and Hygiene. 2011;85(3):514–517. doi: 10.4269/ajtmh.2011.10-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onifade D. A., Bayer A. M., Montoya R., et al. Gender-related factors influencing tuberculosis control in shantytowns: a qualitative study. BMC Public Health. 2010;10(1):p. 381. doi: 10.1186/1471-2458-10-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubaihayo J., Tumwesigye N. M., Konde-Lule J., et al. Trends and predictors of mortality among HIV positive patients in the era of highly active antiretroviral therapy in Uganda. Infectious Disease Reports. 2015;7(3):p. 5967. doi: 10.4081/idr.2015.5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dembele M., Saleri N., Carvalho A. C., et al. Incidence of tuberculosis after HAART initiation in a cohort of HIV-positive patients in Burkina Faso. International Journal of Tuberculosis and Lung Disease. 2010;14(3):318–323. [PubMed] [Google Scholar]
- 35.Pathmanathan I., Dokubo E. K., Shiraishi R. W., et al. Incidence and predictors of tuberculosis among HIV-infected adults after initiation of antiretroviral therapy in Nigeria, 2004-2012. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173309.e0173309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miranda A., Morgan M., Jamal L., et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995-2001. PLoS One. 2007;2(9) doi: 10.1371/journal.pone.0000826.e826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore R. D., Keruly J. C. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clinical Infectious Diseases. 2007;44(3):441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 38.Hunt P. W., Deeks S. G., Rodriguez B., et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17(13):1907–1915. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 39.Baker J. V., Peng G., Rapkin J., et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;48(5):541–546. doi: 10.1097/qai.0b013e31817bebb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichtenstein K. A., Armon C., Buchacz K., et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clinical Infectious Diseases. 2010;51(4):435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 41.Melkamu H., Seyoum B., Dessie Y. Determinants of tuberculosis infection among adult HIV positives attending clinical care in western Ethiopia: a case-control study. AIDS Research and Treatment. 2013;2013:7. doi: 10.1155/2013/279876.279876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assebe L. F., Reda H. L., Wubeneh A. D., Lerebo W. T., Lambert S. M. The effect of isoniazid preventive therapy on incidence of tuberculosis among HIV-infected clients under pre-ART care, Jimma, Ethiopia: a retrospective cohort study. BMC Public Health. 2015;15(1):p. 346. doi: 10.1186/s12889-015-1719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akolo C., Woldehanna S. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.cd000171.pub2.CD000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawn S. D., Kranzer K., Wood R. Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clinics in Chest Medicine. 2009;30(4):685–699. doi: 10.1016/j.ccm.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yirdaw K. D., Jerene D., Gashu Z., et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104557.e104557 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.