Abstract
Piper is the largest genus of the Piperaceae family. The species of this genus have diverse biological activities and are used in pharmacopeia throughout the world. They are also used in folk medicine for treatment of many diseases in several countries including Brazil, China, India, Jamaica, and Mexico. In Brazil, Piper species are distributed throughout the national territory, making this genus a good candidate for biological activity screening. During our studies with Piper essential oils, we evaluated its activity against Rhizopus oryzae, the main agent of mucormycosis. The main compounds of seven Piper essential oils analyzed were Piper callosum—safrole (53.8%), P. aduncum—dillapiole (76.0%), P. hispidinervum—safrole (91.4%), P. marginatum—propiopiperone (13.2%), P. hispidum—γ-terpinene (30.9%), P. tuberculatum—(E)-caryophyllene (30.1%), and Piper sp.—linalool (14.6%). The minimum inhibitory concentration of Piper essential oils against R. oryzae ranged from 78.12 to >1250 μg/mL. The best result of total inhibition of biofilm formation was obtained with Piper sp. starting from 4.88 μg/mL. Considering the bioactive potential of EOs against planktonic cells and biofilm formation of R. oryzae could be of great interest for development of antimicrobials for therapeutic use in treatment of fungal infection.
1. Introduction
Piper is the largest genus of the Piperaceae family. The species of this genus have diverse biological activities and are used in pharmacopeia throughout the world. They are also used in folk medicine for treatment of many diseases in several countries including Brazil, China, India, Jamaica, and Mexico. In Brazil, Piper species are distributed throughout the national territory. Among the aromatic flora of the Amazon region, there are more than a dozen species that provide essential oils that are used by the population for therapeutic purposes. The tea of the decoction of Piper hispidum leaves is useful for the treatment of malaria. Piper marginatum is used as a tonic, carminative, stimulant, diuretic, and sudorific agent against stomach, liver and gallbladder pain, toothaches, and snake and insect bites [1]. Regasini et al. [2] related trypanocidal activity of the Piper tuberculatum extract.
Zygomycosis, also referred to as phycomycosis or mucormycosis, is an aggressive and rapidly progressive infection that primarily occurs in immunocompromised patients. Members of the genera Rhizopus, Mucor, and Absidia are the organisms most commonly isolated from patients with zygomycosis. Rhizomucor, Cunninghamella, Apophysomyces, and Saksenaea are other zygomycetes that have been implicated in human diseases. Amphotericin B, as well as its lipid formulation, has been essential for treatment for several decades [3].
The purpose of the present work was to evaluate the anti-Rhizopus oryzae activity of Piper aduncum, P. hispidinervum, P. callosum, P. hispidum, P. tuberculatum, P. marginatum, and Piper sp. essential oil of leaves.
2. Materials and Methods
2.1. Plant Material and Essential Oil Extraction
Plant material was obtained from EMBRAPA Experimental Farm, Amazonas, Brazil. A voucher of each specimen was deposited at Federal Agrotechnical School of Machado Herbarium (Table 1). Leaves of Piper species were collected between 8 and 9 a.m., dried at room temperature, and coarsely ground into powder just before distillation. The oil was obtained by hydrodistillation in a modified Clevenger apparatus for 5 h [4].
Table 1.
Deposit number and deposit location of plant material.
| Plant material | Deposit number | Deposit location | Name of herbarium |
|---|---|---|---|
| Piper aduncum | 10,480 | INPA1 | INPA herbarium |
| Piper tuberculatum | 6,797 | IFAM2 | EAFM herbarium |
| Piper hispidum | 6,796 | IFAM | EAFM herbarium |
| Piper marginatum | 6,798 | IFAM | EAFM herbarium |
| Piper callosum | 6,794 | IFAM | EAFM herbarium |
| Piper hispidinervum | IFAM | EAFM herbarium | |
| Piper sp. | IFAM | EAFM herbarium |
1National Institute of Amazonas Research; 2Federal Institute of Amazonas.
2.2. Essential Oil Analyses
Sample of each Piper essential oil was analyzed in an Agilent 6890 N gas chromatograph fitted with a 5% diphenyl-95% dimethylpolysiloxane capillary column (DB-5MS, 30 m × 0.25 mm × 0.25 μm). The results were compared to data from the literature [5].
2.3. Antifungal Activity Assay
The antifungal activity of Piper essential oils was evaluated against R. oryzae (UCP1506). The strain used belongs to the culture collection of the “Universidade Católica de Pernambuco,” located in the Nucleus of Research in Environmental Sciences, Catholic University of Pernambuco, Brazil, NPCIAMB/UNICAP. The culture collection is registered in the WFCC.
The microdilution broth method was used according to CLSI reference document M38-A [6] for filamentous fungi. Briefly, the cells were grown in RPMI-MOPS (pH 7.2) for 18 h at 30°C in the presence of different concentrations (1.22 to 1250 μg/mL) of each essential oil. Positive and negative growth controls were performed. Amphotericin B (Sigma) was used as a reference drug, and stock solution was made at 20 mg/mL in sterile distilled water. All experiments were performed in duplicate and repeated twice.
In order to evaluate the fungicide/fungistatic properties of Piper essential oils, a 10 μL aliquot was collected from the inhibited cultures and dropped on the surface of potato dextrose agar. The absence or presence of growth in the solid medium was evaluated after 48 h incubation period at 30°C.
2.4. Biofilm Formation
The influence of Piper essential oils on biofilm formation was determined as described by Singh et al. [7]. Briefly, spores of R. oryzae were put in 96-well microtiter plate at 5 × 104 cells per mL in RPMI and treated with twofold serial dilution of each Piper essential oil. After incubation for 18 h at 30°C, the culture media was removed and the wells were washed twice with PBS 0.01 M and pH 7.2. Biofilms were stained with 200 µL of 0.1% safranin for 5 min. Then, the supernatants were removed, and the wells were washed twice with PBS. Finally, 200 µL of 30% glacial acetic acid was added to the microplates in order to elute safranine from the matrix. Biofilm formation was estimated by spectrophotometry (SpectraMax M5) at 490 nm.
2.5. Red Blood Cell Lysis Assay
The hemolytic activity was evaluated by Franca Rodrigues et al. [8] by mixing 80 μL of a 5% suspension of fresh human red blood cells (O+) in PBS with 20 μL of different concentrations of Piper sp. essential oil and incubating at 37°C for 1 h. The reaction was slowed by adding 200 μL of PBS, and the suspension was centrifuged (1000 g for 10 min). The supernatant was transferred to a 96-well plate, and cell lysis was quantified by spectrophotometrical measurement of absorbance at 540 nm, as previously described. The maximal lysis and blank control were obtained by replacing the extract sample with an equal volume of PBS or distilled water, respectively.
2.6. Rhizopuspepsin Inhibition
In order to evaluate a possible mode of action of the Piper essential oils, the inhibition of rhizopuspepsin (Sigma) activity was determined as previously described by Buroker-Kilgore and Wang with some modifications [9]. First, 59 μL of the rhizopuspepsin solution was mixed with 1 μL inhibitor, 20 μL BSA (1 mg/mL), and 20 μL buffer (pH 3.0). After 1 h incubation at 37°C, 100 μL of Bradford solution (0.025% Coomassie Blue G-250, 11.75% ethanol, and 21.25% phosphoric acid) previously diluted (1 : 1) was added. Negative control was performed by adding the substrate immediately after the incubation period. Finally, the plate was read on a spectrophotometer (SpectraMax M5) at 595 nm. One unit of enzyme activity was defined as the total enzyme that causes an increase of 0.001 in unit of absorbance under the conditions of the standard assay. The inhibitors tested were Piper essential oils (48 μg/mL) and 10 mM Pepstatin A (standard inhibitor).
2.7. Antioxidant Activity of Piper spp. Essential Oils
The antioxidant activity was evaluated qualitatively [10, 11] by application of 0.5 μL of each essential oil and 7-hydroxycalamenene (as standard) on a plate of silica gel 60 F254 and eluted with hexane-ethyl acetate (9 : 1). The plates were treated with a 0.2% methanolic solution of DPPH and read just after spraying and after 45 min.
3. Results and Discussion
The average oil yield obtained was 0.65% (dry wt.). The compounds present in the essential oils from Piper species used are shown in Table 2. Quantitative and/or qualitative variations were observed among samples of Piper.
Table 2.
Main components from Piper spp. essential oils.
| Area (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak | LRI calc | LRI lit | Identification | P. callosum | P. aduncum | P. hispidinervum | P. marginatum | P. hispidum | P. tuberculatum | Piper sp. |
| 1 | 924 | 924 | α-Thujene | 0.1 | 0.1 | 0.1 | ||||
| 2 | 931 | 932 | α-Pinene | 12.2 | 1.5 | 0.1 | 2.0 | 1.3 | 9.4 | |
| 3 | 946 | 946 | Canphene | 0.4 | 0.1 | 0.1 | ||||
| 4 | 971 | 969 | Sabinene | 3.0 | 0.1 | |||||
| 5 | 975 | 974 | β-Pinene | 7.7 | 3.5 | 1.5 | 1.1 | 15.0 | ||
| 6 | 985 | 981 | 6-Methyl-5-hepten-2-one | 0.6 | ||||||
| 7 | 989 | 990 | Myrcene | 0.6 | 0.1 | 1.3 | 1.2 | |||
| 8 | 1004 | 1002 | α-Phellandrene | 0.1 | 1.5 | 0.5 | ||||
| 9 | 1009 | 1008 | δ-3-Carene | 0.2 | 11.3 | 0.4 | ||||
| 10 | 1014 | 1014 | α-Terpinene | 0.7 | 0.2 | 14.4 | ||||
| 11 | 1022 | 1022 | p-Cimene | 0.3 | 0.3 | 0.3 | 0.3 | 12.1 | ||
| 12 | 1025 | 1024 | Limonene | 0.7 | 0.4 | 0.2 | 1.6 | |||
| 13 | 1026 | 1025 | β-Phellandrene | 1.0 | 1.4 | |||||
| 14 | 1028 | 1026 | 1,8-Cineole | 3.7 | ||||||
| 15 | 1034 | 1032 | (Z)-β-Ocimene | 0.4 | 0.3 | 6.0 | ||||
| 16 | 1044 | 1044 | (E)-β-Ocimene | 0.8 | 0.7 | 8.3 | ||||
| 17 | 1055 | 1054 | γ-Terpinene | 1.8 | 0.3 | 30.9 | ||||
| 18 | 1085 | 1086 | Terpinolene | 0.5 | 1.2 | 0.3 | 7.3 | |||
| 19 | 1098 | 1098 | Linalool | 0.3 | 0.2 | 1.1 | 14.6 | |||
| 20 | 1134 | 1135 | trans-Pinocarveol | 0.2 | ||||||
| 21 | 1152 | 1155 | Isoborneol | 0.1 | ||||||
| 22 | 1173 | 1174 | Terpinen-4-ol | 0.7 | 1.0 | |||||
| 23 | 1182 | 1179 | p-8-Cymenol | 1.0 | ||||||
| 24 | 1187 | 1186 | α-Terpineol | 0.5 | 0.1 | |||||
| 25 | 1193 | 1194 | Myrtenol | 0.1 | ||||||
| 26 | 1314 | 1285 | Safrole | 53.8 | 91.4 | 4.6 | ||||
| 27 | 1332 | 1335 | δ-Elemene | 0.3 | ||||||
| 28 | 1370 | 1374 | α-Copaene | 0.5 | 0.5 | 4.8 | 0.5 | 1.3 | ||
| 29 | 1379 | 1387 | β-Bourbonene | 0.9 | ||||||
| 30 | 1385 | 1387 | β-Cubebene | 0.3 | ||||||
| 31 | 1387 | 1389 | β-Elemene | 0.6 | 3.0 | |||||
| 32 | 1402 | 1403 | Methyl eugenol | 7.6 | 5.4 | |||||
| 33 | 1413 | 1417 | (E)-Caryophyllene | 0.7 | 6.0 | 0.3 | 6.3 | 5.3 | 30.1 | 14.4 |
| 34 | 1423 | 1430 | β-Copaene | 0.3 | 2.8 | |||||
| 35 | 1438 | 1439 | Aromadendrene | 1.4 | ||||||
| 36 | 1447 | 1452 | α-Humulene | 0.1 | 0.9 | 0.7 | 0.4 | 7.1 | ||
| 37 | 1450 | — | n.i. | 0.3 | ||||||
| 38 | 1456 | 1457 | Croweacin | 0.9 | ||||||
| 39 | 1468 | 1471 | 4,5-Di-epi-aristolochene | 0.3 | ||||||
| 40 | 1475 | 1476 | β-Chamigrene | 1.6 | ||||||
| 41 | 1480 | 1489 | β-Selinene | 1.7 | 8.1 | 2.6 | 5.5 | |||
| 42 | 1489 | 1498 | α-Selinene | 9.0 | 1.7 | 5.0 | ||||
| 43 | 1499 | 1500 | Epizonarene | 0.1 | ||||||
| 44 | 1471 | 1478 | γ-Muurolene | 0.4 | 1.6 | |||||
| 45 | 1474 | 1484 | Germacrene D | 1.0 | 0.6 | 2.9 | ||||
| 46 | 1488 | 1493 | Epi-cubebol | 0.4 | ||||||
| 47 | 1490 | 1494 | Bicyclogermacrene | 0.5 | 1.0 | 3.9 | ||||
| 48 | 1491 | 1494 | Sarisan | 0.3 | ||||||
| 49 | 1495 | 1500 | Pentadecane | 0.3 | 0.2 | |||||
| 50 | 1498 | 1505 | Germacrene A | 0.2 | ||||||
| 51 | 1500 | 1500 | α-Muurolene | 0.2 | ||||||
| 52 | 1500 | 1506 | β-Bisabolene | 9.1 | ||||||
| 53 | 1509 | 1514 | Cubebol | 0.8 | ||||||
| 54 | 1510 | — | n.i. | 1.6 | ||||||
| 55 | 1518 | 1517 | Myristicin | 2.4 | 2.0 | |||||
| 56 | 1513 | 1513 | γ-Cadinene | 0.4 | 3.5 | |||||
| 57 | 1516 | 1520 | 7-epi-α-Selinene | 0.2 | ||||||
| 58 | 1501 | 1511 | δ-Amorphene | 0.3 | ||||||
| 59 | 1518 | 1522 | δ-Cadinene | 0.4 | 0.8 | 1.1 | 1.2 | |||
| 60 | 1530 | 1545 | Propiopiperone | 13.2 | 1.2 | |||||
| 61 | 1544 | 1548 | Elemol | 1.1 | ||||||
| 62 | 1554 | 1555 | Elemicin | 1.4 | 2.7 | |||||
| 63 | 1559 | 1561 | (E)-Nerolidol | 0.5 | 1.0 | 6.5 | 13.8 | |||
| 64 | 1571 | 1577 | Spathulenol | 0.5 | 0.7 | 4.1 | 2.2 | 2.5 | ||
| 65 | 1576 | 1582 | Caryophyllene oxide | 1.5 | 1.8 | 13.3 | 10.1 | |||
| 66 | 1580 | 1601 | α-Cedrol | 3.1 | ||||||
| 67 | 1582 | 1590 | Globulol | 1.2 | ||||||
| 68 | 1584 | 1592 | Viridiflorol | 1.2 | ||||||
| 69 | 1593 | 1624 | Selina-6-en-4-ol | 7.3 | ||||||
| 70 | 1606 | — | n.i. | 0.6 | ||||||
| 71 | 1618 | — | n.i. | 0.1 | ||||||
| 72 | 1625 | 1620 | Dillapiole | 76.0 | ||||||
| 73 | 1625 | 1627 | 1-epi-Cubenol | 0.9 | ||||||
| 74 | 1631 | 1642 | 2-Hydroxy-3,4-methylenedioxypropiophenone | 1.0 | ||||||
| 75 | 1637 | 1638 | epi-α-Cadinol | 0.5 | ||||||
| 76 | 1640 | 1644 | α-Muurolol | 0.2 | ||||||
| 77 | 1648 | 1649 | β-Eudesmol | 0.2 | 0.9 | |||||
| 78 | 1651 | 1658 | Selin-11-en-4α-ol | 2.0 | ||||||
| 79 | 1652 | 1652 | α-cadinol | 1.2 | 1.5 | 3.0 | ||||
| 80 | 1655 | 1658 | neo-Intermedeol | 0.5 | ||||||
The essential oils of P. aduncum, P. hispidinervum, P. callosum, P. marginatum, P. hispidum, P. tuberculatum, and Piper sp. were analyzed by GC and GC-MS, and the percentage of identified components is given in Table 2.
The major compounds of P. aduncum and P. hispidinervum were identified as dillapiole (76%) and safrole (91.4%), respectively. In the oil of P. callosum, the main components were safrole (53.8%) and α-pinene (12.2%). Major components of P. marginatum were propiopiperone (13.2%) and δ-3-carene (11.3%). P. hispidum presented the terpinene isoforms γ-terpinene (30.9%) and α-terpinene (14.4%) as main compounds. β-Pinene (15%) and caryophyllene oxide (13.3%) were the major constituents of P. tuberculatum, while the sesquiterpenes linalool (14.6%) and nerolidol (13.8%) were identified in the Piper sp. oil.
Dillapiole has been described as acaricidal (Rhipicephalus (Boophilus) microplus), larvicidal and insecticidal (Anopheles marajoara, Aedes aegypti, and Solenopsis saevissima), and antifungal (Aspergillus fumigatus) agent. Safrole demonstrated antileishmanial (L. major, L. mexicana, L. braziliensis, and L. donovani) activity. Propiopiperone exhibited antifungal activity against Cladosporium cladosporioides and C. sphareospermum. Oyedemi et al. [12] showed the activity of γ-terpinene against Proteus vulgaris and Escherichia coli. Our group previously described [13] the activity of (+)-β-pinene against Cryptococcus neoformans and Candida albicans. Other promising activity described by our group [14] was linalool-rich essential oil of Lippia alba against two dermatophytes Trichophyton rubrum and Epidermophyton floccosum [12–21].
The results of the MIC assay of Piper essential oils and amphotericin B against R. oryzae are shown in Table 3.
Table 3.
MIC values (μg/ml) of Piper essential oils and amphotericin B against R. oryzae.
| Essential oil | MIC | MFC |
|---|---|---|
| Piper aduncum | >1250 | ND |
| Piper hispidinervum | 1250 | ND |
| Piper callosum | >1250 | ND |
| Piper marginatum | 156.25 | >1250 |
| Piper hispidum | 312.5 | >1250 |
| Piper tuberculatum | 625 | >1250 |
| Piper sp. | 78.12 | >156.5 |
| Amphotericin B | 0.98 | 1.95 |
| Posaconazole | 1.56 | 1.56 |
MIC: minimal inhibition concentration; MFC: minimal fungicide concentration; ND: not determined.
Sartoratto et al. [22] considered MIC values between 50 and 500 µg/mL as strong activity, MIC values between 600 and 1500 µg/mL as moderate activity, and above 1500 µg/mL as weak activity [21]. According to this classification, it could be stated that Piper sp., P. marginatum, and P. hispidum essential oils present high activity, P. tuberculatum and P. hispidinervum present moderate activity, and P. aduncum and P. callosum against R. oryzae planktonic cells present weak activity.
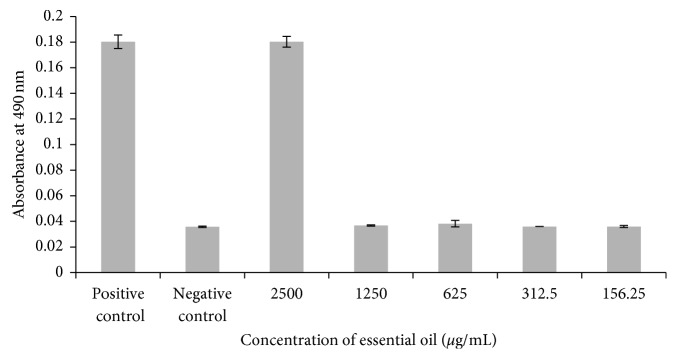
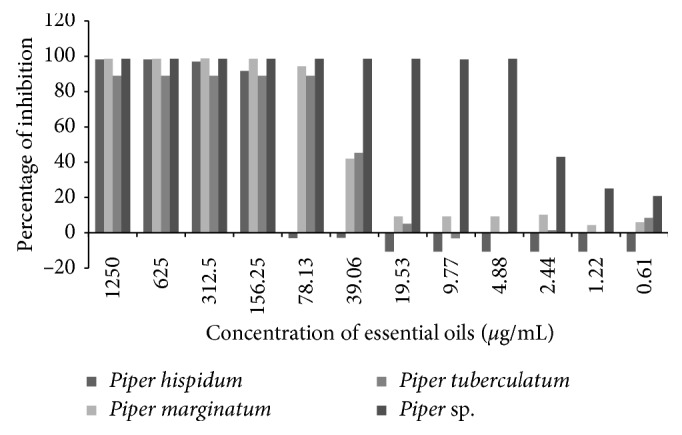
Based on previous MIC results, the essential oils tested on biofilm formation were P. hispidum, P. marginatum, and P. tuberculatum; Piper sp. Rhizopus oryzae biofilm formation in the presence of each Piper essential oil was inhibited in lower concentration than MIC for all species tested (Figure 1).
Figure 1.
Effect of Piper essential oils against R. oryzae biofilm formation. The plates were incubated at 30°C for 18 h.
In their natural environments, most of bacteria and fungi change from a planktonic to a sessile state forming the so-called biofilms. Biofilms are sessile microbial and fungal communities that are strongly attached to surfaces and to each other; in such phase, they are protected by a polymeric extracellular matrix (ECM), constituted primarily of polysaccharides. According to Singh et al. [7], the major compounds of biofilm matrix are GlcN and GlcNAc. The cell wall of zygomicetes is also mainly formed by GlcN and GlcNAc polymer constituents of chitosan and chitin, respectively. Then, our results on MIC and inhibition of biofilm formation could be associated with each other. The essential oil of Piper sp. showed the most active agent against the two cell forms, planktonic and biofilm [7, 23].
Piper sp. essential oil was the most active agent against planktonic cells and biofilm formation (78.12 and 4.88 μg/mL, resp.). However, this essential oil displayed hemolytic activity (Figure 2) at higher concentration (2500 μg/mL), making it a promising antifungal candidate.
Figure 2.
Hemolytic assay after treatment with various concentrations of Piper sp. essential oil.
Other important mechanism of action is the inhibition of rhizopuspepsin and/or saps, a class of enzymes secreted for R. oryzae and other Rhizopus species [24]. The results in Figure 3 showed inhibition of proteolytic activity of rhizopuspepsin when Piper essential oils were used, mainly P. hispidum and P. tuberculatum which inhibited 11.8% and 12.05% of enzymatic activity, respectively.
Figure 3.
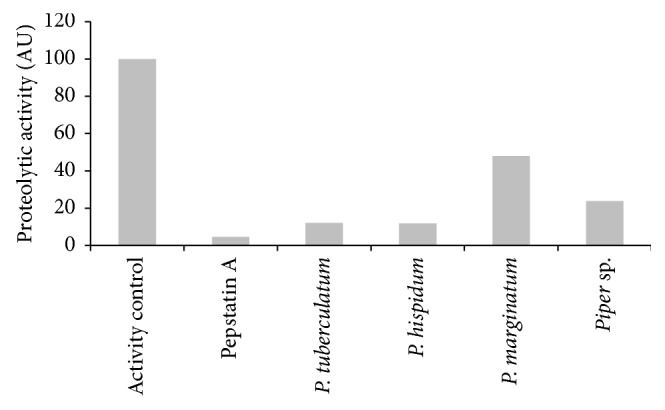
Proteolytic activity of rhizopuspepsin after overnight treatment with 48 µg/ml of Piper essential oils. The plates were incubated at 37°C.
The antioxidant activity was evaluated after TLC of Piper essential oils. It was not possible to identify regions containing substances with activity even after 45 min of application of DPPH. Terpenes are the most significant class of compounds present in essential oils. Among them, several monoterpene hydrocarbons, oxygenated monoterpenes, and sesquiterpenes are often reported as weak antioxidant agents [25]. However, due to the complexity of essential oils' composition, some antioxidant activity was expected. Thus, further investigation will be necessary in order to evaluate other antioxidant methods.
4. Conclusion
This study showed the promising anti-Rhizopus oryzae activity of Piper tuberculatum, P. hispidum, and Piper sp. against planktonic cells, biofilm formation, and rhizopuspepsin which makes these essential oils useful in formulating strategies to limit the growth of R. oryzae.
Acknowledgments
This study was supported by the Brazilian agencies Rio de Janeiro State Research Foundation (FAPERJ), Coordination for Improvement of Higher Education Personnel (CAPES), and National Council for Scientific and Technological Development (CNPq).
Conflicts of Interest
The authors declare that they have no conflicts of interest concerning this article.
References
- 1.Takeara R., Gonçalves R., dos Santos Ayres V. F., Guimarães A. C. Biological properties of essential oils from the Piper species of Brazil: a review. In: El-Shemy H., editor. Aromatic and Medicinal Plants–Back to Nature. https://www.intechopen.com/books/aromatic-and-medicinal-plants-back-to-nature/biological-properties-of-essential-oils-from-the-piper-species-of-brazil-a-review. [Google Scholar]
- 2.Regasini L. O., Cotinguiba F., Passerini G. D., et al. Trypanocidal activity of Piper arboreum and Piper tuberculatum (Piperaceae) Revista Brasileira de Farmacognosia. 2009;19(1):199–203. doi: 10.1590/s0102-695x2009000200003. [DOI] [Google Scholar]
- 3.Azevedo M. M., Almeida C. A., Chaves F. C., et al. Effects of 7-hydroxycalamenene isolated from Croton cajucara essential oil on growth, lipid content and ultrastructural aspects of Rhizopus oryzae. Planta Medica. 2014;80(7):550–556. doi: 10.1055/s-0034-1368441. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb O. R., Magalhães M. T. Modified distillation trap. Chemist Analyst. 1960;49(4):p. 114. [Google Scholar]
- 5.Adams R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th. Miami, FL, USA: Carol Stream: Allured Publishing Corporation; 2007. [Google Scholar]
- 6.Clinical and Laboratory Standards Institute (CLSI) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard M38. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2002. [Google Scholar]
- 7.Singh R., Shivaprakash M. R., Chakrabarti A. Biofilm formation by zygomycetes: quantification, structure and matrix composition. Microbiology. 2011;157(9):2611–2618. doi: 10.1099/mic.0.048504-0. [DOI] [PubMed] [Google Scholar]
- 8.Franca Rodrigues K. A., Amorim L. V., Dias C. N., Moraes D. F. C., Carneiro S. M. P., Amorim Carvalho F. A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. Journal of Ethnopharmacology. 2015;160:32–40. doi: 10.1016/j.jep.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Buroker-Kilgore M., Wang K. K. A coomassie brilliant blue G-250-based colorimetric assay for measuring activity of calpain and other proteases. Analytical Biochemistry. 1993;208(2):387–392. doi: 10.1006/abio.1993.1066. [DOI] [PubMed] [Google Scholar]
- 10.Calvin A., Potterat O., Wolfender J. L., Hostettmann K., Dyatmyko W. Use of On-flow LC/1H NMR for the study of an antioxidant fraction from Orophea enneandra and isolation of a polyacetylene, lignans, and a tocopherol derivative. Journal of Natural Products. 1998;61(12):1497–1501. doi: 10.1021/np980203p. [DOI] [PubMed] [Google Scholar]
- 11.Azevedo M. M. B., Chaves F. C. M., Almeida C. A., et al. Antioxidant and antimicrobial activities of 7-hydroxy-calamenene-rich essential oils from Croton cajucara Benth. Molecules. 2013;18(1):1128–1137. doi: 10.3390/molecules18011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyedemi S. O., Okoh A. I., Mabinya L. V., Pirochenva G., Afolayan A. J. The proposed mechanism of bactericidal action of eugenol, ∝-terpineol and g-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. African Journal of Biotechnology. 2009;8(7) [Google Scholar]
- 13.Silva A. C. R., Lopes P. M., Azevedo M. M. B., Costa D. C. M., Alviano C. S., Alviano D. S. Biological activities of a-pinene and β-pinene enantiomers. Molecules. 2012;17(6):6305–6316. doi: 10.3390/molecules17066305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa D. C. M., Vermelho A. B., Almeida C. A., et al. Inhibitory effect of linalool-rich essential oil from Lippia alba on the peptidase and keratinase activities of dermatophytes. Journal of Enzyme Inhibition and Medicinal Chemistry. 2014;29(1):12–17. doi: 10.3109/14756366.2012.743537. [DOI] [PubMed] [Google Scholar]
- 15.Silva W. C., de Souza Martins J. R., de Souza H. E. M., et al. Toxicity of Piper aduncum L. (Piperales: Piperaceae) from the Amazon Forest for the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Veterinary Parasitology. 2009;164(2–4):267–274. doi: 10.1016/j.vetpar.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.de Almeida R. R. P., Souto R. N. P., Bastos C. M., da Silva M. H. L., Maia J. G. S. Chemical variation in Piper aduncum and biological properties of its dillapiole-rich essential oil. Chemistry and Biodiversity. 2009;6(9):1427–1434. doi: 10.1002/cbdv.200800212. [DOI] [PubMed] [Google Scholar]
- 17.Souto R. N. P., Harada A. Y., Andrade E. H. A., Maia J. G. S. Insecticidal activity of Piper essential oils from the Amazon against the fire ant Solenopsis saevissima (Smith) (Hymenoptera: Formicidae) Neotropical Entomology. 2012;41:510–517. doi: 10.1007/s13744-012-0080-6. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira R. G., Monteiro M. C., da Silva J. K. R., Maia J. G. S. Antifungal action of the dillapiole-rich oil of Piper aduncum against dermatomycoses caused by filamentous fungi. British Journal of Medicine and Medical Research. 2016;15:1–10. doi: 10.9734/bjmmr/2016/26340. [DOI] [Google Scholar]
- 19.Monzote L., García M., Montalvo A. M., Scull R., Miranda M. Chemistry, cytotoxicity and antileishmanial activity of the essential oil from Piper auritum. Memã³rias Do Instituto Oswaldo Cruz. 2010;105(2):168–173. doi: 10.1590/s0074-02762010000200010. [DOI] [PubMed] [Google Scholar]
- 20.da Silva J. K. R., Silva N. N., Santana J. F. S., Andrade E. H. A., Maia J. G. S., Setzer W. N. Phenylpropanoid-rich essential oils of Piper species from the Amazon and their antifungal and anti-cholinesterase activities. Natural Product Communications. 2016;11:1907–1911. [PubMed] [Google Scholar]
- 21.da Silva J. K., da Trindade R., Alves N. S., Figueiredo P. L., Maia J. G. S., Setzer W. N. Essential oils from neotropical Piper species and their biological activities. International Journal of Molecular Sciences. 2017;18(12):p. 2571. doi: 10.3390/ijms18122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartoratto A., Machado A. L. M., Delarmelina C., et al. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Brazilian Journal of Microbiology. 2004;35(4):275–280. doi: 10.1590/s1517-83822004000300001. [DOI] [Google Scholar]
- 23.Nazzaro F., Fratianni F., Coppola R., Feo V. D. Essential oils and antifungal activity. Pharmaceuticals. 2017;10(4):p. 86. doi: 10.3390/ph10040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos A. L. S. D. Aspartic proteases of human pathogenic fungi are prospective targets for the generation of novel and effective antifungal inhibitors. Current Enzyme Inhibition. 2011;7(2):96–118. doi: 10.2174/157340811796575281. [DOI] [Google Scholar]
- 25.Ruberto G., Baratta M. T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chemistry. 2000;69(2):167–174. doi: 10.1016/s0308-8146(99)00247-2. [DOI] [Google Scholar]