Graphical abstract
Keywords: Nutty flavor, Corynbacterium glutamicum, Pyrazines, Enzymatic hydrolyzed soybean, Encapsulation
Abstract
The main objective of this study was to evaluate the ability of Corynbacterium glutamicum to produce a safe nutty like flavor from enzymatic soybean meal hydrolysate (E-SH) and to investigate the effect of encapsulation and storage on the quality of the produced nutty flavoring. C. glutamicum was incubated with E-SH, supplemented and un-supplemented with a mixture of threonine and lysine. The generated volatiles of each culture were subjected to odor sensory analysis. The volatile compounds were analyzed by headspace solid phase microextraction (HS-SPME) and gas chromatography coupled with mass spectrometry (GC-MS). The sample showed the best nutty aroma and highest content of the most odorant compounds of nutty flavor was subjected to toxicity test and encapsulated in Arabic gum using spray drier. The stability of the encapsulated flavoring was evaluated during storage. A high correlation was found between the culture growth and consumed sugars. The odor intensity of the generated nutty-chocolate like aroma showed a gradual increase during incubation time. Pyrazines and 2/3- methylbutanal showed the highest content at the end of fermentation time. Encapsulation gave rise to a significant decrease in the branched aldehydes, which are responsible for the chocolate note of the flavoring sample. The high odor intensity of the stored sample was correlated to the significant increase in the pyrazines. The results of GC–MS analysis confirmed those of odor sensory evaluation of the nutty-like flavor.
Introduction
The abundant industrial production of food products has led to a great demand for flavoring compounds. Nutty flavor is one of the most popular flavors to the consumers. However, the production of nutty flavor by direct extraction from plant sources is very expensive. The flavor characteristics of pyrazines could be generally described as nutty, roasted and toasty, dependent on the nature of the alkyl substituent [1]. Chemical methods of pyrazines synthesis have been reported [2]. However, consumers prefer natural products even though they are much more expensive than their corresponding chemicals. Therefore, many investigations have been directed towards the search of other strategies to produce natural flavors.
Microorganisms are essential for the development of the desired flavors by bioconversion of natural precursors of flavoring substances that can be labeled as natural and represent as such an interesting area in the field of food science [3]. Organic nitrogen sources were found to be necessary for healthy growth and accumulation of the volatile compounds. Pyrazines production by microorganisms from natural raw materials becomes more appropriated for its bio/or natural properties [4].
Enzyme hydrolysate of soybean meal supplemented with vitamin was found to be essential for efficient production of tetramethyl pyrazine (TTMP) when fermented by Bacillus mutant [5]. TTMP and 2,5-dimethylpyrazine (2,5-DMP) are the main pyrazines produced by Bacillus subtilis from fermented cocoa bean and considered as important contributors to its flavor [6]. Several alkylpyrazines were produced by Bacillus subtilis grown in solid substrate conditions using soybean suspended in water supplemented with threonine and acetoin [7] as precursors of 2,5-DMP and TTMP, respectively.
Biotechnological use of C. glutamicum has been impressive progress for the production of various chemicals [8]. Considerable quantity of alkypyrazines had been produced by C. glutamicum with trimethylpyrazine (TMP), TTMP and acetoin as main compounds [9]. In our previous study C. glutamicum was used for the bioproduction of beef-like flavor from enzymatic hydrolysate of mushroom and soybean meal enriched with cysteine, as a precursor of beef aroma. The results confirmed the essential role of the precursors on the production of the desired flavor [10].
Although there are several studies dealing with the bioproduction of the pyrazine derivatives [5], [7], no one has been evaluated their quality as flavoring agents. Microencapsulation by using spray drying is the most commonly technique used for the production of dry flavorings that are easy to handle and incorporate into dry food mixture. Flavor retention and stability against oxidation are strongly influenced by the carrier material [11]. Gum Arabic is the most common used carrier in food industry [12].
The main purpose of the present study was the bioproduction of economic and safe nutty flavoring by C. glutamicum. The enzymatic hydrolysate of soy bean meal was used as the main source of free amino acids and carbohydrates that are required for the bioproduction of nutty flavor. Addition effect of amino acids that are considered as precursors of pyrazines on the volatiles released during fermentation of enzymatic soy bean hydrolysate was investigated.
The Flavor and Extract Manufactures Association had recommended the pyrazines as safe (GRAS, generally regarded as safe) flavoring agents in food [13]. Therefore, the nutty flavor that exhibited the best quality was subjected to toxicological study to confirm its safety. The present study was extended to evaluate the effect of encapsulation in gum Arabic and storage on the odor quality and retention of the volatile compounds of the nutty flavorings.
Material and methods
Materials
Plant materials and chemicals
Defatted soybean meal (48% protein, 28.6% total sugar, 9.7% reducing sugar, 6% lipid, 9.0% ash, and 8.4% moisture) was obtained from Food Technology Research Institute, Agric. Res. Center, Giza, Egypt. Amino acids; threonine and lysine, authentic compounds, and standard n-paraffin (C8-C22) were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Flavourzyme (from Aspergillus oryzae) and Alcalase (from Bacillus Licheniformis) were obtained from Novo Nordisk A/S (NOVO ALLE, DK - 2880, Bagsvaerd, Denmark). Glucose, agar and H2SO4 were purchased from Merck Company, Germany. Peptone, yeast extracts and di-ammonium phosphate was purchased from Loba Chemie, Bombay, India. DNS was purchased from Sigma Aldrich Chemical Co. Filter papers (Whatman No. 1, 15 cm diameter) Whatman International Ltd Maidstone, England.
Experimental animals
Fifty-six senile albino mice (50% male and 50% female) of body weight ranging from 23 to 25 g. were purchased from Animal House of National Research Centre, Cairo, Egypt to be used in the acute toxicity test. The mice were housed in stainless steel cages. Each group consisted of 4 male and 4 female mice were kept in a cage (i.e. 8 mice per cage). Water and food were provided ad libitum. The animals were housed at 26 ± 2 °C and 55 ± 10% relative humidity. The acute toxicity test was implemented according to the Medical Research Ethics Committee for institutional and national guide for the care and use of laboratory animals, National Research Centre; Cairo, Egypt (Publication No. 85-23, revised 1985).
Bacteria
C. glutamicum 1220T, collected from Microbiological Resources Center; Cairo, Egypt (MIRCEN), was cultured and maintained on nutrient agar slant (13 g/L yeast extract, 10 g/L peptone) at 28 °C for 24 h. The direct microscopic method (optical light microscope (10 × 90) Olympus CH40, New York, USA) was carried out for examining the morphological feature of vegetative cells using production medium for 3 days and Gram staining.
Methods
Production of enzymatic hydrolysate
The enzymatic hydrolysate of soybean meal was prepared according to Aaslyng et al. [14]. Flavourzyme and Alcalase were used for the hydrolysis of protein. The prepared hydrolysate was used as the main substrate for the production of nutty-like flavor by C. glutamicum. To determine the content of free amino acids, the hydrolysate was subjected to centrifugation and the precipitate was washed with 300 mL of tap water and centrifuged again. The combined hydrolysates in water were filtered, freeze-dried (Snijders Scientific b.v. Model L45 Fm-Ro, Tilburg, Holland), and stored immediately in closed glass bottles at −10 °C pending further analysis. Composition and content of free amino acids of the enzymatic hydrolyzed protein (E-HVP) was determined as described in previous study using LC3000 amino acid analyzer (Eppendorf–Biotronik, Maintal, Germany) [15].
Production medium and batch culture of bacterial strain
C. glutamicum was first grown on nutrient broth (YP) medium for 12 h in 250 mL shaking flask with agitation, then inoculated (2%) into the GYP medium, which composed of (g/L) glucose, 100; yeast extract, 10; peptone, 30; and di-ammonium phosphate, 30 at pH 7.2; autoclaved at 121 °C for 20 min. The glucose was autoclaved separately. GYP medium was inoculated (6%) into production medium, which composed of 50 mL sterile soybean hydrolysate supplemented with 5 g of a sterile mixture of threonine and lysine (at equal molar ratio) at pH 8 and incubated with shaking (150 rpm) at 28 °C for 3, 5, 7, and 9 days. Each fermented medium was cooled in an ice bath and filtrated. The residual was washed with 100 mL distilled water and filtrated again.
Determination of reducing and total sugars
Reducing sugars was determined in the filtrate according to dinitro salicylic acid (DNS) method [16] and total sugar according to phenol-H2SO4 method [17] using glucose as standard.
Biomass determination
The growth of the two fermented cultures during incubation period was measured as dry weight of mycelium. The mycelium of each flask was filtered, using filter paper (Whatman No. 1; 15 cm diameter), washed three times with distilled water, and dried for 24 h at 85 °C. Each filtrate was subjected to the following analysis.
Odor sensory analysis
Evaluation of odor quality of the nutty-like aroma generated by C. glutamicum fermented on the soybean meal hydrolysate was carried out during incubation period 9 days. The evaluation was conducted by a well-trained panel consisting of 10 member (6-female and 4-male) drawn from Food Technology and Nutrition Division, National Research Center, Cairo, Egypt. All panelists had experience with odor sensory analysis “>20 h”. Preliminary description odor sensory analysis had been carried out by the panelists through three sessions each spent 2 h to determine the odor sensory attributes of the sample. Two descriptions were selected (nutty and chocolate) and used for the quantitative odor analysis. The panelists were trained for additional 3 h to identify and define the intensity of nutty-like aroma in terms of appropriate reference samples (roasted peanut and raw chocolate). The panelist sniffed and scored the intensity of the perceived nutty-like aroma of each culture medium on the 3rd, 5th, 7th, and 9th days on a category scale 0 (not perceptible) to 10 (strongly perceptible). Each sample was evaluated in triplicate.
Acute oral lethal toxicity test
Acute oral lethal toxicity test for nutty flavor (E microbiology) was carried out according to Goodman et al. [18]. The animals were divided into seven groups; each of 8 mice. Seven dose levels ranging from 0.5 to 12 g nutty-like flavor/kg mouse body weight were given orally to the mice of the different groups. Mortality counts were recorded among each group (if any) in the next 24 h.
Preparation of encapsulated nutty-like flavor
Arabic gum at concentration of 10%, w/w was dispersed in the filtrate, vigorously homogenized (10,000 rpm/3 min) at 25 °C and then subjected to spray drying in Buchi, B-290 model mini spray dryer-Switzerland, equipped with 0.5 mm diameter nozzle. Encapsulation process was conducted as previously described [15]. The spray dried powders were filled immediately in airtight, self-sealable polyethylene pouches and stored at −10 °C until further studies.
Headspace solid phase microextraction(HS-SPEM)
A divinylbenzene/carboxen/polydimethyl siloxane (DVB/CAR/PDMS) fiber (coating thickness: 50/30 µm) was used in solid-phase microextraction analysis (Supleco, 57348-U, Bellefonte, PA, USA). This fiber showed a high ability to extract the alkylpyrazines [19]. The optimum extraction conditions (time and temperature) of the target volatile compounds were investigated. Each target compound was spiked to 5 mL of the filtrate placed in a 10 mL headspace glass vial sealed with a PTFE faced silicon septum (Supelco, Bellefonte, PA, USA) at concentration 1 µg/ mL. The extraction efficiency of each compound at various extraction temperatures was determined. The results revealed that 60 °C was the most adequate temperature for optimum extraction. The times of extraction from 20 to 70 min were investigated (data not shown). Extraction time 60 min showed the best result therefore was chosen for SPME of the volatiles in headspace of each sample.
The combined filtrates of each culture (50 mL) with 9.72 µg of 3-heptanol was placed in a 100 mL headspace glass vial sealed with a PTFE faced silicon septum (Supelco, Bellefonte, PA, USA). Extraction was performed by exposing the SPME fiber to the headspace of each sample for 60 min at 60 °C, then it was inserted into the GC injection port for desorption (260 °C/5 min in splitless mode). Before use, the fiber was conditioned in the injection port of the GC (270 °C/1 h) as recommended by manufacture. Extraction was carried out in triplicate for each sample.
Gas chromatography–mass spectrometry (GC–MS) analysis
Analysis of the volatile compounds was performed by a gas chromatography (Hewlett-Packard model 5890, USA) coupled to a mass spectrometer (Hewlett-Packard-MS 5970, USA). The injection was conducted in the splitless mode for 5 min at 260 °C. The GC was equipped with a fused silica capillary column DB5 (60 m × 0.32 mm i.d. × 0.25 μm film thickness). The oven temperature was held initially at 50 °C for 5 min and then programmed from 50 to 250 °C at a rate of 4 °C/min. Helium was used as the carrier gas, at flow rate of 1.1 mL/min [15]. The mass spectrometer was operating in the electron impact mode (EI) at 70 eV and scan m/z range from 39 to 400 amu. The retention indices (Kovats index) of the separated volatile compounds were calculated with reference to the retention time of a series of n-paraffin (C6-C20) as external standard run at the same conditions. The isolated peaks were identified by matching with data from the library of mass spectra (National Institute of Standard and Technology, NIST) and comparison with those of authentic compounds and published data [20], [21], [22]. The relative concentration of each identified compound was calculated by comparing the peak area of the compound in each chromatogram with that of 3-heptanol, an internal standard compound, on total ion chromatograms (TIC) of GC–MS, assuming all response factors were 1. Each reported concentration is the average of three separate extractions.
Statistical analysis
Analysis were performed in triplicate for each sample for all the tests, except for odor sensory evaluation ten replicates were used. Each data was presented as mean ± standard deviation (±SD). Obtained data were subjected to analysis of variance (ANOVA) by the Statgraphics package (Statistical Graphics Corporation, 1993; Manugistics Inc., USA) followed by the multiple range test L.S.D. (Duncan multiple range test) at the significant level at P < .05.
Results and discussion
Composition of free amino acids
Organic nitrogen sources were found to be very important for bioproduction of the volatile compounds as well as the growth of fermented cultures. Enzymatic hydrolysis of protein results in a release of free amino acids that can be subsequently degraded by bacteria into various flavor compounds [5], [23].
In the present study, the enzymatic hydrolysate of soybean meal was used as a source of nitrogen and sugar that are required for the culture growth. Composition of the free amino acids in the enzymatic hydrolyzed soybean meal is cited in Table 1S (Suppl. materials). A total of 15 amino acids were determined with total concentration 48.52 ± 6.07 mg/100 mL. Phenylalanine was the major free amino acids (8.70 ± 1.09 mg/100 mL) followed by leucine (6.38 ± 0.80 mg/100 mL).
A direct biosynthetic link had been demonstrated early between the bioproduction of pyrazines and the free amino acids valine, leucine and isoleucine [24]. Lysine and l-threonine enhanced the bioproduction of 2,5-dimethylpyrazine by Bacillus cereus and Bacillus subtilis [7], [25]. Free amino acids produced during cocoa fermentation are the main precursors of chocolate flavor [26].
Culture growth
The correlation between the culture growth (dry matter) of C. glutamicum during fermentation of hydrolyzed soybean meal, with and without addition of amino acids, and the content of each of total and reducing sugars is shown in Table 1. It is obvious that there was a high correlation coefficient between the culture growth and sugars (total and reducing) consumed during incubation time (9 days) for each investigated sample. Early study [9] revealed that the biomass growth of fermented soybean was corresponded with sugar consumption. Also, sugar catabolism gave rise to accumulation of acetoin, which is considered as the precursor of TTMP, the potent odorant of roasted nutty flavor [4]. As shown in Table 1, during incubation period the sugars (total and reducing) showed insignificant increase (P > 0.05) in sample supplemented with amino acids compared with the unsupplemented sample. This result is consistent with previous studies [27], [28], which revealed that addition of amino acids gave rise to a decrease in consumed sugars during fermentation.
Table 1.
Correlation between sugar content (total sugar and reducing sugar) and culture growth (dry matter) of fermented soybean hydrolysats (with and without amino acids) during incubation time.
| Incubation time (days) | Without amino acid |
With amino acid |
||||
|---|---|---|---|---|---|---|
| Total sugar | Reducing sugars | Dry matter | Total sugar | Reducing sugars | Dry matter** | |
| 0 | 55.33 ± 2.11 | 13.74 ± 0.88 | 1.3 ± 0.27 | 53.46 ± 2.15 | 14.26 ± 0.97 | 1.4* ± 0.28 |
| 3 | 42.16 ± 1.83 | 12.56 ± 0.97 | 4.8 ± 0.33 | 43.22 ± 1.55 | 12.81 ± 0.88 | 5.2 ± 0.33 |
| 5 | 35.20 ± 1.33 | 9.15 ± 0.67 | 6.3 ± 0.48 | 36.16 ± 1.67 | 9.21 ± 0.48 | 6.6 ± 0.370 |
| 7 | 29.15 ± 0.98 | 7.52 ± 0.63 | 7.1 ± 0.66 | 29.65 ± 1.02 | 7.85 ± 0.37 | 7.6 ± 0.68 |
| 9 | 22.86 ± 0.97 | 5.61 ± 0.33 | 7.6 ± 0.68 | 23.11 ± 0.97 | 5.76 ± 0.28 | 7.9 ± 0.88 |
| r | 0.980 | 0.948 | 0.989 | 0.975 | ||
r: correlation coefficient between the cultures growth (dry matter) and content of each of total sugar and reducing sugar during incubation.
Values are the average of triplicate analysis (g/100 mL fermented culture) ±SD.
Cell dry weight of C. glutamicum culture.
Odor sensory evaluation
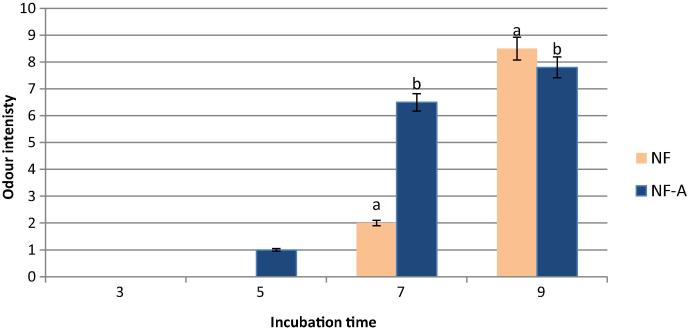
The effect of incubation time on intensity of the nutty-like aromas (NF and NFA) produced by C. glutamicum from the two investigated cultures (soybean hydrolysate and soybean hydrolysate supplemented with amino acids, respectively) is shown in Fig. 1. The odor intensity was scored by 10 panelists, three replicates were applied to assess the results. In general, the aroma perceived was described as nutty like aroma with chocolate note. The aroma was detected after five days in the culture supplemented with amino acids, but at low intensity, followed by a gradual increase during incubation period. The nutty chocolate-like aroma was perceived at low score in NF sample after 7 days. However, it showed a significant (P < 0.05) higher score (8.5) at the end of incubation time (9 days) than NF-A sample.
Fig. 1.
Odor sensory evaluation of nutty-like flavor produced by C. glutamicum from fermented soybean hydrolyzate supplemented (NF-A) and unsupplemented (NF) with amino acid (vertical bars represented ± SD of the means, n = 10). Odor intensity, at each incubation time, followed by same letter means no significant deference at P < .05.
Volatile compounds
The headspace volatiles released during fermentation of the two investigated culture (NF and NF-A) by C. glutamicum were isolated and subjected to GC–MS analysis to explain the variation in odor intensity between them. Table 2 shows the identified volatile compounds and the recovered amount of each of them as well as the description of their odor as reported in literatures. The total volatiles in both cultures showed a gradual increase during incubation period. However, at the end of fermentation time their total content was higher in sample NF than NF-A. This finding may be correlated to the decrease in pH during amino acids catabolism [29]. The main identified compounds, presented in Table 2, are two branched aldehydes, acetoin and six pyrazine derivatives. The total yield of the two branched aldehydes, 2-methylbutanal and 3-methylbutanal, showed a gradual increase during incubation period, however their total yield was higher in NF (3.22 mg/L) sample than NF-A (1.16 mg/L) at the end of fermentation. These compounds are the biodegradation products of isoleucine and leucine respectively [30], they are described to have malty-dark chocolate note [31]. As shown in Table 1S leucine (6.38 ± 0.80 mg/100 mL) was the second major compound in the enzymatic hydrolyzed soybean meal. These results confirm the chocolate note of the perceived nutty flavor.
Table 2.
Volatile compounds identified in nutty chocolate-like aroma generated by C. glutamicum from enzymatic soybean meal hydrolysate supplemented (NF-A) and unsupplemented (NF) with amino acids.
| Peak No | Components | Time of fermentation |
Description | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KIa | 3 days |
5 days |
7 days |
9 days |
|||||||
| NF | NF-A | NF | NF-A | NF | NF-A | NF | NF-A | ||||
| 1 | 2-Methylbutanal | 639 | 0.06a ± 0.01 | 0.02a ± 0.00 | 0.09a ± 0.01 | 0.02a ± 0.00 | 0.82b ± 0.10 | 0.12b ± 0.02 | 1.40c ± 0.18 | 0.53c ± 0.07 | Malty chocolate1 |
| 2 | 3-Methylbutanal | 682 | 0.14a ± 0.02 | 0.07a ± 0.01 | 0.19a ± 0.12 | 0.09a ± 0.01 | 0.27b ± 0.03 | 0.10a ± 0.01 | 1.82c ± 0.23 | 0.83b ± 0.11 | Malty chocolate1 |
| 3 | Acetoine | 714 | 0.55a ± 0.07 | 0.42a ± 0.05 | 0.44b ± 0.06 | 0.31b ± 0.04 | 0.25c ± 0.03 | 0.11c ± 0.01 | – | – | Butter2 |
| 4 | 2-Methylpyrazine | 834 | – | – | – | 0.03b ± 0.00 | – | 0.12 ± 0.02 | 0.01a ± 0.00 | 0.17d ± 0.02 | Earthy nutty3 |
| 5 | 2,5-Dimethylpyrazine | 918 | – | 0.02a ± 0.00 | 0.02a ± 0.00 | 0.51b ± 0.06 | 0.04a ± 0.00 | 0.78c ± 0.10 | 0.06a ± 0.01 | 0.55b ± 0.07 | Chocolate nutty3 |
| 6 | 2,6-Dimethylpyrazine | 923 | – | – | – | – | 0.03a ± 0.00 | 0.08b ± 0.01 | 0.01a ± 0.00 | 0.03c ± 0.00 | Nutty herbal3 |
| 7 | 2,3-Dimethylpyrazine | 933 | – | – | – | – | 0.01a ± 0.00 | 0.07b ± 0.01 | 0.05a ± 0.01 | 0.08b ± 0.01 | Nutty3 |
| 8 | Trimethylpyrazine | 1030 | – | 0.07a ± 0.006 | 0.03b ± 0.00 | 0.16b ± 0.02 | 0.07c ± 0.01 | 0.26c ± 0.03 | 0.11d ± 0.01 | 0.17b ± 0.02 | Chocolate nutty3 |
| 9 | Tetramethylpyrazine | 1098 | – | – | – | 0.03a ± 0.00 | – | 1.80b ± 0.23 | 3.70b ± 0.47 | 3.23c ± 0.41 | Roasted nutty4 |
| Total yield | 0.75 ± 0.01 | 0.60 ± 0.08 | 0.77 ± 0.10 | 1.15 ± 0.15 | 1.49 ± 0.19 | 3.44 ± 0.44 | 7.16 ± 0.91 | 5.59 ± 0.71 | |||
Values are the average of triplicate analysis (mg/L fermented cultures) ±SD.
Mean values in the same row for each culture followed by different superscript lower case letters are significantly different at P < 0.05.
Retention indices.
Rodriguez-Campos et al. [41].
Forster et al. [35].
Bonvechi [42].
Afoakwa et al. [7].
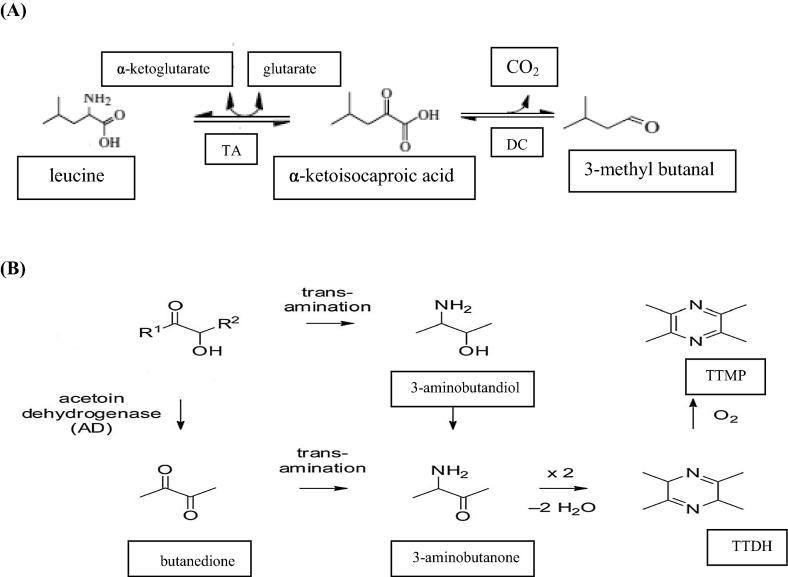
Different bacterial strains had been screened for their ability to produce 3-methylbutanal from leucine [32]. Among them only Lactococcus lactis subsp. B1157 and Corynebacterium ammonia genes strain B1506 showed high ability to convert leucine to 3-methylbutanal. As shown in Fig. 2A, during fermentation leucine converts by a transamination reaction to α-ketoisocaproic acid, which is the central intermediate in amino acid catabolism. This compound either transaminated back to the corresponding amino acid (leucine) or decarboxylated directly or indirectly to the corresponding aldehyde, 3-methylbutanal [32], [33].
Fig. 2.
(A) Reaction scheme of simplified leucine degradation pathways [32], (B) reaction scheme of biosynthetic pathway of TTMP from acetoin [9].
Acetoin showed a gradual decrease, in both cultures, during incubation period whereas, the pyrazines showed an opposite trend (Table 2). Acetoin is a biodegradation product of sugar [34], it possesses buttery flavor [35]. 2-Methylpyrazine, 2,5-dimethylpyrazine, 2,6-dimethylpyrazine, 2,3-dimethylpyrazine, trimethylpyrazine and TTMP were identified in the present study. The bioproduction of TTMP was faster in NF-A culture; it comprised 1.8 ± 0.23 mg/L of the total volatiles, after incubation for 7 days, followed by a significant (P < 0.05) increase after 9 days. TTMP was detected in NF culture only at the end of fermentation time (9 days), but with higher concentration (3.70 ± 0.46) mg/L than in NF-A (3.23 ± 0.40 mg/L). In previous study [5], TTMP comprised 98.98% of the total volatiles produced from soytone, an enzymatic soybean hydrolysate, supplemented with vitamin by Bacillus mutant. Whereas, 2,3,5-trimethylpyrazine and 2-ethyl-3,5,6-trimethylpyrazine were considered as impurities, they comprised 0.09% and 0.02%, respectively. Bioproduction of TTMP by Bacillus subtilis was enhanced by enrichment the ground soybean suspended in water with acetoin [7]. As shown in Table 2 the other pyrazines identified in present study were detected in much less concentration than TTMP. However, their presence confirmed the results of odor sensory analysis (Table 2).
Several bacterial strains having the ability to generate the precursors required for the bioproduction of pyrazines such as α-acetolactate, acetoin, free amino acids, ammonia [36]. The pathway to biosynthesis of TTMP by C. glutamicum from acetoin was proposed by Dickschat et al. [9]. As shown in Fig. 2B, acetoin was oxidized to butandione by acetoin dehydrogenase (AD) and transaminated to 3-aminobutanone. Two units of 3-aminobutanone were consequently condensed to produce tetramethyldihydropyrazine (TTMDHP) which oxidized spontaneously to TTMP. Alternatively, a transamination reaction with acetoin may proceed at first to 3-aminobutan-2-ol which can be oxidized to 3-aminobutanone and complete the reaction as mentioned above. The bioproduction of trimethylpyrazine from acetoin by C. glutamicum was proposed by Dickschat et al. [9]. It may be formed from one unit of acetoin and C2 building blocks such as glycol aldehyde or C3 unit such as hydroxy acetone which were absent in GC–MS data because they coelute with the solvent.
Methylpyrazine and 2,5-dimethylpyrazine can be formed by Corynebacterium glutamicum from acetoin with C2+C3 unit blocks [9]. Combination of acetoin with other α-hydroxyketones could contribute to the bioproduction of 2,3 or 2,6-dimethylpyrazine [36]. As shown in Table 2, supplementation of the soybean enzymatic hydrolysate with amino acids (lysine and threonine) resulted in a higher production of 2,5-dimethylpyrazine in NF-A culture compared with the unsupplemented culture. Addition of lysine (1–2%) to the Bacillus cereus culture enhanced the production of 2,5-dimethylpyrazine [25]. Enrichment of the ground soybean suspended in water with l-threonine improved the production of 2,5-dimethylpyrazine by Bacillus subtilis. However, the results showed that the maximum concentration of the recovered 2,5-dimethylpyrazine was limited [7].
The aforementioned results revealed that the best nutty chocolate-like flavor was generated from soybean hydrolysate (NF) incubated for 9 days. So, it was selected and subjected to toxicity study and encapsulation in Arabic gum.
Toxicity test
The nutty chocolate like flavor produced from enzymatic hydrolyzed soybean meal fermented by C. glutamicum for 9 days showed very high safety. The highest safe dose demonstrated in the current study was 10 g/kg mouse body weight. There was no observed death among the different mice groups (6 groups) treated by the different doses from 0.5 to 10 mg/kg mouse body weight. The only death was observed in the seventh group that was treated by 12 g/kg mouse body weight which showed death of one mouse. The dose level of 10 g/kg mouse body weight (the safest dose) when translated to human dose, adopting interspecies conversion tables [37], was found to be about 78 g/70 kg man body weight.
As shown in Table 2 the pyrazines comprised the highest yield (3.94 mg/L) in the investigated flavor (NF). The available studies concerning the toxicity of the pyrazines reported that the mouse acute oral LD50 values are greater than 2000 mg/kg [38]. Short and long-term subacute chronic studies showed no adverse effect. Furthermore, the in vitro and in vivo carcinogenicity, mutagenicity and genotoxicity tests confirmed the safety of the pyrazines [38]. TTMP which comprised the highest yield of the investigated flavor (Table 2) have been used as medicaments for several diseases [39].
Effect of encapsulation and storage on the nutty-like flavor
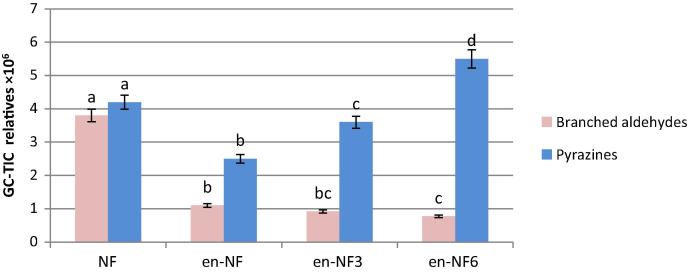
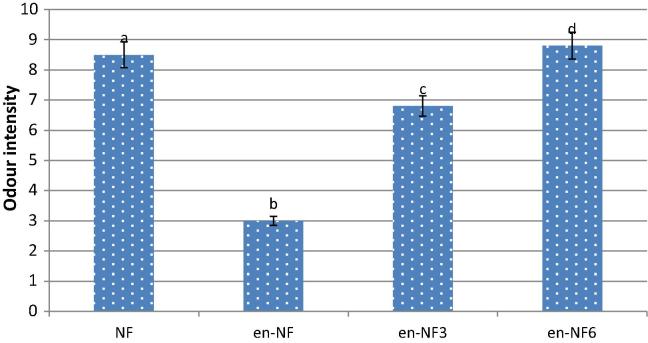
In general, encapsulation resulted in a significant (P < .05) decrease (55%) in the total volatiles. However, as shown in Fig. 3 the total content of the branched aldehydes (2/3-methylbutanal) showed a higher (71%) decrease than that of the pyrazines (40%). It is well documented that during encapsulation the high temperature (150 °C) and presence of oxygen catalyze the dehydration and oxidative reaction and subsequently lead to the decrease in the compounds originally encapsulated [40]. The two branched aldehydes could undergo further reaction during encapsulation including melanodins formation that would occur at high temperature [18]. Lotfy et al. [15] correlated the decrease in the more volatile compounds in the headspace of the encapsulated beef-like flavorings to their high volatility in addition they can undergo further reaction to produce volatile and non volatile compounds that would occur at high temperature [14]. The results of the odor sensory evaluation (Fig. 4) confirm these results. It is obvious that encapsulation resulted in more than 63% decrease in the odor intensity of the perceived flavor (Fig. 4). Storage of the encapsulated flavor showed a gradual increase in the total pyrazines reaching more than twice their total content before storage while the branched aldehydes showed insignificant decrease (Fig. 3). The results of the odor sensory evaluation confirm those obtained by GC–MS analysis (Fig. 4). The panelists described the released volatiles of the encapsulated flavoring as nutty-like aroma. Storage resulted in a significant (P < 0.05) increase in the perceived aroma.
Fig. 3.
Total yields of the branched aldehydes and pyrazines before and after encapsulation and storage (vertical bars represented ± SD of the means, n = 3). Relative areas of each chemical group followed by same letter means no significant difference at P < 0.05.
Fig. 4.
Odor evaluation of nutty-like flavor before and after encapsulation and storage (vertical bars represented ± SD of the means, n = 10). Odor intensity followed by different letter means significant difference at P < 0.05.
Conclusions
From the aforementioned results and those obtained in previous study [10]. It can be concluded that, for the same microorganism the selection of the appropriate substrate is very important to produce the desired flavor. In present study, the enzymatic soybean hydrolysate was used as a main source of the free amino acids that are required for the biosynthesis of the nutty like aroma. Supplementation of the enzymatic soybean hydrolysate with a mixture of threonine and lysine, as precursors of alkylated pyrazines, resulted in faster production of nutty chocolate-like aroma. However, the odor intensity and total volatiles produced at the end of fermentation time (9 days) was higher in the unsupplemented culture.
The toxicity study confirmed the safety of using the alkylated pyrazines as flavoring agents. The high yield of 2/3-methylbutanal confirmed the chocolate note of the biosynthesized nutty flavor. Storage of the encapsulated nutty flavorings improved its quality. The results of GC–MS analysis confirmed those of odor sensory evaluation.
Conflict of interest
The authors have declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jare.2018.01.003.
Appendix A. Supplementary material
References
- 1.Masuda H., Mihara S. Olfactive properties of alkylpyrazines and 3-substituted 2-alkylpyrazines. J Agric Food Chem. 1988;36:584–587. [Google Scholar]
- 2.Shoji T, Nakaishi T, Mikata M. Process for producing pyrazine compounds, U.S.1997; Patent 5,693,806.
- 3.Fadel H.H.M., Mahmoud M.G., Asker M.S., Lotfy S.N. Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC-107 in solid state fermentation on sugarcane bagasse. Electron J Biotechnol. 2015;18:5–9. [Google Scholar]
- 4.Schrader J. Microbial Xavour production. In: Berger R.G., editor. Flavours and fragrances: chemistry, bioprocessing and sustainability. Springer; Berlin: 2007. pp. 507–574. [Google Scholar]
- 5.Xiao Z.J., Xie N.Z., Liu P.H., Hua D.L., Xu P. Tetramethylpyrazine production from glucose by a newly isolated Bacillus mutant. Appl Microbiol Biotechnol. 2006;73:512–518. doi: 10.1007/s00253-006-0491-6. [DOI] [PubMed] [Google Scholar]
- 6.Afoakwa E.O., Peterson A., Flower M., Ryan A. Flavour formation and character in cocoa and chocolate: a critical review. Crit Rev Food Sci Nutr. 2008;48:840–857. doi: 10.1080/10408390701719272. [DOI] [PubMed] [Google Scholar]
- 7.Larroche C., Besson I., Gros J.B. High pyrazine production by Bacillus subtilis in solid substrate fermentation on ground soybean. Process Biochem. 1999;34:667–674. [Google Scholar]
- 8.Sagong H.-Y., Kim K.-J. Structural basis for redox sensitivity in Corynebacterium glutamicum diaminopimelate epimerase: an enzyme involved in L-lysine biosynthesis. Sci Rep. 2017;7:42318. doi: 10.1038/srep42318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickschat J.S., Wickel S., Bolten C.J., Nawrath T., Schulz S., Wittmann C. Pyrazine biosynthesis in Corynebacterium glutamicum. Eur J Org Chem. 2010;14:2687–2695. [Google Scholar]
- 10.Fadel H.H.M., Lotfy S.N., Mahmoud M.G., Asker M.M.S. Bioproduction of beef-like flavor by Corynebacterium glutamicum 1220t based on enzymatic hydrolyzate of mushroom and soybean. Res J Pharm Biol Chem Sci. 2016;7:2372–2381. [Google Scholar]
- 11.Kanakdande D., Bhosale R., Singhal R.S. Stability of cumin oleoresin microencapsulated in different combination of gum Arabic, maltodextrin and modified starch. Carbohydr Polym. 2007;67:536–541. [Google Scholar]
- 12.Qi Z.H., Xu A. Starch based ingredients for flow encapsulation. Cereal Food World. 1999;44:60–465. [Google Scholar]
- 13.Adams T.B., Doull J., Feron V.J., Goodman J.I., Marnett L.J., Munro I.C. The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. Food Chem Toxicol. 2002;40:429–451. doi: 10.1016/s0278-6915(01)00123-5. [DOI] [PubMed] [Google Scholar]
- 14.Aaslyng M.D., Elmore J.S., Mottram D.S. Comparison of the aroma characteristics of acid-hydrolyzed and enzyme hydrolyzed vegetable proteins produced from soy. J Agric Food Chem. 1998;46:5225–5231. [Google Scholar]
- 15.Lotfy S.N., Fadel H.H.M., El-Gorab A.H., Shaheen M.S. Stability of encapsulated beef-like flavourings prepared from enzymatically hydrolyzed mushroom proteins with other precursors under conventional and microwave heating. Food Chem. 2015;187:7–13. doi: 10.1016/j.foodchem.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 16.Miller L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. [Google Scholar]
- 17.Dubois M., Gills K.A., Hamltton J.K., Rebers P.A., Smoth F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 18.Goodman AG, Goodman LS, Gilman A. Principles of toxicology. In: Goodman, Gilman, editors. The pharmacological basis of therapeutics. 6th ed. New York: Macmillan; 1980. p. 1602–15.
- 19.Baneras L., Trias R., Godayol A., Cerdan L., Nawrath T., Schulz S. Mass spectrometry identification of alkyl-substituted pyrazines produced by pseudomonas spp. isolates obtained from wine corks. Food Chem. 2013;138:2382–2389. doi: 10.1016/j.foodchem.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 20.Fadel H.H.M., Abdel Mageed M.A., Abdel Samad A.K., Lotfy S.N. Cocoa substitute: evaluation of sensory qualities and flavourstability. Eur Food Res Technol. 2006;223:125–131. [Google Scholar]
- 21.Fan W., Xu Y., Zhang G.Y. Characterization of pyrazines in some Chinese liquor and their approximate concentrations. J Agric Food Chem. 2007;5:9956–9962. doi: 10.1021/jf071357q. [DOI] [PubMed] [Google Scholar]
- 22.Yu A.N., Zhang A.D. The effect of pH on the formation of aroma compounds produced by heating a model system containing L-ascorbic acid with L-threonine/L-serine. Food Chem. 2010;119:214–219. [Google Scholar]
- 23.Van Kranenburg R., Kleerebezem M., van HylckamaVlieg J., Ursing B.M., Boekhorst J., Smit B.A. Flavour formation from amino acids by lactic acid bacteria: predictions from genome sequence analysis. Int Dairy J. 2002;12:111–121. [Google Scholar]
- 24.Demain A.L., Jackson M., Trcnner N.R. Thiamine dependent accumulation of tetramethylpyrayine accumulation a mutation in the isoleucine-valine pathway. J Bacteriol. 1967;94:323–326. doi: 10.1128/jb.94.2.323-326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams A., De-Kimpe N. Formation of pyrazines and 2-acetyl-1-pyrroline by Bacillus cereus. Food Chem. 2007:1230–1238. [Google Scholar]
- 26.Rohan T.A., Stewart T. The precursors of chocolate aroma: production of reducing sugars during fermentation of cocoa. J Food Sci. 1967;32:339–402. [Google Scholar]
- 27.Hernandez-Orte P., Cacho J.F., Ferreira V. Relation between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solution and chemometric study. J Agric Food Chem. 2002;50:2891–2899. doi: 10.1021/jf011395o. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez-Orte P, Ibarz M.J., Cacho JF, Ferreira V. Addition of amino acids grape juice of the merlot variety: effect on amino acid uptake and aroma generation during alcoholic fermentation. Food Chem. 2006;98:300–10.
- 29.Yvon M., Rijnen L. Cheese flavour formation by amino acid catabolism. Int Dair J. 2001;11:185–201. [Google Scholar]
- 30.Smit A.B., Engels W.J.M., Smit G. Branched chain aldehydes: production and breakdown pathways and relevance for flavour in foods. Appl Microbiol Biotechnol. 2009;81:987–999. doi: 10.1007/s00253-008-1758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aprotosoaie A.C., Lucaand S.V., Miron A. Flavor chemistry of cocoa an cocoa products – an overview. Compr Rev Food Sci Food Saf. 2017;15:73–91. doi: 10.1111/1541-4337.12180. [DOI] [PubMed] [Google Scholar]
- 32.Smit A.B., Engels W.J.M., Smit G. Diversity of L-leucine catabolism in various microorganisms involved in dairy fermentations, and identification of the rate- controlling step in the formation of the potent flavour component 3-methylbutanal. Appl Microbiol Biotechnol. 2004;64:396–402. doi: 10.1007/s00253-003-1447-8. [DOI] [PubMed] [Google Scholar]
- 33.Amárita F., Requena T., Taborda G., Amigo L., Peláez C. Lactobacillus casei and Lactobacillus plantarum initiate catabolism of methionine by transamination. J App Microbiol. 2001;90:971–978. doi: 10.1046/j.1365-2672.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 34.Dettwiler B., Dunn I.J., Heinzle E., Prenosil J.E. A simulation model for the Continuous production of acetoin and butanediol using Bacillus subtilis with integrated pervaporation separation. Biotechnol Bioeng. 1993;41:791–800. doi: 10.1002/bit.260410805. [DOI] [PubMed] [Google Scholar]
- 35.Foṏrster A.H., Beblawy S., Golitsch F., Johannes G.J. Electrode-assisted acetoin production in a metabolically engineered Escherichia coli strain. Biotechnol Biofuels. 2017;10:65. doi: 10.1186/s13068-017-0745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owens J.D., Allangheny N., Kipping G., Ames J.M. Formation of volatile compounds during Bacillus subtilis fermentation of soya beans. J Sci Food Agric. 1997;74:132–140. [Google Scholar]
- 37.Paget P, Barnes T. Evaluation of drug activities Pharmacometrics. In: Laurrence DH, Bacharach AL, editors. London and New York: Academic Press; 1964. p. 135–40.
- 38.Müller R., Rappert S. Pyrazines: occurrence, formation and biodegradation. Appl Microbiol Biotechnol. 2010;85:1315–1320. doi: 10.1007/s00253-009-2362-4. [DOI] [PubMed] [Google Scholar]
- 39.Tsai T.H.T., Liang C. Pharmacokinetics of tetramethylpyrazine in rat blood and brain using microdialysis. Int J Pharm. 2001;216:61–66. doi: 10.1016/s0378-5173(01)00572-5. [DOI] [PubMed] [Google Scholar]
- 40.Clark B.C., Jones B.B., Jacobucci J.A. Characterization of the hydroperoxides derived from singlet oxygen oxidation of (+)-limonene. Tetrahedron. 1981;37:405–409. [Google Scholar]
- 41.Rodriguez-Campos J., Escalona-Buend́ıa H.B., Contreras-Ramos S.M., Orozco- Avila I., Jaramillo Flores E., Lugo-Cervantes E. Effect of fermentation time and drying temperature on volatile compounds in cocoa. Food Chem. 2012;132:277–288. doi: 10.1016/j.foodchem.2011.10.078. [DOI] [PubMed] [Google Scholar]
- 42.Bonvechi J.S. Investigation of aromatic compounds in roasted cocoa powder. Eur Food Res Technol. 2005;221:19–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.