Abstract
Background:
The randomised phase 2 CABOSUN trial comparing cabozantinib with sunitinib as initial therapy for advanced renal cell carcinoma (RCC) of intermediate or poor risk met the primary end-point of improving progression-free survival (PFS) as assessed by investigator. We report PFS by independent radiology review committee (IRC) assessment, ORR per IRC and updated overall survival (OS).
Patients and methods:
Previously untreated patients with advanced RCC of intermediate or poor risk by IMDC criteria were randomised 1:1 to cabozantinib 60 mg daily or sunitinib 50 mg daily (4 weeks on/2 weeks off). Stratification was by risk group and presence of bone metastases.
Results:
A total of 157 patients were randomised 1:1 to cabozantinib (n = 79) or sunitinib (n = 78). Median PFS per IRC was 8.6 months (95% confidence interval [CI] 6.8—14.0) versus 5.3 months (95% CI 3.0—8.2) for cabozantinib versus sunitinib (hazard ratio [HR] 0.48 [95% CI 0.31—0.74]; two-sided p = 0.0008), and ORR per IRC was 20% (95% CI 12.0—30.8) versus 9% (95% CI 3.7—17.6), respectively. Subgroup analyses of PFS by stratification factors and MET tumour expression were consistent with results for the overall population. With a median follow-up of 34.5 months, median OS was 26.6 months (95% CI 14.6—not estimable) with cabozantinib and 21.2 months (95% CI 16.3—27.4) with sunitinib (HR 0.80 [95% CI 0.53—1.21]. The incidence of grade 3 or 4 adverse events was 68% for cabozantinib and 65% for sunitinib.
Conclusions:
In this phase 2 trial, cabozantinib treatment significantly prolonged PFS per IRC compared with sunitinib as initial systemic therapy for advanced RCC of poor or intermediate risk.
Keywords: Cabozantinib, First-line, Sunitinib, IMDC risk groups, Advanced renal cell carcinoma
1. Introduction
Despite many available treatment options, advanced renal cell carcinoma (RCC) remains essentially incurable. International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria were developed in the era of targeted therapy to classify patients into prognostic groups based on the number of established risk factors (poor risk: 3—6, intermediate risk: 1—2, and favourable risk: 0) [1]. Patients with intermediate or poor risk disease (70—80% of all patients with advanced RCC) have shorter survival duration compared with favourable risk patients and have the greatest need for more effective therapies.
VEGFR-targeted therapy is the current standard first-line treatment for patients with advanced RCC based on improvements in progression-free survival (PFS) in phase 3 clinical trials, with sunitinib and pazopanib as the most commonly used therapies [2]. Patients eventually develop disease progression, with median PFS ranging from 8 to 11 months for sunitinib and pazopanib in populations that include patients of all risk groups [3—5]. Duration of PFS is shorter in intermediate and poor risk patients; for example, in a mixed population of intermediate or poor risk patients treated with targeted therapy, median PFS can be less than 6 months, based on data from the IMDC [6].
The VEGF-signalling pathway is upregulated in clear cell RCC due to inactivation of the VHL tumour suppressor gene [7], providing a molecular rationale for the use of VEGF-targeted therapies in this setting. Targeting oncogenic pathways involved in RCC in addition to VEGF-signalling might result in therapeutic benefit. Two relevant targets are MET and AXL, as both are upregulated as a result of VHL loss and have been associated with tumour progression, resistance to VEGF-pathway inhibition in preclinical models, and poor prognosis in patients with RCC [8—11].
Cabozantinib is an oral inhibitor of MET, AXL, and VEGFR2 [12] that is approved for treatment of patients with advanced RCC after prior antiangiogenic therapy based on results from the phase 3 METEOR trial [13,14]. The randomised, open-label phase 2 CABOSUN trial (Alliance for Clinical Trials in Oncology study A031203) compared cabozantinib versus sunitinib as initial targeted therapy in patients with metastatic RCC of intermediate or poor risk by IMDC criteria. The CABOSUN study met the primary end-point of improving investigator-assessed PFS with cabozantinib compared with sunitinib; median PFS per investigator was 8.2 months with cabozantinib versus 5.6 months with sunitinib (hazard ratio [HR] = 0.66, 95% confidence interval [CI] 0.46—0.95, one-sided log-rank p = 0.012) [15]. A retrospective analysis of PFS and objective response rate (ORR) by a central, blinded independent radiology review committee (IRC) was performed to determine if independent assessment supports the investigator results.
We report results of independent assessment of PFS and ORR as well as updated overall survival (OS) for the CABOSUN trial in patients with advanced RCC of intermediate or poor risk. Subgroup analyses of PFS based on stratification factors and tumour MET expression level are also presented.
2. Methods
2.1. Study design and participants
CABOSUN (Alliance for Clinical Trials in Oncology A031203) is a randomised, phase 2 trial conducted at 77 investigative centres in the United States. Eligible patients were 18 years of age or older with advanced or metastatic RCC with a clear-cell component and measurable disease per investigator without previous systemic treatment for RCC. Patients were required to have had intermediate or poor risk disease per IMDC criteria, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0—2, and adequate organ function and no uncontrolled significant illnesses. Patients with treated, stable brain metastases were allowed. Patients were required to have archival tumour tissue but could choose not to consent to analysis of this tissue for MET expression. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and the protocol was approved by the institutional review board or ethics committee at each centre. Each participant signed an institutional review board—approved, protocol-specific informed consent form in accordance with federal and institutional guidelines. Safety was monitored by the Alliance Data and Safety Monitoring Board (DSMB); the majority of the voting DSMB members are not affiliated with the Alliance, and voting quorums for a DSMB meeting require that the majority of voting members not belong to the Alliance.
2.2. Randomisation and blinding
Patients were randomised 1:1 to receive cabozantinib or sunitinib. Randomisation was stratified by IMDC risk group (intermediate or poor), and the presence of bone metastases (yes or no) using the dynamic allocation method. Randomisation occurred at the time of enrolment using the web-based National Cancer Institute Oncology Patient Enrollment Network (OPEN) system. Patients and investigators were not blinded to treatment. The central IRC was blinded to treatment assignment. Aggregate efficacy analyses were not performed until analysis of the primary end-point was triggered, with the exception of a single planned futility analysis of PFS.
2.3. Procedures
Patients were randomly assigned to receive cabozantinib 60 mg once daily or to receive sunitinib 50 mg once daily for 4 weeks, followed by a 2-week break. Cabozantinib was provided by Exelixis, Inc. (South San Francisco, CA, USA) and sunitinib was purchased commercially. Dose could be modified by dose holds or reductions to manage adverse events. Dose reductions were to 40 mg and 20 mg for cabozantinib and to 37.5 and 25 mg for sunitinib. Patients continued treatment until radiographic disease progression as assessed by the investigator, intolerance to therapy, or withdrawal of consent. Crossover between treatment groups was not prescribed by the protocol. One treatment cycle was defined as 6 weeks. Safety was assessed every cycle and included adverse events, physical examination and standard laboratory tests. Treatment-emergent adverse events were reported and graded by the investigator according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Some adverse events (alanine aminotransferase increased, aspartate aminotransferase increased, blood bilirubin increased, electrocardiogram QT prolonged, fatigue, hypertension, neutrophil count decreased, palmar-plantar erythrodysesthesia syndrome, platelet count decreased, diarrhoea and pancreatitis) were solicited at every visit. For solicited adverse events, grade and relationship to treatment were reported. For unsolicited adverse events, grade 1 or 2 events were only required to be reported if the investigator considered them to be treatment related.
Magnetic resonance imaging or computed tomography scans were performed at baseline and every 12 weeks thereafter until progression or until 5 years after randomisation. Radiographic images were collected from the sites for retrospective assessment by a blinded central independent review committee. Investigators and the IRC assessed tumour response and progression using Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) [16]. Partial and complete responses required confirmation ≥4 weeks after initial response. Survival status was determined every 6 months after discontinuation of study treatment.
Archival tumour tissue was analysed by immunohistochemistry to determine MET expression level, based on published procedures [14,17,18]. Formalin-fixed paraffin-embedded tumour blocks were analysed by the Department of Oncologic Pathology at Dana-Farber Cancer Institute using the SP44 antibody (Ventana Medical Systems, Inc, Tucson, AZ, USA) and MET status was categorised as positive or negative based on a cutoff of ≥50% of tumour cells stained 2 + or 3 + for positive status.
2.4. Outcomes
The primary end-point was PFS assessed by investigator. Secondary end-points were ORR per investigator, OS, and safety. PFS, ORR and best change in tumour target lesions by independent review were post-hoc retrospective analyses which were initiated after analysis of the primary end-point.
2.5. Statistical analyses
The study was designed to test the hypothesis that cabozantinib increased the primary end-point of investigator-assessed PFS compared with sunitinib. The alternative hypothesis was that the hazard ratio for PFS was 0.67 favouring the experimental arm (cabozantinib) over the control arm (sunitinib). With 123 PFS events, the log-rank test had 85% power to detect a HR of 0.67 with a one-sided type I error of 0.12 (equivalent under assumptions of exponential event-time distributions and proportional hazard to an increase in median PFS from 8 months in the sunitinib arm to 12 months in the cabozantinib arm). The planned sample size was 150 patients based on additional assumptions of an accrual rate of 5.8 patients per month for 24 months, 20 months of follow-up after closure to randomisation, and a 7% ineligibility rate. A single futility analysis was conducted when the investigator-determined events reached 50% of the required total.
PFS was defined as the time from randomisation to the earlier of radiographic progression per RECIST version 1.1 or death due to any cause [15]. For retrospective analyses of PFS per IRC, censoring rules per the Food and Drug Administration (FDA) Guidance on Oncology Endpoints [19] were employed. For patients who had not experienced an event at the time of data cutoff, had two or more missing adequate tumour assessments immediately before radiographic progression or death, or had received systemic subsequent anticancer therapy, event-time was censored at the time of their most recent adequate tumour assessment before the date these criteria were met. The application of these FDA-recommended censoring rules for PFS, which were not applied in the previous report of investigator-assessed PFS [15], necessarily reduces the number of events available for analysis. To increase the number of events included in the current analysis, the data cutoff for radiographic end-points was extended from April 11, 2016 (the date of the 123rd investigator-determined event in the previous report) to September 15, 2016. A supportive analysis of investigator-assessed PFS was also conducted with FDA censoring rules using the September 15, 2016 cutoff date.
OS was defined as the time from randomisation to death due to any cause and was analysed with a data cutoff of July 01, 2017. For patients who were alive at the time of data cutoff or were permanently lost to follow-up, duration of OS was right censored at the earliest of date of withdrawal of consent from all followup, data cutoff date, or the date the patient was last known to be alive.
Analyses of efficacy end-points were performed in all randomised patients based on the intent-to-treat principle. Safety was assessed in patients who received at least one dose of study treatment. Analyses of PFS used a stratified log-rank statistic to compare the two treatment arms; stratification factors were those used for the randomisation (IMDC risk group [intermediate or poor] and bone metastases [yes or no]). The Kaplan—Meier method [20] was used to estimate the median PFS and median OS and associated 95% confidence intervals. Hazard ratios were estimated using a Cox proportional-hazard regression model and adjusted with the stratification factors [21]. For subgroup analyses of PFS, all hazard ratios are unstratified. ORR was the proportion of patients with confirmed complete or partial responses per RECIST. Point estimates of ORR with 95% confidence intervals were calculated using exact methods. Although the original statistical design used one-sided p-values, all p-values presented herein are two-sided for consistency with previously published results for cabozantinib in RCC. HRs and CIs for the secondary endpoints are to be considered descriptive, and p-values are not reported because the study did not have prespecified hypotheses for secondary end-points, and no adjustments were made for multiple testing. SAS software (version 9.1 or higher) was used for all analyses.
2.6. Role of the funding source
The study was designed by the Alliance for Clinical Trials in Oncology, endorsed by the ECOG—American College of Radiology Imaging Network Group and approved by the Cancer Therapy Evaluation Program of the National Cancer Institute part of the National Institutes of Health (the funder). The Alliance Statistics and Data Center performed patient registration, data collection, and all previously published statistical analyses and interpretations [15]. Exelixis initiated the analysis of PFS and tumour response by the central IRC and arranged for collection of radiographic images for IRC assessments. All other data presented herein were collected by the Alliance, and quality of those data was ensured by review by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. Statistical analyses presented herein were conducted by Exelixis in collaboration with the Alliance. The authors had access to the raw data. The first draft was written by the corresponding author in collaboration with Exelixis. Medical writing support as well as cabozantinib supply for the study were provided by Exelixis. All authors gave final approval to submit the manuscript, and the corresponding author had final responsibility for the decision to submit for publication.
3. Results
From July 9, 2013 to April 6, 2015, 157 patients were randomised 1:1 to receive cabozantinib (n = 79) or sunitinib (n = 78). Overall, baseline demographics and characteristics were balanced between treatment groups (Table 1). Eighty-one percent of patients were intermediate risk and 19% were poor risk according to IMDC criteria. Twenty-five percent of patients had not had prior nephrectomy, 36% had bone metastases and 73% had two or more metastatic sites. Tumour MET status was determined for 83% of patients; 39% of all randomised patients were MET positive and 44% were MET negative.
Table 1.
Baseline characteristics.
| Characteristic | Cabozantinib (N = 79) | Sunitinib (N = 78) |
|---|---|---|
| Age (years) | 63 (56—69) | 64 (57—71) |
| Sex | ||
| Male | 66 (84%) | 57 (73%) |
| Female | 13 (16%) | 21 (27%) |
| Ethnic origin | ||
| White | 70 (89%) | 75 (96%) |
| Black or African American | 3 (4%) | 2 (3%) |
| Other | 7 (9%) | 1 (1%) |
| ECOG performance status | ||
| 0 | 36 (46%) | 36 (46%) |
| 1 | 33 (42%) | 32 (41%) |
| 2 | 10 (13%) | 10 (13%) |
| IMDC risk group | ||
| Intermediate | 64 (81%) | 63 (81%) |
| Poor | 15 (19%) | 15 (19%) |
| Bone metastasis per IxRS | ||
| Yes | 29 (37%) | 28 (36%) |
| No | 50 (63%) | 50 (64%) |
| Prior nephrectomy | ||
| Yes | 57 (72%) | 60 (77%) |
| No | 22 (28%) | 18 (23%) |
| MET statusa | ||
| Positive | 32 (41%) | 30 (38%) |
| Negative | 39 (49%) | 30 (38%) |
| Missing | 8 (10%) | 18 (23%) |
| Sum of diameters of lesions per RECIST per investigator (cm) | 7.2 (4.3—11.7) | 8.1 (4.7—13.4) |
| Number of metastatic sites per investigator | ||
| 1 | 17 (22%) | 26 (33%) |
| 2 | 37 (47%) | 20 (26%) |
| ≥3 | 25 (32%) | 32 (41%) |
| Metastatic sites per investigator | ||
| Nodal | 45 (57%) | 42 (54%) |
| Lung | 55 (70%) | 54 (69%) |
| Liver | 15 (19%) | 20 (26%) |
| Bone | 31 (39%) | 30 (38%) |
| CNS/brain | 3 (4%) | 2 (3%) |
Data are n (%) or median (IQR).
Abbreviations: IMDC, International Metastatic Renal Cell Carcinoma Database Consortium criteria; IxRS, interactive web/voice response system.
Based on tumour MET levels by immunohistochemistry.
As of the September 15, 2016 data cutoff date for PFS per IRC, 10 (13%) patients in the cabozantinib group and 2 (3%) patients in the sunitinib group remained on study treatment (Fig. 1). Median duration of follow-up through this date was 25.0 months (IQR 21.9—30.9). For the IRC assessment, radiographic tumour images were available for 156 of 157 patients (one site with one patient in the sunitinib group declined to participate in radiographic image collection). For these 156 patients, 100% of the known tumour images through the data cutoff were retrieved.
Fig. 1. Trial profile through September 15, 2016.
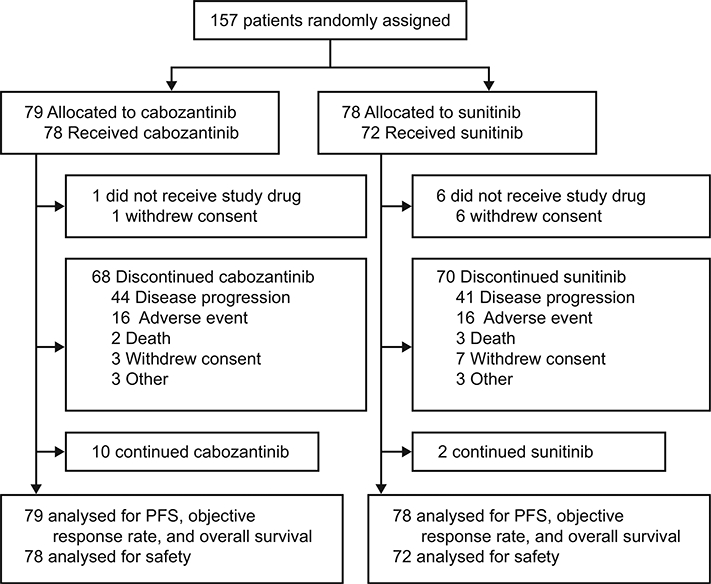
PFS, progression-free survival.
As of the September 15, 2016 cutoff date, 92 PFS events were observed (43 with cabozantinib and 49 with sunitinib) as assessed by the IRC. Cabozantinib significantly improved PFS per IRC (Fig. 2); median PFS per IRC was 8.6 months (95% CI 6.8—14.0) with cabozantinib versus 5.3 months (95% CI 3.0—8.2) with sunitinib (HR 0.48 [95% CI 0.31—0.74]; p = 0.0008). Results per investigator assessment using the same cutoff date and censoring rules were consistent with those from the independent assessment; median PFS per investigator was 8.3 months (95% CI 6.5—12.4) with cabozantinib versus 5.4 months with sunitinib (95% CI 8.2; HR 0.56 [95% CI 0.37—0.83]; p = 0.0042).
Fig. 2. Kaplan—Meier plot of progression-free survival per independent radiology review committee through September 15, 2016.
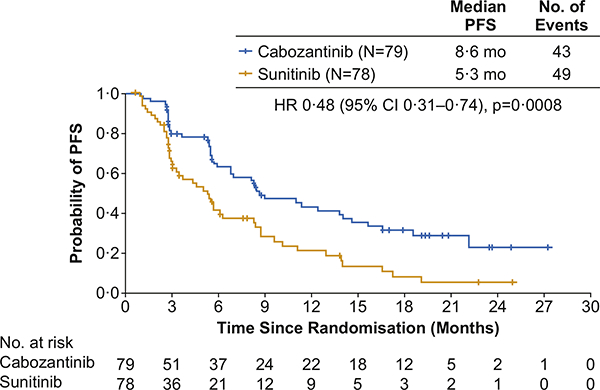
All 157 randomised patients were included in the analysis. HR, hazard ratio; PFS, progression-free survival.
Subgroup analyses of PFS per IRC assessment based on the stratification factors and MET expression level are presented in the appendix (Appendix p 4). The relative treatment effect in subgroups based on stratification factors was consistent with the result for the overall population. For MET-positive patients (n = 62), median PFS was 13.8 months (95% CI 5.7—22.1) with cabozantinib and 3.0 months (95% CI 2.5—5.4) with sunitinib (HR 0.32 [95% CI 0.16—0.63]). For MET-negative patients (n = 69), median PFS was 6.9 months (95% CI 14.6) with cabozantinib and 6.1 months (95% CI 3.6—9.6) with sunitinib (HR 0.67 [95% CI 0.37—1.23]).
As of September 15, 2016, any reduction in tumour target lesions by IRC assessment was observed in 63 (80%) of 79 patients with cabozantinib compared with 39 (50%) of 78 patients with sunitinib (Fig. 3). Confirmed objective responses per IRC were observed in 16 patients (20% [95% CI 12.0—30.8]) in the cabozantinib group and 7 patients (9% [95% CI 3.7—17.6]) in the sunitinib group (Table 2). All responses were partial responses. The disease control rate (complete responses + partial responses + stable disease) was 75% (59 of 79 patients) with cabozantinib and 47% (37 of 78 patients) with sunitinib. Using the same cutoff date, confirmed objective responses per investigator assessment were observed in 26 patients (33% [95% CI 22.7—44.4]) in the cabozantinib group and 9 patients (12% [95% CI 5.4—20.8]) in the sunitinib group (Appendix p 5). One confirmed complete response per investigator was observed with cabozantinib; all other responses were partial responses. The disease control rate per investigator was 76% (60 patients) with cabozantinib and 49% (38 patients) with sunitinib.
Fig. 3. Best target lesion change from baseline.
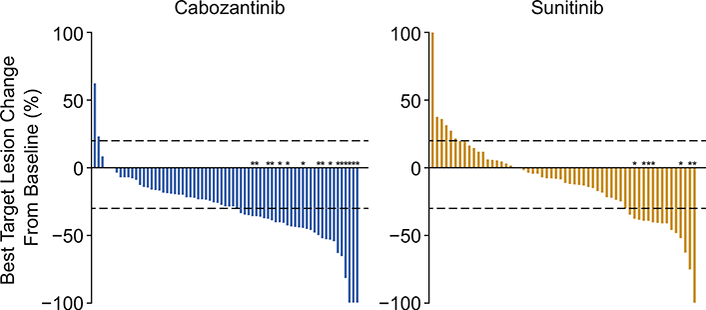
*Confirmed partial responses. Six patients in the cabozantinib group and 18 patients in the sunitinib group were unevaluable because they had no adequate post-baseline imaging assessments (Table 2).
Table 2.
Tumour response per independent radiology review committee.
| Tumour response | Cabozantinib (N = 79) | Sunitinib (N = 78) |
|---|---|---|
| Objective response rate (95% CI) | 20% (12%—31%) | 9% (4%—18%) |
| Best overall response | ||
| Confirmed partial response | 16 (20%) | 7 (9%) |
| Stable disease | 43 (54%) | 30 (38%) |
| Progressive disease | 14 (18%) | 23 (29%) |
| Unevaluable or missinga | 6 (8%) | 18 (23%) |
Data are % or n (%) and are as of September 15, 2016. All responses were partial responses.
Unevaluable or missing for the following reasons: cabozantinib: adverse event (5), withdrew consent (1); sunitinib: adverse event (6), death (2), disease progression (1), withdrew consent (9).
For ORR per IRC, images for six patients in the cabozantinib group and 18 patients in the sunitinib group were unevaluable or missing for tumour response. These patients discontinued or did not receive study treatment for the following reasons: adverse event (5 in the cabozantinib group versus 6 in the sunitinib group), withdrew consent (1 versus 9), death (0 versus 2) and disease progression (0 versus 1). Baseline characteristics and disposition for the patients who had unevaluable or missing post-baseline radiographic images compared with those whose images were adequately assessed are summarised in the appendix (Appendix p 6). Some characteristics differed; for example, in the sunitinib group, among those who had unevaluable or missing post-baseline radiographic images, 28% of patients had poor risk disease, and 22% of patients had an ECOG PS of 2 compared with 17% with poor risk disease and 10% with ECOG PS of 2 in those whose images were adequately assessed.
As of the July 01, 2017, data cutoff date for OS with a median follow-up of 35.4 months (IQR 31.4—40.4), 90 deaths had occurred (43 of 79 patients in the cabozantinib group and 47 of 78 patients in the sunitinib group). Median OS was 26.6 months (95% CI 14.6—not estimable) with cabozantinib and 21.2 months (95% CI 16.3—27.4) with sunitinib. The stratified HR for death was 0.80 (95% CI 0.53—1.21; Fig. 4). Subsequent anticancer therapy (surgery, radiotherapy, or systemic therapy) was received by 51 (65%) patients in the cabozantinib group and 50 (64%) patients in the sunitinib group with systemic therapy received by 48 (61%) and 48 (62%), respectively (Appendix p 7). Systemic therapies included tyrosine kinase inhibitors (38 [48%] in the cabozantinib group versus 37 [47%] in the sunitinib group), mTOR inhibitors (15 [19%] versus 18 [23%]) and PD-1 checkpoint inhibitors (14 [18%] versus 15 [19%]).
Fig. 4. Kaplan—Meier plot of overall survival through July 01, 2017.
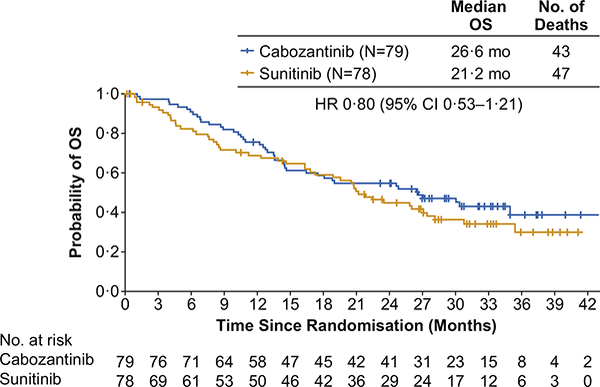
All 157 randomised patients were included in the analysis. HR, hazard ratio; OS, overall survival.
As of the September 15, 2016 data cutoff date, median duration of exposure was 6.5 months (IQR 2.8—16.5) for cabozantinib-treated patients (n = 78) and 3.1 months (IQR 2.0—8.2) for sunitinib-treated patients (n = 72). Dose reductions occurred for 36 cabozantinib-treated patients (46%) and 25 sunitinib-treated patients (35%). The median average daily dose was 50.3 mg (IQR 41.8—60.0) for cabozantinib and 44.7 mg (IQR 35.1—50.0) for sunitinib (while on study treatment). Treatment discontinuation due to an adverse event occurred for 16 patients (21%) with cabozantinib and 16 patients (22%) with sunitinib.
Adverse events of any grade regardless of causality were recorded for 75 (96%) cabozantinib-treated patients and 71 (99%) sunitinib-treated patients (Table 3). Grade 3 or 4 adverse events occurred for 53 (68%) cabozantinib-treated patients and 47 (65%) sunitinib-treated patients. The most common grade 3 or 4 adverse events were hypertension (22 [28%] in the cabozantinib group versus 15 [21%] in the sunitinib group), diarrhoea (8 [10%] versus 8 [11%]), fatigue (5 [6%] versus 12 [17%]) and platelet count decreased (1 [1%] versus 8 [11%]). Grade 5 adverse events occurred for 3 (4%) patients with cabozantinib and 7 (10%) patients with sunitinib. Two grade 5 adverse events (renal failure acute and sepsis) were considered related to cabozantinib and four grade 5 adverse events (angiopathy, sepsis, respiratory failure, sudden death) were considered related to sunitinib.
Table 3.
All-causalitv adverse events.
| Adverse event | Cabozantinib (N = 78) | Sunitinib (N = 72) | ||||
|---|---|---|---|---|---|---|
| Grade 1—2 | Grade 3 | Grade 4 | Grade 1—2 | Grade 3 | Grade 4 | |
| Any adverse event | 19 (24%) | 45 (58%) | 8 (10%) | 17 (24%) | 42 (58%) | 5 (7%) |
| Diarrhoeaa | 49 (63%) | 8 (10%) | 0 | 31 (43%) | 8 (11%) | 0 |
| AST increaseda | 45 (58%) | 1 (1%) | 1 (1%) | 20 (28%) | 2 (3%) | 0 |
| Fatiguea | 45 (58%) | 5 (6%) | 0 | 37 (51%) | 12 (17%) | 0 |
| ALT increaseda | 39 (50%) | 3 (4%) | 1 (1%) | 20 (28%) | 0 | 0 |
| Decreased appetite | 33 (42%) | 4 (5%) | 0 | 22 (31%) | 1 (1%) | 0 |
| Dysgeusia | 32 (41%) | 0 | 0 | 21 (29%) | 0 | 0 |
| Hypertensiona | 30 (39%) | 22 (28%) | 0 | 17 (24%) | 14 (19%) | 1 (1%) |
| Platelet count decreaseda | 29 (38%) | 1 (1%) | 0 | 36 (50%) | 6 (8%) | 2 (3%) |
| PPESa | 27 (35%) | 6 (8%) | 0 | 21 (29%) | 3 (4%) | 0 |
| Anaemia | 25 (32%) | 1 (1%) | 0 | 31 (43%) | 2 (3%) | 0 |
| Stomatitis | 25 (32%) | 4 (5%) | 0 | 17 (24%) | 4 (6%) | 0 |
| Nausea | 23 (29%) | 2 (3%) | 0 | 25 (35%) | 3 (4%) | 0 |
| Weight decreased | 22 (28%) | 3 (4%) | 0 | 12 (17%) | 0 | 0 |
| Dyspepsia | 21 (27%) | 0 | 0 | 12 (17%) | 0 | 0 |
| Hypothyroidism | 18 (23%) | 0 | 0 | 4 (6%) | 0 | 0 |
| Blood creatinine increased | 17 (22%) | 2 (3%) | 0 | 13 (18%) | 2 (3%) | 0 |
| Vomiting | 17 (22%) | 1 (1%) | 0 | 14 (19%) | 2 (3%) | 0 |
| Dizziness | 16 (21%) | 1 (1%) | 0 | 16 (22%) | 0 | 0 |
| Dysphonia | 16 (21%) | 1 (1%) | 0 | 1 (1%) | 1 (1%) | 0 |
| Hyperglycaemia | 16 (21%) | 0 | 0 | 7 (10% | 4 (6%) | 0 |
| Neutrophil count decreaseda | 12 (15%) | 0 | 0 | 22 (31%) | 3 (4%) | 0 |
| White blood cell count decreased | 9 (12%) | 0 | 0 | 23 (32%) | 2 (3%) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; PPES, palmar-plantar erythrodysesthesia syndrome.
Treatment-emergent adverse events are summarised as of September 15, 2016. Adverse events that were reported as grade 1 or 2 in at least 20% of the patients in either study group are shown, irrespective of whether the event was considered by the investigator to be related to the study treatment. Some adverse events were solicited at every visit. For unsolicited adverse events, grade 1 or 2 events were only required to be reported if they were considered related by the investigator. Patients are counted once at the highest grade for each preferred term. The severity of adverse events was graded by the investigator according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Solicited adverse event.
4. Discussion
Cabozantinib significantly prolonged PFS per IRC assessment compared with sunitinib as initial targeted therapy in patients with advanced RCC of intermediate or poor risk by IMDC criteria. Median PFS per IRC was 8.6 months with cabozantinib versus 5.3 months with sunitinib (HR 0.48). Subgroup analyses of PFS based on stratification factors showed a relative treatment effect consistent with the overall population results. The ORR per IRC was about two times higher with cabozantinib than with suntinib. OS analysis showed a HR <1, observationally favouring cabozantinib, although the 95% CI included 1.
The randomised, phase 2 CABOSUN trial was designed to evaluate whether cabozantinib increased PFS compared with sunitinib. Consistent with the phase 2 design, the primary end-point was investigator-assessed PFS with a one-sided p-value and high critical value of 0.12. The retrospective blinded independent assessment by a central IRC was initiated after the study met the primary end-point and used censoring rules per FDA guidance for the analysis. The application of FDA censoring resulted in fewer events in the IRC analysis compared with the previous investigator analysis despite additional follow-up of approximately 5 months. Missing or inadequate tumour assessments immediately preceding an event or receipt of subsequent systemic therapy caused censoring in the current analysis but not in the previous analysis. The results by IRC assessment were highly statistically significant (two-sided p-value = 0.0008) and consistent with the previous investigator assessment [15] as well as the investigator assessment reported here, which was conducted with the same data cutoff and FDA censoring rules.
The ORR was improved with cabozantinib compared with sunitinib for both the IRC and investigator assessments. Although the ORR with cabozantinib was higher when assessed by the investigator, the disease control rate with cabozantinib was similar by both assessments, indicating a shift from confirmed partial response to stable disease in the IRC assessment. In addition, the majority of patients in the cabozantinib group had regression in tumour target lesions by IRC, and the percentage who experienced tumour reduction was about 1.5 times higher than that observed with sunitinib, similar to the original investigator review [15].
More patients did not have adequate tumour assessments in the sunitinib group (n = 18) compared with the cabozantinib group (n = 6) primarily because of early withdrawn consent, death or disease progression. To evaluate whether this difference might have affected the radiographic efficacy end-points, baseline characteristics of the 18 patients in the sunitinib group whose radiographic images were missing or unevaluable for tumour response were compared with those of the remaining 60 sunitinib group patients with adequate assessments. A higher percentage of patients with missing or unevaluable assessments were classified as poor risk, ECOG PS 2 and/ or had other factors associated with poor prognosis than the patients who were adequately assessed. Therefore, the missing tumour assessment data for these patients are unlikely to have unfavourably affected the radiographic efficacy outcomes in the sunitinib group.
The median PFS per IRC of sunitinib in this study (5.3 months) and ORR per IRC (9%) were lower than that those previously reported for sunitinib in patients with advanced RCC [15]. In the COMPARZ trial, median PFS per IRC with sunitinib was 9.5 months and ORR per IRC was 25% [3]; however, as discussed in Choueiri et al. [15], the present study included only patients with intermediate or poor risk disease, whereas the COMPARZ trial included patients of all risk groups (favourable, intermediate or poor). Patients with intermediate or poor risk disease treated with targeted therapy have shorter duration of PFS [6]. Median PFS per IRC for suntinib in the CABOSUN study was also shorter than the value of 8.4 months for suntinib reported for patients with intermediate or poor risk RCC in the CheckMate-214 study [22,23].
Cross study comparisons are confounded by uncontrolled variables in patient characteristics and physician practice. The CABOSUN study included a relatively high incidence of patients with poor prognostic features not explicitly included in the IMDC criteria, including the presence of bone metastases [24], lack of prior nephrectomy [25], greater number of metastatic sites, and worse ECOG PS [26,27]. Furthermore, PFS may be shorter in the cooperative group setting, which is more similar to real-world experience compared with industry-sponsored trials. For example, in the CALGB 90206 trial of interferon-alpha with or without bevacizumab in untreated patients with advanced RCC, median PFS was 1.7 months shorter in the combination arm on the CALGB study [28] compared with the value reported for this combination in the phase 3 industry-sponsored Avoren study [29].
Updated OS included an additional 9.5 months of follow-up compared with the previous analysis and showed a hazard ratio <1, observationally favouring cabozantinib over sunitinib, consistent with the previous results [15]. This phase 2 study was designed to evaluate the primary end-point of PFS, and the secondary endpoint of OS was not powered for determination of survival differences. Importantly, improved PFS has been shown to correlate with prolonged survival in RCC by several retrospective studies, and PFS has been used as the primary end-point for many pivotal clinical studies in RCC [30,31].
Updated safety was consistent with that reported at the earlier cutoff and with the known safety profiles of both study treatments [4,14,15]. The most common adverse events were diarrhoea, hypertension, fatigue, and AST increased with cabozantinib and fatigue, platelet count decreased and diarrhoea with sunitinib. In this study, some adverse events were solicited, which may have increased the reported incidence of these events. Conversely, adverse events that were unsolicited and grade 1 or 2 did not have to be reported unless considered treatment related by the investigator, which would likely decrease the recorded incidence of these events. Dose reductions to manage adverse events were frequent in both treatment groups, and the incidence of treatment discontinuation due to adverse events was similar with both study treatments.
The combination of nivolumab and ipilimumab was recently shown to significantly improve OS compared with sunitinib in patients of intermediate or poor IMDC risk in a phase 3 study of previously untreated patients with advanced RCC [23]. PFS was also improved in these patients, although the result was not statistically significant [22,23]. The improvement in PFS was only observed in the subgroup of intermediate or poor risk patients with high tumour PD-L1 expression (HR 0.46 for PD-L1 expression ≥ 1% versus HR 1.00 for PD-L1 <1%). Furthermore, PFS was shorter for the combination regimen compared with sunitinib in patients of favourable IMDC risk (HR 2.17; p < 0.0001), who have lower tumour PD-L1 expression levels. These results suggest that patient characteristics may be important in the selection of first-line treatment and that regimens that combine checkpoint inhibitors with cabozantinib should be explored as first-line therapy.
The observed improvement in PFS with cabozantinib compared with sunitinib may be due, in part, to inhibition of MET and AXL by cabozantinib in addition to VEGF receptors. Subgroup analyses of PFS based on MET expression level favoured cabozantinib over sunitinib (HR < 1) regardless of MET status. Although the HR more strongly favoured cabozantinib for MET-positive versus MET-negative patients, subgroup sizes were small and analyses were descriptive.
Cabozantinib treatment resulted in clinically meaningful and statistically significant prolongation of PFS per IRC compared with sunitinib as initial targeted therapy in patients with advanced RCC in this phase 2 trial. The independent assessment confirms the investigator-assessed results for PFS and supports that cabozantinib is a potential treatment option as initial therapy for patients with advanced RCC of intermediate or poor risk.
Supplementary Material
Acknowledgements
The authors thank the patients, their families, the investigators and site staff, the Alliance for Clinical Trials in Oncology, the Cancer Therapy Evaluation Program (John Wright), and the study teams participating in this trial. Research reported in this publication was supported by National Institutes of Health (award numbers U10CA180821 and U10CA180882 [to the Alliance for Clinical Trials in Oncology], U10CA180791, U10CA180833, U10CA180850, U10CA180857, U10CA180867) and Exelixis, Inc. The content is the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. TKC is supported in part by the Dana-Farber/Harvard Cancer Center Kidney SPORE and Program, the Kohlberg Chair at Harvard Medical School and the Trust Family, Michael Brigham, and Loker Pinard Funds for Kidney Cancer Research at the Dana-Farber Cancer Institute. Patient treatment at Memorial Sloan Kettering Cancer Center was supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). Medical writing support was provided by Julie C Lougheed, PhD (Exelixis) with editorial assistance by Fishawack Communications (Conshohocken, PA, USA), which was funded by Exelixis.
Appendix
Contributors
TKC, SH, BS, MDM, OH, TO, JP, EJS, SD, DRF, DG and MJM were involved in study design. TKC was the study chair. Patients were recruited and accrued by TKC, MDM, OH, MW, TO, JP, EJS, SD, DRF, DG and MJM. SH, BS and CH were study statisticians. TKC, MDM, OH, MW, TO, JP, EJS, SD, DRF, CS, DG and MJM were involved in data collection. TKC, CH, SH, BS, MDM, OH, TO, JP, EJS, SD, DRF, MM, CS, DG and MJM contributed to data analysis. All authors contributed to manuscript preparation.
Conflict of interest statement
TKC reports personal fees for an advisory/consulting role from Pfizer, GlaxoSmithKline, Novartis, Merck, Bayer, Eisai, Roche, Prometheus Labs Inc., Foundation Medicine Inc., Bristol-Myers Squibb, and research funding from Pfizer, GlaxoSmithKline, Novartis, Bristol-Myers Squibb, Merck, Exelixis Inc., Roche, AstraZeneca, Tracon and Peloton. MDM reports attendance at advisory boards for Pfizer and Exelixis, Inc., outside the submitted work. OH reports relevant financial activities outside the submitted work, and participation at an advisory board for Pfizer. MJM reports attendance at advisory boards for Bayer, Astellas and Progenics, personal fees and research support from Progenics, and research support from Endocyte, outside the submitted work. DRF reports research support from Seattle Genetics and Novartis, outside the submitted work. DG reports personal fees from Dendreon, Novartis, Sanofi, Bayer, Medivation, Biopharm, Axess Oncology, Exelixis, Inc., Pfizer, GlaxoSmithKline, Astellas Pharma, Innocrin Pharma, Bristol-Myers Squibb, Genentech, Janssen, Acceleron Pharma, Celgene, Merk Sharp & Dohme, and Myovant Sciences, Inc, and research funding from Dendreon, Novartis, Bayer, Exelixis, Inc., Pfizer, Astellas Pharma, Innocrin Pharma, Bristol-Myers Squibb, Genentech, Janssen, Millennium, Acerta Pharma, outside the submitted work. CH, MM and CS are the employees of Exelixis, Inc. All other authors declare no competing interests.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ejca.2018.02.012.
Trial Registration Number: NCT01835158.
References
- [1].Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013;14(2):141—8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Choueiri TK, Motzer RJ. Systemic therapy for metastatic renalcell carcinoma. N Engl J Med 2017;376(4):354—66. [DOI] [PubMed] [Google Scholar]
- [3].Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369(8):722—31. [DOI] [PubMed] [Google Scholar]
- [4].Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356(2):115—24. [DOI] [PubMed] [Google Scholar]
- [5].Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28(6):1061—8. [DOI] [PubMed] [Google Scholar]
- [6].Ko JJ, Choueiri TK, Rini BI, Lee JL, Kroeger N, Srinivas S, et al. First-, second-, third-line therapy for mRCC: benchmarks for trial design from the IMDC. Br J Cancer 2014;110(8):1917—22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shen C, Kaelin WG Jr. The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol 2013;23(1):18—25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gibney GT, Aziz SA, Camp RL, Conrad P, Schwartz BE, Chen CR, et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol 2013;24(2):343—9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.[] Gustafsson A, Martuszewska D, Johansson M, Ekman C, Hafizi S, Ljungberg B, et al. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res 2009;15(14):4742—9. [DOI] [PubMed] [Google Scholar]
- 10.[] Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci USA 2014;111(37):13373—8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016;35(21):2687—97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10(12):2298—308. [DOI] [PubMed] [Google Scholar]
- [13].Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373(19):1814—23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17(7):917—27. [DOI] [PubMed] [Google Scholar]
- [15].Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol 2017;35(6):591—7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2): 228—47. [DOI] [PubMed] [Google Scholar]
- [17].Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebocontrolled phase 2 study. Lancet Oncol 2013;14(1):55—63. [DOI] [PubMed] [Google Scholar]
- [18].Spigel DR, Ervin TJ, Ramlau RA, Daniel DB, Goldschmidt JH Jr, Blumenschein GR Jr, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31(32):4105—14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. 2007 https://www.fda.gov/downloads/ Drugs/Guidances/ucm071590.pdf. May. ed.
- [20].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53(282):457—81. [Google Scholar]
- [21].Cox DR, Oakes D. Analysis of survival data. London: Chapman & Hall; 1984. [Google Scholar]
- [22].Escudier B, Tannir NM, McDermott DF, Frontera OA, Melichar B, Plimack ER, et al. CheckMate 214: efficacy and safety of nivolumab + ipilimumab (N+I) v sunitinib (S) for treatment-naїve advanced or metastatic renal cell carcinoma (mRCC), including IMDC risk and PD-L1 expression subgroups. Ann Oncol 2017;28(Suppl. 5). LBA5. [Google Scholar]
- [23].Motzer RJ, Tannir NM, McDermott DF, Frontera OA, Melichar B, Plimack ER, et al. Nivolumab + Ipilimumab (N+I) vs Sunitinib (S) for treatment-naїve advanced or metastatic renal cell carcinoma (aRCC): results from CheckMate 214, including overall survival by subgroups. J Immunother Cancer 2017; 5(Suppl. 3):89. [Google Scholar]
- [24].McKay RR, Lin X, Perkins JJ, Heng DY, Simantov R, Choueiri TK. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur Urol 2014;66(3):502—9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hanna N, Sun M, Meyer CP, Nguyen PL, Pal SK, Chang SL, et al. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: a national cancer data base study. J Clin Oncol 2016; 34(27):3267—75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Manola J, Royston P, Elson P, McCormack JB, Mazumdar M, Negrier S, et al. Prognostic model for survival in patients with metastatic renal cell carcinoma: results from the international kidney cancer working group. Clin Cancer Res 2011; 17(16):5443—50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Motzer RJ, Bukowski RM, Figlin RA, Hutson TE, Michaelson MD, Kim ST, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer 2008;113(7):1552—8. [DOI] [PubMed] [Google Scholar]
- [28].Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008;26(33):5422—8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, doubleblind phase III trial. Lancet 2007;370(9605):2103—11. [DOI] [PubMed] [Google Scholar]
- [30].Halabi S, Rini B, Escudier B, Stadler WM, Small EJ. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic renal cell carcinoma. Cancer 2014; 120(1):52—60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Delea TE, Khuu A, Heng DY, Haas T, Soulieres D. Association between treatment effects on disease progression end points and overall survival in clinical studies of patients with metastatic renal cell carcinoma. Br J Cancer 2012;107(7):1059—68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


