Graphical abstract
Keywords: Thraustochytrid, Meat-processing industry, Pig slaughtering plant, Environmental pollution, Chemical oxygen demand, Iron
Abstract
Industrial wastewaters from pig slaughtering plants (PSPs) generated in the slaughtering process could have an environmental impact, if discharged to a receiving water body without any treatment. In this study, a Chilean Thraustochytrid (TH) strain, a class of marine protist, was used for the bioremediation of piggery slaughterhouse wastewater (SWW). According to the physicochemical analysis of the residue, it was characterized by an initial chemical oxygen demand (COD) of 9610 mg L−1, 18,625 mg L−1 of oil and grease, 1639 mg L−1 of total nitrogen, 149 mg L−1 of total phosphorus, and 82.41 mg L−1 of total iron. Growth studies were conducted to evaluate the growth and biomass production of the strain on residue-based media and its subsequent bioremediation ability. After 5–7 days of fermentation, the results showed that COD of the medium supernatant was reduced by 56.29% (4200 mg L−1), while oil and grease had a significant decrease about 99% (18 mg L−1), and the content of total nitrogen, total phosphorus, and total iron were also decreased by 63.27% (602 mg L−1), 97.55% (3.65 mg L−1) and 60.35% (30.88 mg L−1), respectively. With these results, it was concluded that VAL-B1 can be used for the bioremediation of industrial wastewater from PSPs, and therefore THs could contribute to regulate the environmental pollution.
Introduction
Food industry generates a great variety of residues and constitutes one of the most harmful productive sector for the environment [1], especially industrial wastewater (IWW), which consists in residual water generated in industrial establishments after being used in different processes, activities or services, and it is considered one of the most important contamination sources in the environmental pollution [2]. There are different types of IWW, based on the industries and the contaminants, in which each sector produces its own particular combination of pollutants [3]. Among these types of IWW, meat-processing sector is one of the most-water consuming industry [4], which produces high volume of slaughterhouse wastewater (SWW) generated from slaughtering process of animals, and contains various and high amounts of proteins, blood, fat, oil, feathers, lard, bones, microorganisms, hair, flesh, manure, etc. [5] and sometimes it can also contain heavy metals, disinfectants, cleaning agents, and pharmaceuticals for veterinary purposes [6]. The main pollutant in slaughterhouse discharges is organic matter, in which the chemical oxygen demand (COD) ranges between 500 and 15,900 mg L−1 [7]. Due to these characteristics, SWW could have a significant environmental impact if are discharged to a receiving environment (e.g., oceans, seas, lakes or groundwater) without a previous treatment. Within the possible treatments which could be applied for the management of these type of IWW, bioremediation is a technique that has acquired an increasing importance for waste treatment, which involves the use of microorganisms, plants, fungus, etc. to remove or neutralize pollutants from a polluted site, either by absorbing, degrading, removing or transforming the toxic compounds into harmless or less toxic metabolic products [8], [9]. In the recent years there has been an increasing interest on a marine eukaryotic protist belonging to the phylum Labyrinthulomycota, which is classified as an oleaginous microorganism [10], [11], called thraustochytrid (TH). THs grow on a heterotrophic medium (e.g., with glucose, fructose and starch as carbon sources and yeast extract, monosodium glutamate and peptone as nitrogen sources), and commonly require micronutrients, such as, vitamin B1 (thiamine) and vitamin B12 (cobalamine) for normal growth, and more recently, it has been reported that culture medium containing trace elements (e.g., iron and zinc) have a positive effect on microorganism growth [12]. On the other hand, this microorganism is known for its ability to produce biomass rich in lipids, with high content of polyunsaturated fatty acids (PUFAs), such as, docosahexanoic acid (DHA) and eicosapentaenoic acid (EPA). In addition, it has been demonstrated that THs are able to use different residues as a substrate for their growth and biomass production, for example, okara, a residue of soy milk [13], crude glycerol [14], coconut water [15] and bread crumbs [16]. However, since most of the investigations are focused on the biotechnological application of the microorganism (PUFAs production), the bioremediation potential of THs has been scarcely evaluated, and there are few studies in which researchers observed that THs can act favorably on the pollutant load of residues and decrease the concentration of physicochemical parameters, such as COD [17], [18], total nitrogen, nitrate, ammonia and phosphate content on different residues of food industry [19], [20]. Thus, the aim of this work was to evaluate the bioremediation ability of a native strain of TH in piggery SWW. Here, we determined the appropriate concentration for the optimal use of residue and biomass production by the microorganism, and evaluated the physicochemical parameters before and after the cultures. This is the first report that uses a Chilean thraustochytrid strain for the bioremediation of piggery slaughterhouse wastewater.
Material and methods
Chemicals
Monosodium glutamate (MSG), glucose (Gluc), C2H6O, CH3OH, HCl, NaOH, CHCl3, C6H14, CaCl2×2H2O, KCl and standard solutions of Zn, Fe, Cu were purchased from Merck (Darmstadt, Germany). NaCl, MgCl×6H2O, MgSO4×7H2O, streptomycin sulfate, Penicillin G were purchased from Sigma-Aldrich (Steinheim, Germany). Agar, peptone, yeast extract (YE), NaHCO3 and FeCl3×6H2O were purchased from Becton Dickinson and Co. (New Jersey, USA), Oxoid (Wade Road, Basingstoke, United Kingdom), Himedia (Mumbai, India), Synth (Diadema, Brazil) and Scharlau (Sentmenat, Spain), respectively, and FAME standard mixtures were obtained from Sigma-Aldrich (Missouri, USA).
Residue
The residue was obtained from a piggery cold meat factory, and corresponds to the animal blood mixed with water used for cleaning of facilities, and with solid residues (animal’s fat, hair, meat residue, etc.) generated in the pig slaughtering process. The residue was homogenized on a magnetic stirrer, and filtered with cheesecloth, and then by using filter paper (Advantec, No. 1, Tokyo, Japan) for its use during this study.
Microorganism
A Chilean strain from Thraustochytriidae family, Thraustochytrium kinney VAL-B1 (Fig. 1) (GenBank accession number: KF709393), isolated from Carvallo beach’s coastal zone at Valparaíso, Chile (geographic coordinates: 33°1′9″S 71°38′30″W) was used [21]. The strain was kept at 4 °C in B1 solid medium (for 1 L of artificial seawater (ASW): peptone 1 g, YE 1 g, agar 10 g; pH 6.5) containing streptomycin sulfate and penicillin G (300 mg L−1).
Fig. 1.

Epifluorescence microscopy of Thraustochytrium kinney VAL-B1 showing (a) vegetative cells attached to (b) pine polen (Leica DM IL, Germany). Scale bar 10 µm.
Culture conditions
The inoculum was prepared by transferring cells from agar B1 solid medium to 50 mL of sterile medium B2 (for 1 L ASW: glucose 10 g, YE 2 g, MSG 2 g). The incubation in Erlenmeyer flasks was held for 48 h at 25 °C with orbital shaking at 180 rpm, according to Hinzpeter et al. [22].
Selection of the appropriate concentration for the optimal use of residue
The residue-based media were prepared by adding YE-MSG (both at 2 g L−1), and the residue at different dilutions (25, 50, 80 and 100% of residue) with ASW (for 1 L: NaCl 27.5 g, MgCl2×6H2O 5.38 g, MgSO4×7H2O 6.78 g, KCl 0.72 g, NaHCO3 0.2 g and CaCl2×2H2O 1.4 g, at 29 PSU), followed by the adjusting of pH at 6.5 and autoclaving (121 °C, 15 min). Sterile growth media (100 mL) were inoculated (inoculum size used was 5% of the total volume of the media, 5% v/v, and optical density of inoculum was 0.6 at 600 nm), and incubated at 25 °C in Erlenmeyer shaking flasks at 180 rpm (LabTech LSI-3016A, United Kingdom), for 5 days.
Analytical methods
Biomass production
Growth was quantified by measuring the accumulation of the TH biomass throughout the fermentation. Total biomass was recovered by centrifugation (Hermle Z326k, Wehingen, Germany) at 4000 g (6000 rpm), 4 °C for 15 min, and washed three times with sterile distilled water. The cell pellets were lyophilized (Thermo Savant ModulyoD-230, New York, USA) and their weight were gravimetrically determined. The biomass samples were stored at −20 °C until fatty acids extraction.
Characterization of residue
Characterization of the residue before the biological treatment (raw SWW) was based on sampling procedure during normal operation of the plant, while for the characterization of the residue after the biological treatment, fermentations of 1 L were conducted in a 1-L stirred culture vessel (Nalgene 2605-0001, Thermo Scientific, Massachusetts, USA), containing 900 mL of residue-ASW salts (residue at the selected concentration for its optimal use mixed with artificial sea salts), YE-MSG (both at 2 g L−1) and 100 mL of inoculum, adjusting the pH and salinity of the medium before being autoclaved. Cultures were incubated for 5 days at 25 °C and 180 rpm, without external agitation. After fermentation, the supernatant samples were collected by one centrifugation cycle at 4000 g (6000 rpm), 4 °C for 15 min, and stored for further analyses (proximate and trace elements analysis).
Proximate analysis
Proximate composition in the residue (raw SWW) and in the supernatant (after the treatment) of 5-day cultures (1 L) were determined by the Laboratory of Environmental Tests of Universidad de La Frontera (Temuco, Chile). Total Nitrogen test was carried out by Kjeldahl method according to Standard Methods 4500-Norg D, while the Nitrite and Nitrate content were determined according to Standard Methods 4110-B (ion chromatography with chemical suppression of eluent conductivity method) and 4500-D (nitrate electrode method), respectively [23]. The content of oil and grease in the samples was determined by gravimetric method according to Standard methods 5520 [23], while the total phosphorus analysis was assayed according to the Chilean norm (NCh) NCh2313/15 [24].
Trace elements analysis
Residue samples were analyzed for dissolved concentrations of Fe, Cu and Zn according to NCh 2313/10 [25] in a flame atomic absorption spectrometer (PerkinElmer AAnalyst 200, Waltham, Massachusetts, USA), equipped with double beam and hollow cathode lamps for respective metallic elements. Air-acetylene was used as fuel. Flame atomic absorption spectrometer determinations for digested samples of the residue (raw SWW), and from medium supernatants (of 5-days cultures) were carried out according to the instrumental operating conditions as recommended by the manufacturer.
Fatty acids analysis
Samples of lyophilized biomass (30–50 mg) were used for direct transesterification [26]. Fatty acid methyl esters (FAMEs) in the hexane layer were collected on chromatography vials by centrifuging (Digisystem DSC-200A-2, New Taipei City, Taiwan) at 4 °C and 4500 rpm for 5 min, and were stored at −20 °C until analyzed. The resulting FAMEs were analyzed by Gas Chromatography (Agilent 7890A, Santa Clara, California, USA). The FAMEs’ peaks were identified and quantified using fatty acid standards (Supelco 37 Component FAME Mix 47885-U, Sigma-Aldrich, Missouri, USA).
Chemical oxygen demand (COD)
A COD curve was carried out by preparing cultures in a one L stirred culture vessel (Nalgene 2605-0001, Thermo Scientific, Waltham, Massachusetts, USA), containing 900 mL of residue-ASW salts (residue at the selected concentration for its optimal use), YE-MSG (both at 2 g L−1) and 100 mL of inoculum, adjusting the pH and salinity of the medium before being autoclaved. Cultures were incubated for 7 days at 25 °C and 180 rpm. COD was measured on medium supernatant containing the wastewater before and after the cultivation (collecting supernatant every 24 h for analysis) by photometric method according to NCh2313/24 [27] using COD kits (Merck, Darmstadt, Germany). The organic matter of the sample (3-mL aliquot) was oxidized with a hot sulfuric solution of potassium dichromate at 150 °C for 2 h in COD tubes. After that, the COD tubes were removed from the oven allowing them to cool down on a test-tube rack for 10 min, swirled and returned to the rack for complete cooling to room temperature (30 min, approximately). COD levels were determined by measuring the absorbance of the digested assay solution with a spectrophotometer at 600 nm. A 1 cm path length was maintained by using a standard cuvette (2.5 mL sample size).
Trace elements supplementation in culture media
In order to observe the effect of Fe on the growth of TH strain, cultures of 100 mL were performed, in which different treatments were applied to the media, having 3 replicates, as follows: (1) cultures supplemented with FeCl3×6H20 at different concentrations (5, 10 and 50 mg L−1), YE-MSG (both at 2 g L−1) in ASW, with the addition/omittion of glucose (Gluc) (5 g L−1); and (2) cultures supplemented with FeCl3×6H2O at the concentrations indicated above in ASW, without the addition of carbon and nitrogen sources (YE-MSG).
In addition, 3 control experiments were performed, having 3 replicates, as follows: (1) cultures with YE-MSG and Gluc in ASW, (2) cultures with YE-MSG in ASW, and (3) cultures in ASW. The concentration of Gluc and YE-MSG used in all the tests in this section was 5 and 2 g L−1, respectively, and the inoculum size was 5 mL. For the preparation of cultures, all glassware was soaked in HCl overnight, and then rinsed with iron-free ultrapure water, according to Nagano et al. [12]. Glucose, FeCl3×6H2O and ASW were prepared with ultrapure water (Milli-Q, Merck Millipore, Massachusetts, USA) and autoclaved separately. Incubation was carried out at 25 °C in orbital shaking at 180 rpm, for 5 days.
Statistics and calculations
Statistical data processing was conducted with Minitab v17.3 software. One-way analysis of variance (ANOVA) test, followed by Tukey multiple comparison tests were used to analyze data. Significance of the effects was determined at 0.05 confidence level. Results are presented as mean ± S.D from replicate (triplicate) assays.
Results and discussion
Biomass production at different residue concentration
As shown in Table 1, concentration of the carbon source in the growth medium composition had a significant (P < 0.05) effect on biomass production by T. kinney VAL-B1. The highest biomass concentration (3.39 g L−1) was obtained in the fermentations with the residue at 100% (without being diluted with ASW), and then, growth of VAL-B1 strain was not inhibited by residue concentration. Therefore, subsequent analyzes were carried out using media containing 100% of residue and the artificial sea salts mixture. However, the appropriate dilution (concentration) of the residue seems to depend on the type of residue used for fermentation, since different effects have been reported on biomass production. For example, in a research conducted by Liang et al. [19], in which sweet sorghum juice was used for docosahexanoic acid (DHA) production by S. limacinum SR21, it was found that low concentration of substrate stimulated cell growth, whereas high concentration of substrate had an inhibitory effect, obtaining optimal biomass production using this substrate at 50%. A similar behavior was observed by Pyle et al. [14], when they used crude glycerol derived from biodiesel for DHA production by S. limacinum. In this work, it was observed that methanol contained in the residue, negatively affected the biomass productivity and the total fatty acid content, and consequently, DHA production decreased when methanol concentration increased.
Table 1.
Biomass production of Thraustochytrium kinney VAL-B1 at different residue concentrations. Values expressed as mean ± S.D (n = 3).
| Residue concentration (%) | Dry cell biomass (g L−1) |
|---|---|
| 25 | 2.15 ± 0.31c |
| 50 | 2.64 ± 0.07b, c |
| 80 | 2.95 ± 0.12a, b |
| 100 | 3.39 ± 0.17a |
Means within column not sharing a common superscript letter differ significantly according to Tukey’s comparison test (P < 0.05).
Characterization of residue before and after fermentation
Proximate composition, trace elements analysis and fatty acid profiles of the residue before and after culture are shown in Table 2.
Table 2.
Proximate analysis, trace metal analysis and fatty acid composition of the residue before and after fermentation of Thraustochytrium kinney VAL-B1. Values expressed as mean ± S.D (n = 3).
| Parameter | Raw wastewater | Culture supernatant |
|---|---|---|
| Oil and grease (mg L−1) | 18,625 ± 0.041 | 18 ± 0.041 |
| Total phosphorus (mg L−1) | 149 ± 0.159 | 3.65 ± 0.159 |
| Total Kjeldahl nitrogen (mg L−1) | 1571 ± 0.216 | 524 ± 0.16 |
| Nitrite (mg L−1) | <0.1 | <0.1 |
| Nitrate (mg L−1) | 68 ± 0.016 | 78 ± 0.016 |
| Total nitrogen (mg L−1) | 1639 ± 0.014 | 602 ± 0.015 |
| Cu (mg L−1) | 0.711 ± 0.373 | 0.621 ± 0.273 |
| Fe (mg L−1) | 77.883 ± 3.401 | 30.88 ± 18.41 |
| Zn (mg L−1) | 1.124 ± 0.197 | 0.511 ± 0.014 |
| C16:0 (%TFA) | 18.89 ± 8.06 | ND |
| C18:0 (%TFA) | 15.46 ± 9.81 | ND |
| C18:1c (%TFA) | 18.15 ± 5.66 | ND |
| C18:2c (%TFA) | 13.54 ± 9.14 | ND |
| Others (% TFA) | 33.96 | ND |
ND = Not Determined.
TFA = Total Fatty Acids.
Proximate composition
According to the proximate analysis of the residue, the wastewater is characterized by a high content of oil and grease, which was reduced by 99.9% (18 ± 0.041 mg L−1) as a result of a 5-day fermentation. About 98% (3.65 ± 0.159 mg L−1) of total phosphorus content, and 64% (602 ± 0.015 mg L−1) of total nitrogen content also decreased in the culture supernatant, which was found to be equivalent to or higher than others reported in previous studies using other TH strains. For example, the reduction of the total nitrogen content of sorghum juice by 50% [19] or in the shochu residue, reaching a decrease of 67% [17]. In addition, it has been reported that these microorganisms are able to act as bioremediators when performing co-cultivations with fungal and microalgal cells, as shown in the research conducted by Wrede et al. [20], in which they evaluated the ability of A. fumigatus and a TH strain for the treatment of a diluted wastewater from swine lagoon wastewaters, resulting in the reduction of ammonia and phosphate concentration by 86 and 69%, respectively after 48 h of treatment of the residue.
Trace elements composition
According to the trace elements evaluation, among the 3 metals analyzed (Table 2), it was determined that the residue is characterized by a high concentration of Fe (77.88 mg L−1), followed by Zn and Cu, respectively. After fermentation of 5 days and the subsequent analyzes, a significant decrease in Fe concentration was observed (about 60.35%), as compared to its initial value (raw SWW), suggesting that VAL-B1 strain could have used Fe contained in the residue for its growth, since blood is a source rich in iron [28].
Fatty acids composition
According to the fatty acid profile, it can be seen that palmitic acid (C16:0) is the main constituent of the total fatty acids, followed by oleic acid (C18:1), stearic acid (C18:0) and linoleic acid (C18:2). These results are consistent with the fact that C18:1 and C16:0 have been reported as the most abundant fatty acids in food processing effluents [29], [30]. In addition, the fatty acid content and composition present in pig and other animals (e.g., cows, sheeps, lambs, etc.) is directly influenced by diet [31], [32], [33], [34] and specifically, C18:2 and C18:3 are exclusively derived from the diet [35], [36], [37].
Chemical oxygen demand (COD)
Changes in biomass concentration were observed during 7 days of fermentation (Fig. 2) in media containing the residue at 100% of concentration, in which the highest biomass content was detected on the fifth day of cultivation. The initial COD measured in the residue was 9610 mg L−1 (data not shown), which is similar to those reported in the literature for SWW samples [38], [39]. After 1 day of cultivation, COD levels in the medium supernatant increased, which could be due to the presence of organic matter from the residue-based medium and the inoculum medium, and the adaptation period of the microorganism, where THs are adjusting to their new conditions. In addition, it should be considered the condition of the microbial cells themselves, and their consequent ability to grow and transform or degrade the organic matter of the medium.
Fig. 2.

Growth and COD curves of Thraustochytrium kinney VAL-B1 in culture supernatant. Circles, culture biomass (residue concentration at 100%); triangles, COD in culture supernatant. Initial COD of residue: 9610 ± 98.99 (data not shown). Values expressed as mean ± S.D (n = 3).
On the other hand, COD of the medium supernatant decreased to 4200 mg L−1, as a result of fermentation for 7 days, which means a final reduction of 56.30% regarding the initial COD of the residue. In fact, the ability to reduce COD of wastewater samples by these microorganisms, and, at the same time, to take advantage of the nutrients in order to obtain value-added products (e.g., EPA, DHA, etc.), has already been reported in other investigations. For example, in the study carried out by Yamasaki et al. [17] when using shochu residue to produce PUFAs and xanthophylls using a Schizochytrium sp strain, COD of the residue was reduced by 35% (initial COD: 79,000 mg L−1) after a 5-day fermentation. On the other hand, in the study carried out by Quilodrán et al. [18], COD of the beer residue decreased by 27.6% after a 7-day fermentation by T. kinney M12-X1.
Biomass production in cultures supplemented with trace elements
Biomass production by VAL-B1 was significantly (P < 0.05) affected by the media composition and the different treatments given to the cultures. It was observed that biomass production of VAL-B1 strain was higher by increasing the concentration of FeCl3×6H2O in the media (Fig. 3), which is similar to the strain behavior in residue-based media cultivations. In fact, Nagano et al. [12] determined that supplementation of culture medium with trace metals increase the growth of the microorganism, evidencing particular importance of Fe and Zn, since omitting the addition of these trace metals in the culture media, cell growth of all strains analyzed were negatively affected.
Fig. 3.
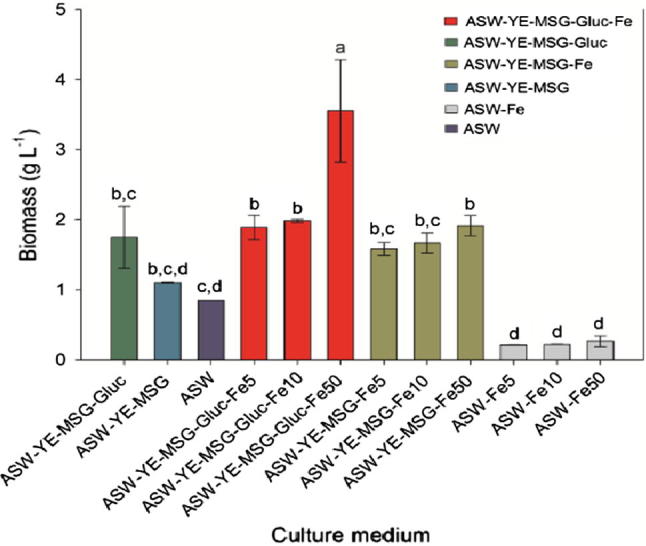
Effect of trace element (Fe) concentration, carbon source (Gluc) and nitrogen sources (YE-MSG) on the growth of T. kinney VAL-B1. Fe5, 10 and 50, FeCl3×6H2O concentration. Values expressed as mean ± S.D (n = 3). Bars denoted with different letters are significantly different according to Tukey’s comparison test (P < 0.05, n = 3), where “a”: medium in which was reached the highest biomass production, and “d”: medium with the lowest biomass production.
On the other hand, it should be noted that nitrogen and carbon sources also influenced the microorganism growth. The highest biomass production (3.55 g L−1) was reached when glucose was added to the medium with FeCl3×6H20 (at 50 mg L−1), whereas cultures without glucose supplementation resulted in less biomass production (1.92 g L−1) at the same FeCl3×6H2O concentration, which was similar to the obtained in fermentations with YE-MSG-Gluc without the supplementation of FeCl3×6H2O (1.75 g L−1). In addition, in the study conducted by Quilodrán et al. [18], the nitrogen source (MSG) had a greater influence on biomass production by M12-X1 strain in medium with beer residue sources (MSG), since the highest biomass production was reached in fermentations with the beer residue supplemented with YE-GMS (2.3 g L−1), whereas the biomass obtained in fermentations for the same residue without the addition of MSG was 1.7 g L−1. This behavior agrees with Iida et al. [40], suggesting that GMS is one of most important components in culture medium for the growth of these microorganisms.
Thus, according to these antecedents, the presence of Fe could contribute positively to the growth of THs, which would make it possible to show that Fe content from the IWW is beneficially used by these microorganisms in order to obtain biomass, and therefore, using this type of wastewater as a substrate is a good resource of nutrients and energy for THs.
These novel findings show that T. kinney VAL-B1 could be a potential bioremediation alternative for the reduction or transformation of the pollutants present in piggery slaughterhouse wastewater, contributing to comply with the regulations established for the discharge of the wastewater on water bodies, and helping to reduce the impacts on water sources. In addition, one advantage of using this microorganism for bioremediation of industrial wastewater is given to the fact that thraustochytrids are able to use the nutrients contained in residues for biomass production, and obtain a value-added product of commercial interest, such as, Omega-3 polyunsaturated fatty acids (DHA and EPA), with potential applications as supplements in animal food.
Conclusions
The results in this study show that the growth of T. kinney VAL-B1 was not inhibited by the amount of residue added to the culture medium, which was favorable, allowing to use the residue without the need for dilutions. Piggery SWW contains nutrients and trace metals which are beneficially usable by THs for their growth and biomass production, while a prolonged fermentation allowed an important decrease of physicochemical parameters like COD, total phosphorus, total nitrogen and oil and grease. Thus, microorganisms like THs are a potential alternative for the treatment of SWW, contributing to regulate potential environmental contamination and comply with the relevant environmental regulations. Further studies will be needed to improve the potential of the bioremediation technique by optimizing culture parameters, as well as to investigate the possibility of carrying out co-cultivation of TH and other microorganisms for wastewater treatment.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This research was financially supported by the internal research project (grant R04/16) and the internal scholarship (grant “Beca de Apoyo a la Finalización de Tesis”), both from the Dirección de Investigación of Universidad de Los Lagos.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Sancho JA, Galindo S. General hygiene in the food industry. Auxiliary maintenance and internal transport operations in the food industry. 1st ed. Spain: Rodio; 2014. [In Spanish].
- 2.Al-Omair M.A., El-Sharkawy E.A. Removal of heavy metals via adsorption on activated carbon synthesised from solid wastes. Environ Technol. 2007;28(4):443–451. doi: 10.1080/09593332808618808. [DOI] [PubMed] [Google Scholar]
- 3.Shi H. Industrial Wastewater-Types, Amounts and Effects. In: Jining C, Yi Q, editors. Point Sources of Pollution: Local Effects and their Control, vol. I. China: EOLSS Publications; 2009. p. 191–192.
- 4.Bustillo-Lecompte C.F., Mehrvar M. Treatment of an actual slaughterhouse wastewater by integration of biological and advanced oxidation processes: modeling, optimization, and cost-effectiveness analysis. J Environ Manage. 2016;182:651–666. doi: 10.1016/j.jenvman.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 5.Massé D.I., Masse L. Characterization of wastewater from hog slaughterhouses in Eastern Canada and evaluation of their in-plant wastewater treatment systems. Can Agric Eng. 2000;42(3):139–146. [Google Scholar]
- 6.Bustillo-Lecompte C.F., Mehrvar M. Slaughterhouse wastewater: treatment, management and resource recovery. In: Farooq R., Ahmad Z., editors. Physico-chemical wastewater treatment and resource recovery. InTech Europe; Rijeka, Croatia: 2017. p. 155. [Google Scholar]
- 7.Bustillo-Lecompte C.F., Mehrvar M. Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: a review on trends and advances. J Environ Manage. 2015;161:287–302. doi: 10.1016/j.jenvman.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Boopathy R. Factors limiting bioremediation technologies. Bioresource Technol. 2000;74(1):63–67. [Google Scholar]
- 9.Sharma B., Dangi A.K., Shukla P. Contemporary enzyme based technologies for bioremediation: a review. J Environ Manage. 2018;210:10–22. doi: 10.1016/j.jenvman.2017.12.075. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A., Barrow C.J., Puri M. Omega-3 biotechnology: thraustochytrids as a novel source of omega-3 oils. Biotechnol Adv. 2012;30(6):1733–1745. doi: 10.1016/j.biotechadv.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Aasen I.M., Ertesvåg H., Heggeset T.M., Brautaset T., Vadstein O., Ellingsen T.E. Thraustochytrids as production organisms for docosahexaenoic acid (DHA), squalene, and carotenoids. Appl Microbiol Biotechnol. 2016;100(10):4309–4321. doi: 10.1007/s00253-016-7498-4. [DOI] [PubMed] [Google Scholar]
- 12.Nagano N., Taoka Y., Honda D., Hayashi M. Effect of trace elements on growth of marine eukaryotes, thraustochytrids. J Biosci Bioeng. 2013;116(3):337–339. doi: 10.1016/j.jbiosc.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Fan K.W., Chen F., Jones E.B.G., Vrijmoed L.L.P. Eicosapentaenoic and docosahexaenoic acids production by and okara-utilizing potential of thraustochytrids. J Ind Microbiol Biotechnol. 2001;27(4):199–202. doi: 10.1038/sj.jim.7000169. [DOI] [PubMed] [Google Scholar]
- 14.Pyle D.J., García R.A., Wen Z. Producing docosahexaenoic acid (DHA)-rich algae from biodiesel-derived crude glycerol: effects of impurities on DHA production and algal biomass composition. J Agric Food Chem. 2008;56:3933–3939. doi: 10.1021/jf800602s. [DOI] [PubMed] [Google Scholar]
- 15.Unagul P., Assantachai C., Phadungruengluij S., Suphantharika M., Tanticharoen M., Verduyn C. Coconut water as a medium additive for the production of docosahexaenoic acid (C22:6n3) by Schizochytrium mangrovei Sk-02. Bioresource Technol. 2007;98(2):281–287. doi: 10.1016/j.biortech.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Thyagarajan T., Puri M., Vongsvivut J., Barrow C.J. Evaluation of bread crumbs as a potential carbon source for the growth of thraustochytrid species for oil and Omega-3 production. Nutrients. 2014;6(5):2104–2114. doi: 10.3390/nu6052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki T., Aki T., Shinozaki M., Taguchi M., Kawamoto S., Ono K. Utilization of shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. J Biosci Bioeng. 2006;102(4):323–327. doi: 10.1263/jbb.102.323. [DOI] [PubMed] [Google Scholar]
- 18.Quilodrán B., Hinzpeter I., Quiroz A., Shene C. Evaluation of liquid residues from beer and potato processing for the production of docosahexaenoic acid (C22:6n–3, DHA) by native thraustochytrid strains. World J Microb Biot. 2009;25:2121–2128. [Google Scholar]
- 19.Liang Y., Sarkany N., Cui Y., Yesuf J., Trushenski J., Blackburn J.W. Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresource Technol. 2010;101(10):3623–3627. doi: 10.1016/j.biortech.2009.12.087. [DOI] [PubMed] [Google Scholar]
- 20.Wrede D., Taha M., Miranda A.F., Kadali K., Stevenson T., Ball A.S. Co-cultivation of fungal and microalgal cells as an efficient system for harvesting microalgal cells, lipid production and wastewater treatment. PlosOne. 2014;9(11):e113497. doi: 10.1371/journal.pone.0113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva D., Roa A., Quevedo R., Quilodrán B. Production of biodiesel from soybean frying oil using native strains of Thraustochytrids. Chilean J Agric Anim Sci. 2015;31(1):29–41. [In Spanish] [Google Scholar]
- 22.Hinzpeter I., Quilodrán B., Stead R., Trujillo L., Shene C. Isolation of thraustochytrid strains in the coastal zone of Puerto Montt, Chile and evaluation of Docosahexaenoic acid (22:6n–3, DHA) production. Afinidad. 2009;66:482–487. [In Spanish] [Google Scholar]
- 23.APHA. Standard Methods for the Examination of Water and Wastewater. 22th ed. Washington: American Public Health Association; 2012. Part 4110-B: Ion Chromatography with Chemical Suppression of Eluent Conductivity. Part 4500–D: NO3- Nitrate Electrode Method. Part 4500-Norg D: Block Digestion and Flow Injection Analysis. Part 5520: Oil and Grease.
- 24.INN. NCh. 2313/15 Of. 2009. Wastewater: Methods of analysis. Part 15: Determination of Total Phosphorus. National Normalization Institute, Santiago, Chile. [In Spanish].
- 25.INN. NCh 2313/10 Of. 1996. Wastewater: Methods of analysis. Part 10: Determination of Heavy Metals: Cadmium, Copper, Total Chromium, Iron, Manganese, Nickel, Lead, Zinc. Method of flame atomic absorption spectrophotometry. National Normalization Institute, Santiago, Chile. [In Spanish].
- 26.Lewis T.E., Nichols P.D., McMeekin T.A. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J Microbiol Meth. 2000;43(2):107–116. doi: 10.1016/s0167-7012(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 27.INN. NCh. 2313/24 Of. 1997. Wastewater: Methods of analysis. Part 24: Determination of Chemical Oxygen Demand (COD). National Normalization Institute, Santiago, Chile. [In Spanish].
- 28.Ofori J.A., Hsieh Y.P. The use of blood and derived products as food additives. In: El-Samragy Y., editor. Food additive. InTech Europe; Rijeka, Croatia: 2012. p. 13. [Google Scholar]
- 29.Pereira M.A., Sousa D.Z., Mota M., Alves M.M. Mineralization of LCFA associated to anaerobic sludge: kinetics, transport limitations, enhancement of methanogenic activity and effect of VFA. Biotechnol Bioeng. 2004;88:502–510. doi: 10.1002/bit.20278. [DOI] [PubMed] [Google Scholar]
- 30.Sousa D.Z., Pereira M.A., Alves J.I., Smidt H., Stams A.J., Alves M.M. Anaerobic microbial LCFA degradation in bioreactors. Water Sci Technol. 2008;57:439–444. doi: 10.2166/wst.2008.090. [DOI] [PubMed] [Google Scholar]
- 31.Wood J.D., Nute G.R., Richardson R.I., Whittington F.M., Southwood O., Plastow G. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 2004;67(4):651–667. doi: 10.1016/j.meatsci.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Straarup E.M., Danielsen V., Høy C.E., Jakobsen K. Dietary structured lipids for post-weaning piglets: fat digestibility, nitrogen retention and fatty acid profiles of tissues. J Anim Physiol An N. 2006;90:124–135. doi: 10.1111/j.1439-0396.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 33.Viera-Alcaide I., Vicario I.M., Graciani Constante E., León-Camacho M. Authentication of fattening diet of Iberian pig according to their triacylglycerols profile from subcutaneous fat. Anal Chim Acta. 2007;596(2):319–324. doi: 10.1016/j.aca.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Dugan M.E.R., Vahmani P., Turner T.D., Mapiye C., Juárez M., Prieto N. Pork as a source of omega-3 (n-3) fatty acids. J Clin Med. 2015;4(12):1999–2011. doi: 10.3390/jcm4121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maw S.J., Fowler V.R., Hamilton M., Petchey A.M. Physical characteristics of pig fat and their relation to fatty acid composition. Meat Sci. 2003;63(2):185–190. doi: 10.1016/s0309-1740(02)00069-4. [DOI] [PubMed] [Google Scholar]
- 36.Wood J.D., Enser M., Fisher A.V., Nute G.R., Sheard P.R., Richardson R.I. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 2008;78(4):343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Domínguez R., Martínez S., Carballo J., Franco I. Fatty acid profile and cholesterol and retinol contents in different locations of Celta pig breed. Grasas Aceites. 2014;65(3):1–13. [Google Scholar]
- 38.Masse L., Massé D.I. Effect of soluble organic, particulate organic, and hydraulic shock loads on anaerobic sequencing batch reactor treating slaughterhouse wastewater at 20 °C. Process Biochem. 2005;40:1225–1232. [Google Scholar]
- 39.Torres-Pérez J., Solache-Ríos M., Martínez-Miranda V. Chemical oxygen demand, total organic carbon and colour reduction in slaughterhouse wastewater by unmodified and iron-modified clinoptilolite-rich tuff. Environ Technol. 2014;35(12):1541–1548. doi: 10.1080/09593330.2013.872198. [DOI] [PubMed] [Google Scholar]
- 40.Iida I., Nakahara T., Yokochi T., Kamisaka Y., Yagi H., Yamaoaka M. Improvement of docosahexaenoic acid production in a culture of Thraustochytrium aureum by medium optimization. J Fermentation Bioeng. 1996;1:76–78. [Google Scholar]



