Abstract
BACKGROUND
Pericallosal artery aneurysm treatment may be challenging using traditional endovascular techniques.
OBJECTIVE
To demonstrate the feasibility, efficacy, and safety of endovascular treatment of pericallosal artery aneurysm using flow diverters.
METHODS
We performed a retrospective review of our institutional database from July 2013 through July 2016 and identified 7 subjects with a pericallosal artery aneurysm treated with the Pipeline embolization device (ev3 Neurovascular, Medtronic, Dublin, Ireland) and at least 1 follow-up angiogram. Technical feasibility, procedural complication, angiographic results, and clinical outcome were evaluated.
RESULTS
Placement of the Pipeline embolization device was successful in all cases without evidence of procedural complication. Five out of 7 subjects showed a complete aneurysm occlusion at 6- to 12-mo follow-up angiogram. The 2 subjects with persistent aneurysm filling showed decreased aneurysm sac volume on follow-up angiograms (96% and 60%). There was no evidence of in-implant stenosis or intimal hyperplasia. No thromboembolic or hemorrhagic complications were seen during the follow-up period. Only 1 patient had a transient change in Modified Rankin scale score from baseline as a result of different unrelated procedure.
CONCLUSION
Our preliminary results demonstrate feasibility of the use of flow diverter stent for treatment of aneurysms of the pericallosal artery with rate of aneurysm occlusion comparable to literature and without evidence of increased procedural or short-term morbidity. A long-term and larger cohort study is needed to validate our findings.
Keywords: Aneurysm, Artery, Flow diverter, Pericallosal
ABBEVIATIONS
- ACT
activated clotting time
- DSA
digital subtraction angiography
- 3-D
3-di-mensional
- EVT
endovascular treatment
- FD
Flow diverter
- ICA
internal carotid artery
- mRS
modified Rankin Scale
- OCT
optic coherence tomography
- PED
PipelineTM Embolization Device
- SEM
scanning electron microscopy
Aneurysms arising from the pericallosal artery may represent a challenge for open surgery or endovascular treatment (EVT). EVT in this region is traditionally performed by primary coiling, stent-assisted coil embolization or balloon remodeling technique in cases of unfavorable aneurysm anatomy.
The PipelineTM Embolization Device (PED; ev3 Neurovascular, Medtronic Inc, Dublin, Ireland) is approved to treat aneurysms ≥10 mm in the internal carotid artery (ICA) located from the petrous to the superior hypophyseal segment. The use of a PED for treatment of aneurysm with a small parent artery and distal to the circle of Willis has been previously demonstrated in a few case series.1,2 However, to the best of our knowledge, only 1 publication has specifically addressed aneurysms of the distal anterior cerebral artery.3
We present a retrospective review of 7 consecutive cases of pericallosal artery aneurysms treated with a PED at our institution, from July 2013 through July 2016. We discuss the feasibility, efficacy, and safety of PED to treat pericallosal artery aneurysms.
METHODS
Subjects
Our hospital institutional review board approved this retrospective study. We performed a retrospective search of our Neurointerventional database. From July 2013 through July 2016, 7 subjects were identified that had an aneurysm of the pericallosal artery and had also undergone placement of a PED (Medtronic) with at least 1 follow-up angiogram. Subject's demographic and relevant medical information, including aneurysm rupture status, comorbidities and risk factors were obtained upon review of Electronic Medical Records.
Imaging Evaluation
Aneurysm height (dome), transverse diameter, and neck sizes as well as proximal and distal parent artery diameter were obtained upon review of procedural images, which included digital subtraction angiography (DSA) and 3-dimensional (3-D) rotational angiography reconstructions (Philips Medical, Best, The Netherlands). Additionally, cone-beam CT with multiplanar 3-D reconstructions after PED placement was obtained for implant to wall apposition evaluation. Follow-up images at 1, 6, and 12 mo included DSA and cone-beam CT with multiplanar 3-D reconstructions for all subjects. Images were reviewed for degree of aneurysm occlusion and presence of in-implant stenosis or intimal hyperplasia.
Endovascular Procedure
Access to the pericallosal artery was carried out in a triaxial fashion for all subjects, with a 6 French Shuttle guide sheath (Shuttle-SL; Cook, Bloomington, Indiana), a 058 Navien intermediate guide catheter (ev3/Covidien, Medtronic, Dublin, Ireland) and Marksman microcatheter (Medtronic) or Excelsior XT-27 microcatheter (Stryker, Kalamazoo, Michigan) over a 0.014-inch Synchro micro guide wire (Stryker). The Shuttle sheath was positioned in the distal common carotid artery while the Navien was placed in the petro-cavernous region. A PED with a 2.5 mm diameter was used for all cases, with length varying from 10 to 20 mm. Pipeline classic was used in 5 cases while PED Flex was used in 2 subjects (Subjects #1 and #3). Subjects received Aspirin 81 or 325 mg and Clopidogrel 75 mg p.o. daily, starting 5 days prior to treatment. Dual antiplatelet therapy was maintained for at least 6 mo following intervention for all patients. Serial P2Y12 Reaction Units (PRU) value checks were performed prior to and after the procedure. Adjustments to the antiplatelet regimen were made in order to ensure PRU value within the desired range (60-200). After confirmation of absence of significant in-implant stenosis on the 6-mo follow-up angiogram (12 mo for 1 patient without a 6-mo follow-up), Clopidogrel was discontinued while Aspirin was maintained. Procedural heparinization was performed and activated clotting time (ACT) maintained above 250 s. Periprocedural complication information was collected.
Outcome
Treatment outcome was assessed by aneurysm occlusion rate, patency of the parent artery and covered side branches, presence of in-implant stenosis or significant intimal hyperplasia. Clinical outcome was evaluated based on available modified Rankin Scale (mRS) at 1-, 6-, and 12-mo follow-up and compared to the patient's neurological function at baseline.
RESULTS
A total of 7 subjects (5 females) were identified. Age ranged from 45 to 68 yr, mean of 60 yr. One subject (Subject #5) had a previously ruptured pericallosal aneurysm that was treated with primary coiling and initially demonstrated complete aneurysm occlusion. However, on the 24-mo follow-up cerebral angiogram, aneurysm regrowth was identified. In view of new growth of previously completely occluded and previously ruptured aneurysm, the subject requested additional treatment after risks and benefits were explained. All other 6 subjects had an unruptured aneurysm that had not been treated previously. Two of these subjects opted for treatment due to family history of ruptured aneurysm (Subjects #3 and #4). One subject had aneurysm growth demonstrated of follow-up angiogram (Subject #2; Figure 1). Two subjects with multiple aneurysm requested treatment of all aneurysms due to anxiety (Subject #1 and #7) and the last subject opted to have his aneurysm treated given young age and added anxiety due to presence of multiple craniocervical fistulas requiring treatment (Subject #6). All subjects were referred to us for endovascular treatment due to patient desire to avoid open surgical approach. Cases were discussed and approved for treatment in multidisciplinary conference. Off-label use of the device and other available treatment options as well as the possibility of close surveillance without treatment were explained to all subjects.
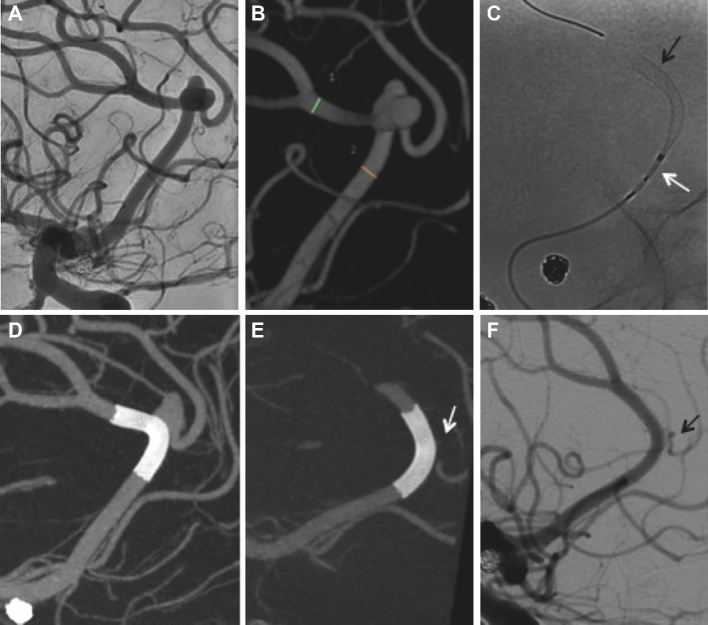
FIGURE 1.
A, Right ICA angiography shows a small right pericallosal artery aneurysm with the neck involving the origin of the right callosomarginal artery. Patient underwent several angiograms for follow-up of this aneurysm and a previously treated large unruptured basilar tip aneurysm with stable size of the pericallosal aneurysm (not shown). B, Five-year follow-up angiogram showed aneurysm growth with new pseudolobulation in its posterior aspect (arrow). C, Immediate postintervention cone-beam CT with multiplanar 3-D reconstruction shows adequate aneurysm neck coverage and slight straightening of the pericallosal artery. D, Twelve-month follow-up angiography shows complete aneurysm occlusion with patency of covered branches.
All aneurysms were saccular in morphology and measured 1.7 to 6.5 mm in height (mean 3.4 mm), 1.8 to 6.9 mm in transverse diameter (mean 4.1 mm), and 1.8 to 6.0 mm at the neck (mean 3.5 mm). Parent artery diameter ranged from 1.6 to 2.6 mm proximal to the aneurysm (mean 2.0 mm) and 1.8 to 2.6 mm distally (mean 1.9 mm). An overview of subject and aneurysm characteristics is found in Table 1.
TABLE 1.
Summary of Patient Data and Aneurysm Characteristics
| Vascular risk | Other brain | Aneurysm | Aneurysm | Aneurysm | Reason for | mRS on | |||
|---|---|---|---|---|---|---|---|---|---|
| Pt# | Gender | Age | factors | aneurysms | Location | size [mm] | characteristics | treatment | admission |
| 1 | F | 59 | HTN, DL, smoking, FMD | Yes | Right pericallosal | 6.5 × 6.9 × 4.6 | Unruptured, Incidental finding | Patient request given multiple aneurysms and size (6.9 mm) | 0 |
| 2 | M | 63 | NS | Yes | Right pericallosal | 2.1 × 4.5 × 2.6 | Unruptured, Incidental finding | New growth in previously stable aneurysm; patient anxiety | 0 |
| 3 | F | 68 | HTN, DL, smoking, CAD | No | Right pericallosal | 3.0 × 5.5 × 6.0 | Unruptured, Incidental finding | Family history of ruptured aneurysm | 0 |
| 4 | F | 61 | DL, carotid stenosis, prior stroke | No | Left pericallosal | 2.3 × 3.0 × 2.6 | Unruptured, Incidental finding | Family history of ruptured aneurysm | 0 |
| 5 | F | 60 | DL | No | Left pericallosal | 1.7 × 1.8 × 1.8 | Ruptured with prior coil embolization | Increasing size of previously ruptured aneurysm | 0 |
| 6 | M | 45 | Smoking | No | Right pericallosal | 3.1 × 2.4 × 2.5 | Unruptured, Incidental finding | Patient anxiety; young age | 0 |
| 7 | F | 65 | NS | Yes | Right pericallosal | 5.2 × 4.6 × 4.6 | Unruptured, Incidental finding | Patient anxiety; multiple aneurysms | 0 |
Pt# = patient number, HTN = hypertension, DL = dyslipidemia, FMF = fibromuscular dysplasia, NS = not significant, CAD = coronary artery disease.
A successful and uncomplicated access with deployment of a single flow diverter (FD) across the neck of the aneurysm was achieved for all subjects. No arterial or aneurysmal wall perforation, thromboembolic, or other periprocedural complication were encountered. Adequate stent to wall apposition was achieved in all cases. No balloon angioplasty was performed.
Two subjects underwent an early follow-up angiogram at 1-mo postimplant placement. For 1 subject, the early angiogram was performed primarily for treatment of a coexistenting left frontal dural arteriovenous fistula. A 90% aneurysmal sac size decrease was seen on this early angiogram. In a second subject, the early follow-up angiogram was performed for endovascular treatment of a second aneurysm. For this subject, there was an aneurysm volume reduction of approximately 60%.
Six of the 7 subjects had a 6-mo postinterventional cerebral angiogram. Complete aneurysm occlusion at 6-mo was seen in 4 out the 6 subjects (66%). Persistent aneurysmal sac filling was seen in 2 subjects, with an aneurysm volume reduction of about 60% and 96%. A 12-mo postintervention cerebral angiogram was available for 3 subjects and 2 demonstrated a complete aneurysm occlusion (Table 2), while 1 subject had a 65% aneurysm sac decrease.
TABLE 2.
Summary of Treatment, Patient Outcome and Follow-Up Results
| PED size | Periprocedural | mRS | Angiography | mRS | Angiography | mRS | Angiography | mRS | |
|---|---|---|---|---|---|---|---|---|---|
| Pt# | [mm]/version | complication | discharge | follow-up 1 mo | 1 mo | follow-up at 6 mo | 6 mo | follow-up 12 mo | 12 mo |
| 1 | 2.5 × 16 Flex | None | 0 | – | – | Complete occlusion, patent PED, no IH | 0 | – | – |
| 2 | 2.5 × 12 Classic | None | 0 | – | – | Not available | – | Complete occlusion, patent PED, no IH | 0 |
| 3 | 2.5 × 12 Flex | None | 0 | – | – | Persistent filling with 60% aneurysmal sac volume reduction, patent PED, no IH | 0 | Persistent filling with 65% aneurysmal sac volume reduction, patent PED, no IH | – |
| 4 | 2.5 × 12 Classic | None | 0 | – | – | Complete occlusion, patent PED, minimal IH | 0 | – | – |
| 5 | 2.5 × 10 Classic | None | 0 | – | – | Complete occlusion, patent PED, no IH | 0 | Complete occlusion, patent PED, no IH | 0 |
| 6 | 2.5 × 20 Classic | None | 0 | Persistent filling with 90% aneurysmal sac volume reduction, patent PED, no IH | 0 | Complete occlusion, patent PED, no IH | 0 | – | – |
| 7 | 2.5 × 16 Classic | None | 0 | Persistent filling with 60% aneurysmal sac volume reduction, patent PED, no IH | 0a | Persistent filling with 96% aneurysmal sac reduction, patent PED, no IH | 0 | – | – |
Pt# = patient number, PED = pipeline embolization device, IH = intimal hyperplasia, mRS = modified Rankin score.
aThere was a transient change to mRS 1 related to a second procedure. However, the patient returned to baseline after a few weeks.
Follow-up angiograms and cone-beam CT in 2 cases showed a mild nonflow limiting narrowing at the origin of the callosomarginal artery, which had been covered by the PED (Medtronic; Figure 2). In a third case, due to a stenosis of the pericallosal artery origin, the decision was made to place the PED spanning from the anterior cerebral artery trunk to the callosomarginal artery, thus covering the pericallosal artery origin in addition to the aneurysm neck, as described in Figure 3. On follow-up study, there was a complete aneurysm occlusion with progressive narrowing of the pre-existing stenosis of the pericallosal artery origin. However, the antegrade distal flow was maintained. Further follow-up is necessary to ascertain long-term patency of covered branches and possible recruitment of collateral flow. Nonetheless, all subjects remain asymptomatic. Neither a significant intimal hyperplasia nor an in-implant stenosis was seen in any of the remaining cases. Two cases of mild nonflow limiting narrowing (approximately 20%) of the parent artery proximal to the implant were seen, corresponding to the subjects with early follow-up angiograms. This finding had spontaneously regressed on the 6-mo follow-up angiogram for both subjects, suggesting this may be a transient phase in the arterial wall remodeling process.
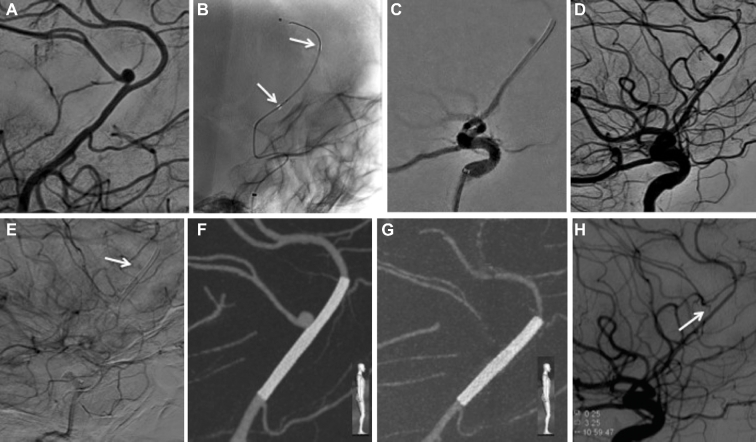
FIGURE 2.
A and B, Right ICA angiography and 3-D rotational angiography reconstruction reveal an anterior cerebral artery azygos configuration with an unruptured bilobed aneurysm located at the pericallosal and callosomarginal bifurcation. C, A 2.5 × 16 mm PED is successfully deployed across the neck of the pericallosal/azygos right anterior cerebral artery (black arrow at distal aspect of PED and white arrow at proximal portion still within delivery system). D, Cone-beam CT with multiplanar 3-D reconstructions confirms proper vessel wall apposition of the PED. E, Six-month follow-up cone-beam CT with multiplanar 3-D reconstruction shows complete occlusion of the bilobed aneurysm (arrow pointing to expected location of aneurysm). F, Corresponding angiography confirms patency of the parent vessel with narrowing of covered branch, which remains patent (arrow). Note the change in configuration with slight straightening of the pericallosal artery at 6-mo follow-up (F) when compared to initial study (A).
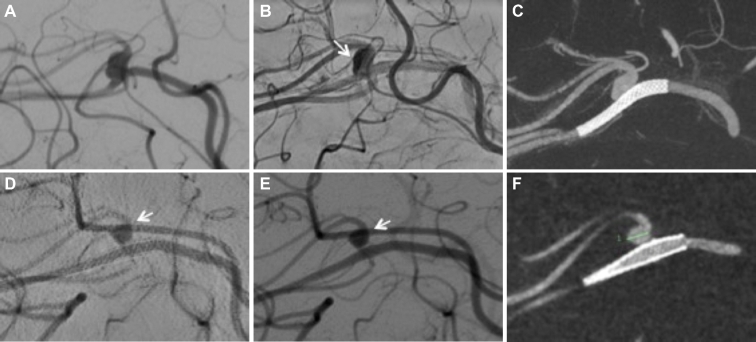
FIGURE 3.
A, Left ICA angiography demonstrates an unruptured 2.3 × 3.0 cm pericallosal aneurysm with a neck width of 3.6 cm and a moderate stenotic proximal parent artery. B, A 2.5 × 12 mm Pipeline Embolization Device (arrows on proximal and distal device markers) was advanced into the callosmarginal artery and successfully placed across the parent artery stenosis and the aneurysm neck. C, Follow-up angiogram demonstrates the proximal and distal edges of the PED within the A2 segment of the left anterior cerebral artery and the left callosomarginal artery, respectively. D, All branches remained patent and no thromboembolic complication is noted. E, Delayed arterial phase shows some faint contrast stagnation within the aneurysm sac (arrow). F, Cone-beam CT with multiplanar 3-D reconstructions confirmed proper wall apposition of the PED. G, Six-month follow-up 3-D cone beam reconstruction images show maintained position and vessel wall apposition of the devices. H, The corresponding angiography demonstrates progression of the narrowing of the origin of the pericallosal artery with maintained antegrade flow (arrow). A patent left callosomarginal artery with complete aneurysm occlusion is also seen.
Clinical follow-up showed no changes in mRS scores at 1, 6, and 12 mo when compared to baseline for 6 subjects. One subject had a transient 1 point increase after intervention for treatment of a second aneurysm with recovery to baseline after a few weeks.
DISCUSSION
Aneurysms of the pericallosal artery are thought to represent 4.4% to 6% of all cerebral aneurysm.4,5 They may represent a challenge to both endovascular and microsurgical approaches, with similar outcomes for unruptures aneurysms.6 The surgical approach is usually performed via an interhemispheric approach with proximal or distal dissection for proximal control. The deep seated location in the interhemispheric fissure with multiple adherences, calcifications, and inadequate aneurysm exposure are among the factors that contribute for the complexity of open surgery.6 From an endovascular standpoint, the challenges are related to the small caliber of the parent vessel and distal access, requiring navigation along multiple vascular curvatures and branching points, and decreasing system responsiveness, resulting in a very limited margin for error. Additionally, wide neck aneurysms may necessitate balloon remodeling or stent assisted coiling requiring dual microcatheter manipulation in a small artery, predisposing to increased friction between catheters and potential hemorrhagic and thromboembolic complications. Infrequently, delayed aneurysm growth or recanalization is observed, especially in coiled ruptured aneurysms as seen in 1 of our patients (Subject #5).
The use of the PED (Medtronic) for treatment of large and giant intracranial wide-neck aneurysms of the ICA was recently approved by the FDA (US Food and Drug Adminstration). However, emerging preclinical evidence suggests that this technology might be most efficacious for small aneurysms with small necks.7 The device promotes flow disruption within the aneurysm, leading to stasis and thrombosis inside the aneurysm sac, while the braided strands serve as scaffolding for neointimal proliferation and vascular remodeling.8 This was shown to result in a 74% rate of complete aneurysm occlusion at 6 mo postprocedure in the Pipeline for Uncoilable or Failed Aneurysms study (PUFS)9 and high occlusion rates have been replicated in several other studies.10-12 This compares favorably to our results that showed a complete aneurysm occlusion in 4 out of 6 subjects at 6 mo (66%), comparable to results from the PUFS trial. Two subjects with a 6-mo follow-up angiogram had a persistent aneurysm sac filling, 1 of which had significant aneurysm sac volume reduction (96%). The 12-mo follow-up angiogram for this subject is pending. The second subject with incomplete aneurysm occlusion at 6-mo had persistent aneurysm filling at 12-mo follow-up (Figure 4). This subject had a wide neck aneurysm at a distal pericallosal artery trifurcation with the neck involving the pericallosal artery and a paracentral lobule branch originating from the aneurysm base. Subsequently after PED placement a persistent inflow was present to the base of the aneurysm and paracentral lobule branch. The aneurysm size decreased to about 60% at 6 mo and to about 65% at 12 mo. Despite this suboptimal result, progressive aneurysm occlusion is still to be expected after 12 mo. Unpublished data from the PUFS trial has shown progressive aneurysm occlusion (86.8% at 1 yr, 93.4% at 3 yr and 95.2% at 5 yr follow-up) with no delayed recanalizations (T. Becske, PUFS collaborator – personal communication). Alternatively, a second PED can potentially be placed in a telescoping fashion as described by Nossek et al3 in order to increase the metal coverage at the aneurysm neck. As with all FD implant studies, the progressive aneurysm occlusion is a function of time13 and, therefore, complete occlusion is still expected for these 2 subjects.
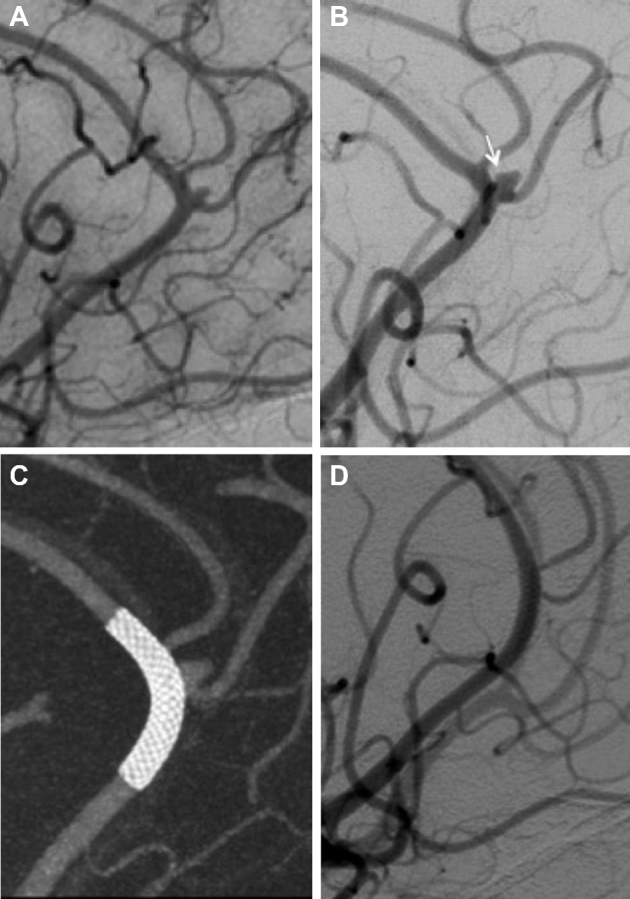
FIGURE 4.
A, Right ICA angiography shows a bilobed aneurysm at the distal right pericallosal artery trifurcation with the neck involving the pericallosal artery and a paracentral lobule branch originating from the aneurysm base (arrow = parietal lobule branch). B, Immediate follow-up angiogram after flow diverter deployment shows contrast stagnation within the aneurysm sac (white arrow = aneurysm sac, black arrows = PED within the pericallosal artery). C, Cone-beam CT with multiplanar 3-D reconstruction postdevice deployment shows adequate coverage of the aneurysm neck. D, Six-month follow-up angiography shows persistent aneurysm filling with an approximate 60% aneurysm sac decrease with patency of a large branch at the aneurysm base (arrow). E, Twelve-month follow-up angiography shows small change compared to prior angriogram with persistent patency of a large branch at the aneurysm base (arrow). Corresponding cone-beam CT with multiplanar 3-D reconstruction demonstrate difference in aneurysm size and shape at 12 mo F when compared to immediate postintervention (C).
Despite limited approval for intracranial aneurysm treatment in terms of anatomical location, off-label use of PED in vessels other than in the ICA has been widely reported in the literature and was found to be feasible.14-17 In particular, the use of PED in small anterior circulation vessels has been addressed in recent publications, with high efficacy and a low rate of complications.1,2 A recent publication also supported the use of PED in distal anterior cerebral artery aneurysms with favorable outcomes.3
The PED can be very effective in treating aneurysms with unfavorable anatomy, such as wide neck aneurysms, when coiling alone would not be feasible, as well as in cases of dysplastic parent artery and fusiform aneurysms due to the remodeling effect it promotes on the parent vessel. Placement of PED can potentially be achieved within a fraction of the time that would be required for assisted coil embolization techniques, resulting in decreased radiation exposure and reduced anesthesia time.18
Although limited by small sample size, our study demonstrated the feasibility of FD treatment for aneurysms of the pericallosal artery. Occlusion rates at 6 mo are similar to those reported in the literature for other locations and very low acceptable perioperative morbidity. In our case series, 2 subjects had mild narrowing of a covered branch without any clinical sequel while another subject had an asymptomatic moderate arterial stenosis. This patient had a very small parent artery measuring proximally 1.4 mm and distally 1.1 mm. The mismatch between the artery and device diameters is unlikely to be the cause of the arterial stenosis, particularly since it has been demonstrated that device oversizing will result in increased porosity and increased flow across the device.19-21 We hypothesize that the increased impedance created by the covered FD, creates a competitive collateral flow resulting in decreased antegrade flow through the artery, which subsequently leads to gradual occlusion of the covered branch origin. Increased neointimal thickness at the level of branching artery ostia in the presence of competitive collateral circulation had been recently demonstrated in a pig model using optic coherence tomography (OCT) and scanning electron microscopy (SEM).22 Two subjects had mild narrowing of the parent artery just proximal to the implant seen on an early follow-up angiogram, without flow limitation or clinical consequences. On 6-mo follow-up angiogram, this had resolved, suggesting this may be a transient remodeling process. Absence of an early follow-up angiogram for the remainder subjects limits the interpretation of this finding.
All subjects tolerated the procedure well, without any complications. No change in mRS was observed after the procedure and during the follow-up period. Only one subject had a transient change in mRS attributable to a second unrelated intervention. The patient returned to baseline after a few weeks.
The use of FDs in the treatment of small aneurysms has been extensively reporter in the literature. Chalouhi et al14 recently described a series of 100 cases of small cerebral aneurysms predominantly in the anterior circulation treated with flow diversion technology with an occlusion rate of 85%, ICA being the parent artery, however, in most of the cases.13
Martínez-Galdámez et al1 published a consecutive series of 25 patients with intracranial aneurysms at the level of the circle of Willis and beyond, including one pericallosal aneurysm, with a complete occlusion rate of 64% at 6 mo. Similarly, Puri et al2 described the use of PED in for treatment of aneurysms in small cerebral arteries with no immediate or delayed complication and 100% occlusion rate at follow-up.
Limitations
The main limitations of our preliminary study are the retrospective nature, small number of cases and limited follow-up. Nevertheless, our results demonstrate the feasibility of FD use for treatment of aneurysms of the pericallosal artery with acceptable rate of aneurysm occlusion at 6 to 12 mo and without evidence of increased procedural or short-term morbidity.
CONCLUSION
We demonstrated the feasibility, efficacy, and safety of the use of the PED for treatment of aneurysms of the pericallosal artery in a small case series. Our initial promising results need to be validated in a large number of patients with long-term follow-up.
Disclosures
Dr Guilherme Dabus is a consultant, speaker, and proctor for Medtronic, Microvention, and Penumbra. He is a shareholder of Medina, Stryker, and InNeuroCo. Dr Peter Kan is a consultant for Medtronic and Stryker neurovascular. Dr Matthew J. Gounis is a consultant for Codman Neurovascular and Stryker Neurovascular, holds stock in InNeuroCo Inc, and has research grants from NIH, Medtronic Neurovascular, Microvention/Terumo, Cerevasc LLC, Gentuity, Codman Neurovascular, Philips Healthcare, Stryker Neurovascular, Tay Sachs Foundation, and InNeuroCo Inc. Dr Ajay K. Wakhloo is a consultant for Codman Neurovascular and Stryker Neurovascular, has research grants from NIH, Philips Healthcare, and Wyss Institute, is a speaker for Harvard Postgraduate Course and Miami Cardiovascular Institute, is co-founder of InNeuroCo Inc, and is a major stockholder in EpiEB and Pulsar Medical. Dr Ajit S. Puri is a consultant for Codman Neurovascular, Stryker Neurovascular, and Covidien, has research grants from Stryker Neurovascular and Covidien, holds stock in InNeuroCo Inc, and is a speaker for Miami Cardiovascular Institute. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Martinez-Galdamez M, Romance A, Vega P et al. Pipeline endovascular device for the treatment of intracranial aneurysms at the level of the circle of Willis and beyond: multicenter experience. J Neurointerv Surg. 2015;7(11):816-823. [DOI] [PubMed] [Google Scholar]
- 2. Puri AS, Massari F, Asai T et al. Safety, efficacy, and short-term follow-up of the use of pipeline embolization device in small (<2.5 mm) cerebral vessels for aneurysm treatment: single institution experience. Neuroradiology. 2015;58(3):267-275. [DOI] [PubMed] [Google Scholar]
- 3. Nossek E, Zumofen DW, Setton A et al. Treatment of distal anterior cerebral artery aneurysms with the pipeline embolization device. J Clin Neurosci. 2017;35(January 2017):133-138. [DOI] [PubMed] [Google Scholar]
- 4. Lehecka M, Dashti R, Lehto H, Kivisaari R, Niemela M, Hernesniemi J. Distal anterior cerebral artery aneurysms. Acta Neurochir Suppl. 2010;107:15-26. [DOI] [PubMed] [Google Scholar]
- 5. Molyneux A, Kerr R, Stratton I et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274. [DOI] [PubMed] [Google Scholar]
- 6. Hui FK, Schuette AJ, Moskowitz SI et al. Microsurgical and endovascular management of pericallosal aneurysms. J Neurointerv Surg. 2011;3(4):319-323. [DOI] [PubMed] [Google Scholar]
- 7. Chung B, Mut F, Kadirvel R, Lingineni R, Kallmes DF, Cebral JR. Hemodynamic analysis of fast and slow aneurysm occlusions by flow diversion in rabbits. J Neurointerv Surg. 2015;7(12):931-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sadasivan C, Cesar L, Seong J et al. An original flow diversion device for the treatment of intracranial aneurysms: evaluation in the rabbit elastase-induced model. Stroke. 2009;40(3):952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Becske T, Kallmes DF, Saatci I et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013;267(3):858-868. [DOI] [PubMed] [Google Scholar]
- 10. Chiu AH, Cheung AK, Wenderoth JD et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol. 2015;36(9):1728-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lylyk P, Miranda C, Ceratto R et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632-642; discussion 642-633; quiz N636. [DOI] [PubMed] [Google Scholar]
- 12. Szikora I, Berentei Z, Kulcsar Z et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010;31(6):1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wakhloo AK, Lylyk P, de Vries J et al. Surpass flow diverter in the treatment of intracranial aneurysms: a prospective multicenter study. AJNR Am J Neuroradiol. 2015;36(1):98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalouhi N, Zanaty M, Whiting A et al. Safety and efficacy of the Pipeline Embolization Device in 100 small intracranial aneurysms. J Neurosurg. 2015;122(6):1498-1502. [DOI] [PubMed] [Google Scholar]
- 15. Kuhn AL, Hou SY, Perras M et al. Flow diverter stents for unruptured saccular anterior circulation perforating artery aneurysms: safety, efficacy, and short-term follow-up. J Neurointerv Surg. 2015;7(9):634-640. [DOI] [PubMed] [Google Scholar]
- 16. Kuhn AL, Kan P, Massari F et al. Endovascular reconstruction of unruptured intradural vertebral artery dissecting aneurysms with the pipeline embolization device. J Neurointerv Surg. 2015;8(10):1048-1051. [DOI] [PubMed] [Google Scholar]
- 17. Lin N, Brouillard AM, Keigher KM et al. Utilization of Pipeline embolization device for treatment of ruptured intracranial aneurysms: US multicenter experience. J Neurointerv Surg. 2015;7(11):808-815. [DOI] [PubMed] [Google Scholar]
- 18. Fiorella D, Lylyk P, Szikora I et al. Curative cerebrovascular reconstruction with the pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg. 2009;1(1):56-65. [DOI] [PubMed] [Google Scholar]
- 19. Berg P, Iosif C, Ponsonnard S, Yardin C, Janiga G, Mounayer C. Endothelialization of over- and undersized flow-diverter stents at covered vessel side branches: an in vivo and in silico study. J Biomech. 2015;49(1):4-12. [DOI] [PubMed] [Google Scholar]
- 20. Mut F, Cebral JR. Effects of flow-diverting device oversizing on hemodynamics alteration in cerebral aneurysms. AJNR Am J Neuroradiol. 2012;33(10):2010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shapiro M, Raz E, Becske T, Nelson PK. Variable porosity of the pipeline embolization device in straight and curved vessels: a guide for optimal deployment strategy. AJNR Am J Neuroradiol. 2014;35(4):727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iosif C, Saleme S, Ponsonnard S et al. Intravascular optical coherence tomography for the evaluation of arterial bifurcations covered by flow diverters. J Neurointerv Surg. 2016;8(12):1283-1287. [DOI] [PubMed] [Google Scholar]