Abstract
Background
Pragmatic studies have gained popularity, thus emphasizing the need for patient-reported outcomes (PRO) to be integrated into electronic health records.
Objective
This study describes the development of a customized short form from the Boston University Osteoarthritis Functional Assessment PRO (BU-OA-PRO) for a specific pragmatic clinical trial.
Methods
A Functional Pain Short Form was created from an existing item bank of deidentified data in the BU-OA-PRO. Item response theory (IRT) methods were used to select items. Reliability was measured with the Cronbach alpha, then with IRT simulation methods. To examine validity, ceiling and floor effects, correlations between the short-form scores and scores from the BU-OA-PRO and the Western Ontario McMasters University Osteoarthritis Index (WOMAC) Pain and Difficulty subscales, and the area under the curve (AUC) were calculated. A minimum detectable change at 90% confidence (MDC90) was calculated based on a calibration sample.
Results
The BU-OA-PRO was reduced from 126 items to 10 items to create the BU-OA Functional Pain Short Form (BU-OA-FPS). The Cronbach alpha indicated high internal consistency (0.91), and reliability distribution estimates were 0.96 (uniform) and 0.92 (normal). Low ceiling effects (4.57%) and floor effects (0%) were found. Moderate-to-high correlations between the BU-OA-PRO and BU-OA-FPS were found with WOMAC Pain (BU-OA-FPS = 0.67; BU-OA-PRO = 0.64) and Difficulty (BU-OA-FPS = 0.73; BU-OA-PRO = 0.69) subscales. The correlation between the BU-OA-PRO and BU-OA-FPS was 0.94. The AUC ranged from 0.80 to 0.88. The MDC90 was approximately 6 standardized points.
Conclusions
The BU-OA-FPS provides reliable and valid measurement of functional pain. Pragmatic studies may consider the BU-OA-FPS for use in electronic health records to capture outcomes.
Pragmatic clinical trials have gained widespread popularity1 and offer opportunities over explanatory trials for uptake into clinical practice because they test if an intervention actually works in real life.2 Pragmatic trials measure a wide spectrum of clinically meaningful patient-centered outcomes that require large sample sizes to overcome the inherent heterogeneity in patients, treatments, and clinical settings.2 By definition, pragmatic trials should mimic everyday clinical settings, and therefore, measurement of outcomes in these trials should not require extensive training or interrupt clinical flow or processes.2
Incorporating patient-reported outcome (PRO) measures into clinical trials is a crucial element for pragmatic research.3 Several systems have been developed that link electronic health records (EHR) to collect PROs for this purpose3 and are becoming increasingly popular.4 However, PRO administration within busy clinical operations is a barrier in the uptake of PROs. To be incorporated into EHR, PROs must not only be valid, reliable, and responsive but must also be practical,3 and results should be readily interpretable and actionable in a clinical practice.5 One way to encourage the design of practical, interpretable, and actionable PROs into clinical practice is by involving input from patients and clinicians during their development.6
Item response theory (IRT) is an increasingly popular modeling process to guide item selection in the development of PROs.7 Although IRT along with computer adaptive testing (CAT) provides a method for developing quantitative PROs,8 implementation of IRT/CAT instruments into EHR for clinical and research purposes is frequently not feasible in many institutions. However, through the use of IRT-calibrated item pools of questionnaire items, customized short-form PROs can be created that can be readily incorporated into EHRs, thus making them practical for administration in clinical practice pragmatic clinical research studies.7
Currently, there is a lack of PRO measures for knee OA function and pain that could be readily implemented into EHR with a number of items small enough for busy clinical practice. There are legacy instruments with adequate psychometric properties, but they are lengthy, such as the Knee injury and Osteoarthritis Outcomes Scale (KOOS), which has 6 different subsections with a total of 42 items,9 and the Western Ontario and McMasters University Osteoarthritis Index (WOMAC), which has 3 subsections and 24 items.10 The KOOS does have a 7-item physical function short form but does not capture pain.11 More recently, the Boston University Osteoarthritis Functional Assessment PRO (BU-OA-PRO) was developed12,13 by asking 328 patients with confirmed knee or hip OA to rate the difficulty and pain they experienced in performing 126 distinct functional tasks and activities. The resultant calibrated item banks were organized into 2 distinct physical function scales: a Functional Difficulty Scale and a Functional Pain Scale. The BU-OA-PROs functional difficulty and functional pain item banks showed strong psychometric properties in this sample of persons with hip OA, knee OA, or both. The full 125-item banks calibrated well with a unidimensional IRT model, providing greater breadth and more precise, more accurate, and more reliable estimates of functional difficulty and functional pain than the WOMAC.9 The purpose of this article is to describe the process used to create a customized short form from the BU-OA-PRO,12,13 the BU-OA-Functional Pain Short Form (BU-OA-FPS), that could be integrated into an EHR for clinical practice or pragmatic studies.
Materials and Methods
Creation of the BU-OA-FPS
All data used in the analyses were from an existing database of deidentified data in the BU-OA-PRO item bank. Details of this item bank used to develop this PRO have been described elsewhere.12,13 Briefly, the mean age of the patient group in the calibration sample was 61.8 (SD = 15.1) years, 64.5% were female, 85.0% were white, and 9.1% were black, and the majority (51.2%) had 4 years of college or greater. Most of the calibration sample had knee OA only (56.7%), 16.2% had hip OA only, and 27.1% had both knee and hip OA. The calibration sample did not demonstrate any differential item functioning by OA status, indicating that the instrument performed similarly across OA patient groups.
To gain an informed understanding of the needs of clinicians, and because of the nature of conducting a pragmatic trial across multiple health care and EHR systems, we conducted informal discussion with patients and clinicians to be considerate of their needs. The pragmatic trial design involved primary care provider (PCP) offices and outpatient physical therapy facilities that cared for people with knee OA and chronic knee pain. Thus, we had informal discussions with PCPs, a PCP office nurse administrator, and physical therapists from some of the outpatient clinics who would be involved in the study. The patients were those who had been treated by PCPs and physical therapists for their knee OA previously, whose ages ranged from about 45 to 75 years, and who had knee OA for greater than 3 years. One patient had eventually undergone total knee arthroplasty. For the patients, we asked them what they thought were the best ways to know whether patients were better off after having treatment for their knee OA and how they would decide the treatment was worth it. They indicated that reduced pain and the ability to perform activities of daily living better without pain, as well as not needing any more knee injections or surgery, would be best. For the clinician stakeholders, we not only asked about how they would know patients were better but also how feasible it would be to include patient-reported questionnaires in their practice. The clinicians acknowledged that reduced pain and improved function would be good indicators of improvement. In addition, they indicated that patient-reported questionnaires would be feasible if they did not take up too much time and if they could be part of the patient record. When asked how many items on a patient-reported measure they would consider to be reasonable, the consensus was 10 to 15 items and no more than 1 instrument.
The BU-OA-PRO includes a Functional Pain Scale and a Functional Difficulty Scale. Although these scales would satisfy the need for measures of both pain and function, a separate short form would have to be developed for each scale. Unfortunately, this would not satisfy the need for a single outcome measure, as stated by clinicians in our informal discussions. Given the high correlation between functional pain and difficulty, we decided to focus on the Functional Pain Scale for the development of the BU-OA-FPS because it queries about pain as it relates to functional task performance. Thus, we would have a single short-form measure that would capture pain and function and would be feasible to incorporate into the EMR.
BU-OA-PRO item reduction
We anticipated in our pragmatic trial that we were developing the short form for a majority of our participants who would be patients with knee OA who were ambulatory and would likely not require assistive devices such as wheelchairs and walkers. Thus, we conducted an initial item reduction of the BU-OA-PRO by removing items that were focused on these devices. Although some individual patients might be using an ambulatory assistive device, the remaining functional task items would be applicable regardless of whether they were using their device or not. We also eliminated items related to sports activities such as running, jumping/landing, and cutting and twisting while running because, in our clinical experience, a low percentage of our population of interest were involved in these higher-level activities. From the remaining items in the BU-OA-PRO, we further reduced the item pool by examining each item's discriminatory and calibration indices from previous IRT analyses. For example, if 2 items were similar on their calibration indices, we excluded the one whose discriminatory index was smaller. This allowed us to maintain a reasonable calibration range and discriminatory values across items. This phase of item reduction left 100 items remaining for further analysis.
Item selection
We used the item information function as the tool to select the short-form items. Item information function is the level of precision that an item could provide in estimating the score at different function levels. The test information function is the summary of a defined set of item information functions. The purpose of selecting the short-form items was to create a set of items, which has sufficiently high score precision (reliability ≥0.9) across the target population (standardized normal distribution). We applied the same iteration procedure described in McDonough et al14 to select the short-form items. We set the number of short-form items at 10, because usually 10 items could provide sufficient score precision without sacrificing practicality.
Psychometric Analysis
We used Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN)15,16 as the guideline to evaluate the BU-OA-FPS. There are 4 criteria included in COSMIN: reliability (internal consistency, measurement error), validity (content, construct, structure, criteria, cross-culture), responsiveness, and interpretability. In reliability, we examined internal consistency (Cronbach alpha) and calculated reliability, examined the score reliability by examining the test information function, and estimated the measurement error and calculated the minimal detectable change (MDC) at 90% confidence (MDC90). In validity, we looked at the content validity (item reduction), criteria validity (the correlation between BU-OA-FPS and BU-OA-PRO), and concurrent validity (the correlation between BU-OA-FPS and WOMAC). Because we used calibration data at 1 item point, we didn’t examine the responsiveness. In interpretability, we reported the demographic characteristics of the study sample and presented the histogram of the score distribution. All analyses were conducted using SAS/STAT 9.1 (SAS Institute Inc, Cary, North Carolina).
Reliability, Minimal Detectable Change, and Validity
Reliability
To determine reliability of the instrument, we first calculated the Cronbach alpha of BU-OA-FPS and then estimated the BU-OA-FPS IRT score reliability based on a simulation method. We considered 2 kinds of true score distributions. One of these is the uniform distribution, where scores range from –5 to 5, and the other is the standardized normative distribution. We generated 1000 true person scores from the true score distributions mentioned above, respectively; then, the item response patterns were simulated based on the generated true score and the short-form item parameters. Finally, the estimated person scores were calculated based on the response patterns and the short-form item parameters, and the reliability was calculated as the squared correlation between the true person scores and estimated person scores. We superimposed the short-form test information function onto the short-form score distribution from the BU-OA-PRO calibration sample and indicated the score range with reliability greater than 0.9 (which is corresponding to test information function value greater than 10), and then calculated the proportion of sample with score reliability greater than 0.9. For the criteria of Cronbach alpha, the reliability above 0.7 is accepted as reliable; above 0.8, highly reliable; above 0.9, perfectly reliable; and the value less than 0.7, not sufficiently reliable.17 We adopted the same criteria for Cronbach alpha to assess the IRT score reliability.
MDC
We calculated the MDC thresholds (MDC90) from standardized short-form scores based on the BU-OA-PRO calibration sample. We used the mean of the estimated standard error of short-form scores as the standard error of measurement (SEM), and MDC90 was calculated as
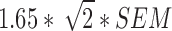 |
Validity
To examine ceiling and floor effects of BU-OA-FPS and WOMAC pain and difficulty scales, we determined the proportion of the sample at the maximum and minimum values of the BU-OA-FPS and the WOMAC,18 as the WOMAC instrument is a gold-standard legacy outcome instrument for hip and knee OA. We calculated the correlations among BU-OA-FPS, BU-OA-PRO, and WOMAC scales. In addition, as a form of “known groups” validation, we calculated area under the curve (AUC) for the ability of the BU-OA-FPS to differentiate different cutpoints for both the WOMAC pain and difficulty scales. We considered the ceiling or floor was presenting if the proportions of ceiling or floor exceeded 15%.19 We used Cohen criteria to establish the strength of correlation: r less than 0.20, no association; r from 0.20 to 0.39, weak; r from 0.40 to 0.59, moderate; r from 0.60 to 0.79, strong; and r from 0.80 to 1.0, very strong.20 The AUC over 0.7 was considered to be adequate.19
Role of the Funding Source
A. Goode was supported by the Foundation for Physical Therapy's Center of Excellence in Physical Therapy Health Services and Health Policy Research and Training Grant. The funder played no role in the design, conduct, or reporting of this study.
Results
Figure 1 contains the 10 items in the BU-OA-FPS. Raw scores on the BU-OA-FPS were transformed to the standardized score with range of scores between 10 and 90 based on full item bank. The transformation table from the raw summed scores to standardized scores is provided in the Appendix.
Figure 1.
Final version of the 10-item Boston University Osteoarthritis Functional Pain Short Form (BU-OA-FPS).
Reliability
The reliability estimates based on uniform and standardized normal distribution are 0.95 and 0.91, respectively, indicating perfectly reliable scales. The solid black line in Figure 2 shows the BU-OA-FPS’s test information function based on the calibration sample on which the BU-OA-PRO was developed, while the gray histogram bars display the person score distribution of the BU-OA-FPS scores in the calibration sample. The light gray shaded rectangle is the area where the BU-OA-PRO would have reliability greater than 0.9. The BU-OA-FPS short form has higher reliability (>0.9) within score range between 38.6∼61.8, and 71.7% of scores on the BU-OA-FPS have been covered by this score range. The Cronbach alpha coefficients indicated relatively high internal consistency for the BU-OA-FPS (0.91) (Tab. 1).
Figure 2.
Test information function and reliability of the Boston University Osteoarthritis Functional Pain Short Form (BU-OA-FPS) using person score distribution of the Boston University Osteoarthritis Patient-Reported Outcome (BU-OA-PRO).
Table 1.
Cronbach Alpha Coefficients and Ceiling and Floor Effect Estimates for the BU-OA-FPS and the WOMAC Pain and Difficulty Scalesa
| Test | No. of Items | Cronbach Alpha | Ceiling Effect N (%)b | Floor Effect N (%)c |
|---|---|---|---|---|
| BU-OA-FPS | 10 | 0.91 | 15 (4.57) | 0 (0) |
| WOMAC-Pain | 5 | 0.82 | 21 (6.4) | 2 (0.61) |
| WOMAC-Difficulty | 17 | 0.95 | 10 (3.05) | 2 (0.61) |
aBU-OA-FPS = Boston University Osteoarthritis Functional Pain Short Form; WOMAC = Western Ontario & McMaster Universities Osteoarthritis Index.
bCeiling effect in BU-OA-FPS or WOMACs means that participants responded to all items as “no pain.”
cFloor effect in BU-OA-FPS or WOMACs means participants responded to all items as “don’t do this activity, because of pain” or “always pain.”
MDC
The mean standard error of the BU-OA-FPS scores is 2.57. MDC90 for the BU-OA-FPS was approximately 6 points on the standardized score.
Validity
There was a very strong correlation between BU-OA-FPS and BU-OA-PRO (r = 0.94). Ceiling effects, which represent when all participants respond to an item as “no pain,” were lower for the BU-OA-FPS (n = 15; 4.57%) compared with the WOMAC pain scores (n = 21; 6.4%), but slightly higher when compared with the WOMAC difficulty scores (n = 10; 3.05%). Floor effects, which represent when participants responded to all items as “don’t do this activity, because of pain” or “always pain,” were lower for the BU-OA-FPS (n = 0; 0%) compared with WOMAC pain scores (n = 2; 0.61%) and difficulty scores (n = 2; 0.61%) (Tab. 1). Based upon our criteria of 15%, ceiling or floor effects would not be present. Table 2 displays the correlations between the WOMAC pain scores (range = 5–25, higher score means more pain) and WOMAC difficulty scores (range = 17–85, higher score means more difficulty) with BU-OA-FPS and BU-OA-PRO in the calibration sample. The BU-OA-FPS performed comparably to the BU-OA-PRO, demonstrating strong correlations with WOMAC pain (BU-OA-FPS = 0.67; BU-OA-PRO = 0.64) and function scores (BU-OA-FPS = 0.73; BU-OA-PRO = 0.69). Figure 3 provides the scatterplots demonstrating the relationship between the WOMAC pain and difficulty scores and the BU-OA-FPS. In addition, the AUC examining BU-OA-FPS vs BU-OA-PRO scores at various cutpoints for WOMAC pain and difficulty scores ranged from approximately 0.80 to 0.88 for both instruments, indicating that scores on the BU-OA-FPS perform similarly to the BU-OA-PRO and are adequately predictive of the WOMAC pain and function scores (Tab. 3).
Table 2.
The Correlationsa Between the BU-OA-FPS Scores and the WOMAC Pain and Difficulty and Boston University Osteoarthritis PRO Scores in the Calibration Samplea
| WOMAC Pain | WOMAC Difficulty | BU-OA-PRO | |
|---|---|---|---|
| BU-OA-FPS | –0.67 | –0.73 | 0.94 |
| BU-OA-PRO | –0.64 | –0.69 | N/A |
aThe negative correlation values are because, in BU-OA-PFS, higher score means higher function or less pain; in WOMAC, higher score means less function and more pain. BU-OA-FPS = Boston University Osteoarthritis Functional Pain Short Form; BU-OA-PRO = Boston University Osteoarthritis Patient Reported Outcome; WOMAC = Western Ontario & McMaster Universities Osteoarthritis Index.
Figure 3.
Scatterplots demonstrating the relationship between the Boston University Osteoarthritis Functional Pain Short Form (BU-OA-FPS) and the Western Ontario & McMaster Universities Osteoarthritis Index (WOMAC) for Difficulty and Pain.
Table 3.
Area Under the Curve (AUC) Values With Different WOMAC Pain and Difficulty Cutpoints for the BU-OA-FPS and Boston University Osteoarthritis PROa
| WOMAC Pain Cutpoints for BU-OA-FPS and BU-OA-PRO | ||
|---|---|---|
| BU-OA-FPS | BU-OA-PRO | |
| Median WOMAC pain score (10b) | 0.831 | 0.817 |
| 55th percentile WOMAC pain score (11) | 0.819 | 0.810 |
| 65th percentile WOMAC pain score (12) | 0.823 | 0.829 |
| 75th percentile WOMAC pain score (13) | 0.813 | 0.824 |
| 85th percentile WOMAC pain score (15) | 0.833 | 0.852 |
| 95th percentile WOMAC pain score (17) | 0.773 | 0.809 |
| WOMAC Difficulty Cutpoints for BU-OA-FPS and BU-OA-PRO | ||
|---|---|---|
| Median WOMAC difficulty score (34b) | 0.883 | 0.850 |
| 55th percentile WOMAC difficulty score (36) | 0.866 | 0.832 |
| 65th percentile WOMAC difficulty score (40) | 0.865 | 0.837 |
| 75th percentile WOMAC difficulty score (44) | 0.874 | 0.866 |
| 85th percentile WOMAC difficulty score (50) | 0.876 | 0.868 |
| 95th percentile WOMAC difficulty score (57) | 0.866 | 0.870 |
aBU-OA-FPS = Boston University Osteoarthritis Functional Pain Short Form; BU-OA-PRO = Boston University Osteoarthritis Patient-Reported Outcome; WOMAC = Western Ontario & McMaster Universities Osteoarthritis Index.
bCutpoint value for WOMAC.
Discussion
The motivation to develop a PRO short form was in planning a pragmatic clinical trial where we determined that the trial outcome measure would have to capture important patient-centered metrics for improved clinical outcome with acceptable psychometric properties, but would also have to be practical for use in the busy, participating clinical practices. We learned from the literature, from our clinical expertise, and from discussions with both patients and clinicians in planning the pragmatic trial that knee pain and functional ability were important patient-centered outcome metrics.21 We also learned that a practical outcome measure would likely be no more than 10 to 15 items in length and had to be easily interfaced with the EHR. The existence of the IRT calibrated 126-item BU-OA-PRO provided an excellent opportunity to customize an outcome measure that would fit all of these requirements for our pragmatic trial.
We utilized IRT to understand the performance of specific items included in the BU-OA-PRO item bank across a wide range of function as reflected by items in the instrument and functional level of participants in the original calibration study.22 This IRT approach provides numerous advantages over classical test theory, including evaluation of the contribution of individual items, and facilitates the identification of the most relevant, precise, and efficient items for the construct of a PRO instrument.22–24 These concepts are important not only in the development of outcome instruments but also in creating short forms of existing instruments.25
There is high interest in the development of PRO short forms that are easily administered in busy clinical practice and integrated into EHR and that assist in the capture of study outcomes. Similar to our study, the KOOS Joint Replacement (KOOS-JR) 7-item short form has been developed from a legacy PRO designed to represent “knee health,” both pain and function.26 However, the population of interest for the KOOS-JR consists of patients with end-stage OA awaiting total joint replacement. Our short form consists of 10 items that demonstrated a high degree of internal consistency of the scores as well as high correlations with the original BU-OA-PRO item banks and the WOMAC scales, which is evidence of high reliability and validity of its ability to characterize level of functional pain secondary to knee OA. The WOMAC is a traditional and widely used PRO with demonstrated acceptable measurement properties, specifically in the knee OA population. It is known through Rasch analyses that items on the WOMAC congregate in the center of a person's functional ability range and tend to have several redundancies.27 This finding is not surprising as classical survey construction methods have addressed coverage dilemmas with focusing on items in the mid range. The findings of our study indicate that, by carefully selecting 10 items using clinical judgment and IRT analytics, the BU-OA-FPS scale is a unidimensional metric that provides a high degree of overlap with the original 126 BU-OA-PRO and covers a wide range of functional pain. As such, the BU-OA-FPS is a very practical, patient-centered outcome metric that shows strong psychometric properties and will perform well in a sample of patients with knee OA.
Although the focus of developing the BU-OA-FPS was for use in patients with knee OA, it should be noted that the calibration sample used to develop the BU-OA-FPS did include some patients with hip OA. The majority of the sample were patients with knee OA, but some patients had hip OA or a combination of knee and hip OA. As noted above, the calibration sample did not demonstrate any differential item functioning by OA status (knee, hip, or combined knee and hip OA), indicating that the BU-OA-PRO used to develop the BU-OA-FPS performed similarly across OA patient groups. Given that the BU-OA-FPS performed similarly to the BU-OA-PRO, one could argue that the short form could also be used for hip OA as well. Further study is recommended to confirm use of the short form for patients with hip OA.
Our study is not without limitations. Our analyses were done on existing data, and we have not yet implemented the form into routine clinical practice or EHR to assess administrative burden. Our first steps described here were necessary so that we could have a short form in which we could be confident when initiating implementation. However, we cannot be certain that our informal discussions with patients and clinicians are representative of the broader population of potential users. We will need to further explore potential barriers and utility of this PRO once we implement it on a broader scale. For now, however, we believe we have justification that the form has adequate reliability and validity to initiate its implementation on a broader scale.
Conclusion
The BU-OA-CAT-FPS is a reliable and valid instrument, and with only 10 items, the short form can be readily implemented into EHR. Further testing of administrative burden and changes over time is needed. Additionally, further testing in different populations of knee and hip OA severity, and studies to compare our short-form instrument performance with new or legacy instruments, are needed. Pragmatic studies may consider the use of the BU-OA-CAT-FPS for implementation in clinical trials that use EHR to capture primary outcomes.
Appendix Conversion Table From the Raw Summed Score to Standardized Score
| Raw Summed Score | Standardized Score | Standardized Score Standard Error |
|---|---|---|
| 0 | 31.28 | 2.51 |
| 1 | 33.19 | 2.75 |
| 2 | 35.27 | 2.69 |
| 3 | 37.25 | 2.53 |
| 4 | 39.18 | 2.40 |
| 5 | 41.00 | 2.34 |
| 6 | 42.69 | 2.29 |
| 7 | 44.22 | 2.23 |
| 8 | 45.59 | 2.16 |
| 9 | 46.85 | 2.08 |
| 10 | 48.01 | 2.00 |
| 11 | 49.08 | 1.94 |
| 12 | 50.09 | 1.90 |
| 13 | 51.06 | 1.88 |
| 14 | 52.00 | 1.87 |
| 15 | 52.94 | 1.89 |
| 16 | 53.87 | 1.92 |
| 17 | 54.81 | 1.96 |
| 18 | 55.77 | 2.00 |
| 19 | 56.76 | 2.06 |
| 20 | 57.78 | 2.12 |
| 21 | 58.84 | 2.19 |
| 22 | 59.97 | 2.26 |
| 23 | 61.17 | 2.34 |
| 24 | 62.47 | 2.44 |
| 25 | 63.90 | 2.54 |
| 26 | 65.49 | 2.69 |
| 27 | 67.28 | 2.88 |
| 28 | 69.36 | 3.17 |
| 29 | 71.81 | 3.57 |
| 30 | 74.73 | 4.06 |
Notes
Dr Jette is a Catherine Worthingham Fellow of the American Physical Therapy Association (APTA)
Author Contributions
Concept/idea/research design: A.P. Goode, G.K. Fitzgerald, A. Jette
Writing: A.P. Goode, A. Jette, G.K. Fitzgerald, P. Ni
Data analysis: A.P. Goode, P. Ni, A. Jette, G.K. Fitzgerald
Project management: G.K. Fitzgerald
Consultation (including review of manuscript before submitting): A.P. Goode, A. Jette, G.K. Fitzgerald, P. Ni
Ethics Approval
There were no data collected from human subjects for the purpose of this study. All data used in the analyses were from an existing database of deidentified data in the BU-OA-PRO item bank.
Funding
A.P. Goode was supported by the Foundation for Physical Therapy's Center of Excellence in Physical Therapy Health Services and Health Policy Research and Training Grant. He also receives funds from the NIH Loan Repayment Program, National Institute of Arthritis Musculoskeletal and Skin Diseases (1-L30-AR057661–01).
Disclosures
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest. All authors report that they have no financial or personal relationships with other people or organizations that could potentially and inappropriately bias their work and conclusions.
References
- 1. Delitto A. Pragmatic clinical trials: Implementation opportunity, or just another fad? Phys Ther. 2016;96:137–138. [DOI] [PubMed] [Google Scholar]
- 2. Patsopoulos NA. A pragmatic view on pragmatic trials. Dialogues Clin Neurosci. 2011;13:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu AW, Jensen RE, Salzberg C, Snyder C. Advances in the use of patient reported outcome measures in electronic health records. Presented at: PCORI National Workshop to Advance the Use of PRO; November 19–20, 2013; Atlanta, GA. [Google Scholar]
- 4. Hsiao CJ, Hing E, Socey TC, Cai B. Electronic health record systems and intent to apply for meaningful use incentives among office-based physician practices: United States, 2001–2011. NCHS Data Brief. 2011;1–8. [PubMed] [Google Scholar]
- 5. Snyder CF, Aaronson NK, Choucair AK et al. Implementing patient-reported outcomes assessment in clinical practice: A review of the options and considerations. Qual Life Res. 2012;21:1305–1314. [DOI] [PubMed] [Google Scholar]
- 6. Concannon TW, Meissner P, Grunbaum JA et al. A new taxonomy for stakeholder engagement in patient-centered outcomes research. J Gen Intern Med. 2012;27:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: Item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007;16:133–141. [DOI] [PubMed] [Google Scholar]
- 8. Linden WJ, van der HR. Handbook of Modern Item Response Theory. New York, NY: Springer-Verlag New York, Inc.; 1997. [Google Scholar]
- 9. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 11. Perruccio AV, Stefan Lohmander L, Canizares M et al. The development of a short measure of physical function for knee OA KOOS-Physical Function Shortform (KOOS-PS)—an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:542–550. [DOI] [PubMed] [Google Scholar]
- 12. Jette AM, McDonough CM, Ni P et al. A functional difficulty and functional pain instrument for hip and knee osteoarthritis. Arthritis Res Ther. 2009;11:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jette AM, McDonough CM, Haley SM et al. A computer-adaptive disability instrument for lower extremity osteoarthritis research demonstrated promising breadth, precision, and reliability. J Clin Epidemiol. 2009;62:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDonough CM, Ni P, Coster WJ, Haley SM, Jette AM. Development of an IRT-Based short form to assess applied cognitive function in outpatient rehabilitation. Am J Phys Med Rehabil. 2016;95:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mokkink LB, Terwee CB, Knol DL et al. Protocol of the COSMIN study: COnsensus-based Standards for the selection of health Measurement INstruments. BMC Med Res Methodol. 2006;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mokkink LB, Terwee CB, Knol DL et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol. 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kline P. The Handbook of Psychological Testing. 2nd ed London, United Kingdom: Routledge; 2000. [Google Scholar]
- 18. Bellamy N, Watson-Buchanan W, Goldsmith C, Campbell J. Validation study of WOMAC: A health status instrument for measuring clincically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 19. Terwee CB, Bot SD, de Boer MR et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. [DOI] [PubMed] [Google Scholar]
- 20. Cohen LH. Measurement of life events. In: Cohen LH, ed. Life Events and Psychological Functioning: Theoretical and Methodological Issues. Newbury, CA: Sage; 1988. [Google Scholar]
- 21. Pham T, van der Heijde D, Altman RD et al. OMERACT-OARSI initiative: Osteoarthritis research society international set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12:389–399. [DOI] [PubMed] [Google Scholar]
- 22. Siemons L, Ten Klooster PM, Taal E, Glas CA, Van de Laar MA. Modern psychometrics applied in rheumatology—a systematic review. BMC Musculoskelet Disord. 2012;13:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reeve BB, Fayers P. Applying item response theory modeling for evaluating questionnaire item and scale properties. Assessing Quality of life in Clinical Trials: Methods of Practice. 2005;232(2):55–73. [Google Scholar]
- 24. McHorney CA, Cohen AS. Equating health status measures with item response theory: Illustrations with functional status items. Med Care. 2000;38(9 Suppl):II43–59. [DOI] [PubMed] [Google Scholar]
- 25. Fries JF, Bruce B, Bjorner J, Rose M. More relevant, precise, and efficient items for assessment of physical function and disability: Moving beyond the classic instruments. Ann Rheum Dis. 2006;65(Suppl 3):iii16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: A short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stucki G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12:331–335. [DOI] [PubMed] [Google Scholar]





