Abstract
Objectives
The aim of this study was to investigate the impact of adrenal venous sampling (AVS) lateralization cutoffs on surgical outcomes.
Patients and Methods
Cosyntropin-stimulated AVS was used to guide surgical management of 377 patients with primary aldosteronism (PA) who were evaluated 6 months after surgery.
Main Outcome Measures
The proportion of patients that achieved clinical benefit and complete biochemical success based on the AVS aldosterone lateralization index (LI) was determined.
Results
Clinical benefit was achieved in 29 of 47 patients with an LI between 2 and 4, in 66 of 101 with an LI between 4 and 10, and in 158 of 203 with an LI > 10 (P < 0.01 for trend). Complete biochemical success was achieved in 27 of 42 with an LI between 2 and 4, in 60 of 76 with an LI between 4 and 10, and in 127 of 155 with an LI > 10 (P = 0.024 for trend). After adjustment for confounders and using those patients with an LI between 2 and 4 as a reference, a clinical benefit was associated only with those with an LI > 10 (OR, 2.30; 95% CI, 1.03 to 5.16), whereas complete biochemical success was associated with those with an LI between 4 and 10 (OR, 2.83; 95% CI, 1.14 to 7.01) or LI > 10 (OR, 3.55; 95% CI, 1.47 to 8.55).
Conclusions
Difference of clinical outcome was relatively small when strict LI diagnostic threshold was used; biochemical cure was sufficiently achieved when an LI > 4 was used. Our study by standardized outcome measures validated that an LI > 4 may be appropriate for determining unilateral disease in PA.
Keywords: adrenal, adrenal venous sampling, aldosterone, primary aldosteronism
We investigated the impact of AVS lateralization cutoffs on surgical outcome by standardized measures and validated that an LI > 4 is appropriate to determine unilateral disease in PA.
Primary aldosteronism (PA) is the most common cause of endocrine hypertension [1, 2], compared with essential hypertension, increasing cardiometabolic complications [3, 4]. Adrenal venous sampling (AVS) is the standard for subtype diagnosis of PA, as recommended by clinical practice guidelines [5], for determining a treatment plan; unilateral hyperaldosteronism can be controlled by surgical treatment and bilateral hyperaldosteronism by specific medical treatments [6]. Although AVS plays an important role in diagnostic strategies for PA, many outstanding issues remain [7], and one of the most crucial is the impact of AVS aldosterone lateralization cutoffs on surgical outcomes.
Several studies have investigated the relationship between the efficacy of unilateral adrenalectomy and AVS [8–10], but results on the effectiveness of surgical treatment have differed widely among studies [11]. Although differences in patient clinical backgrounds in the studies could have influenced the results, one of the most important factors is the lack of a criterion standard for evaluation of postoperative outcomes in PA. However, in recent years, an international consensus on clinical and biochemical outcome measures after adrenalectomy for unilateral PA was established [12]; thus, postoperative outcomes based on AVS results can be assessed according to international standardized criteria.
The aim of the current study was to investigate the impact of lateralization cutoffs in AVS on surgical outcomes based on recent international standardized criteria by analyzing a nationwide multicenter AVS cohort in Japan.
1. Methods
A. Study Design and Patients
This study was conducted as a part of the Japan Primary Aldosteronism Study (JPAS). The details of this study have been described elsewhere [13]. We included patients diagnosed with PA who were adrenalectomized based on cosyntropin-stimulated AVS findings and who underwent follow-up assessments of outcome (clinical and/or biochemical) at 6 months after surgery between January 2006 and December 2016 at 28 participating JPAS centers in Japan. We excluded patients with unsuccessful AVS, those with bilateral aldosterone suppression [14, 15] during AVS (as a sampling error), and those with associated Cushing syndrome. Clinical findings including patients’ characteristics; biochemical, imaging, and AVS results were retrospectively obtained from the JPAS registry. A surgical indication was determined by each investigator based on AVS findings using the guidelines from the Japan Endocrine Society [16] and local reference criteria combined with the clinical background of the patients. This retrospective study was analyzed using the dataset valid in June 2017. The study was conducted according to clinical studies published by the Ministry of Health and Labor, Japan, and was approved by the ethics committee of the National Hospital Organization Kyoto Medical Center, as the project leader, and by the institutional ethics committees of the participating centers. This observational study was registered as UMIN ID 18756.
B. Diagnosis of PA
The diagnosis of PA was made in accordance with the guidelines of the Japan Endocrine Society [16] and the Japan Society of Hypertension [17]. PA was diagnosed by a ratio of plasma aldosterone concentration (PAC) (ng/dL) to plasma renin activity (PRA) (ng/mL/h) [aldosterone to renin ratio (ARR)] >20 and at least one positive result from confirmatory tests, including captopril-challenge, saline-infusion, furosemide-upright, and oral salt-loading. Antihypertensive medications were usually switched to calcium-channel blockers and/or α-adrenergic blockers, as appropriate, until the final diagnosis was made.
C. Adrenal Venous Sampling
AVS was performed in patients who opted for surgery. The details of the AVS procedure have been described previously [18]. In brief, adrenal blood samples were collected sequentially at 24 centers and at 4 centers where simultaneous catheterization was conducted. Cosyntropin was administered by bolus injection alone, bolus injection followed by continuous infusion, or continuous infusion alone throughout the procedure. Adrenal vein cannulation was defined as successful if the selectivity index was >5 [5, 16]. The selectivity index was defined as the ratio of the cortisol concentration in the adrenal vein to that in the inferior vena cava. The lateralization index (LI) was calculated by dividing the aldosterone to cortisol ratio in the dominant adrenal vein by that in the nondominant adrenal vein. The contralateral aldosterone suppression ratio (CLR) was calculated by dividing the aldosterone to cortisol ration in the nondominant adrenal vein by that in the inferior vena cava. Bilateral aldosterone suppression [14, 15] was defined as an aldosterone to cortisol ratio in both the dominant and nondominant adrenal veins lower than that in the inferior vena cava.
D. Definition of Clinical and Biochemical Outcomes
Biochemical and clinical outcomes after unilateral adrenalectomy were evaluated based on the recent international Primary Aldosteronism Surgical Outcome (PASO) consensus. In brief, the biochemical outcome was determined by the postoperative ARR and serum potassium concentration; clinical outcome was determined by postoperative blood pressure. Biochemical and clinical outcomes were classified as complete, partial, or absent success based on the response to surgery. A clinical benefit was defined as either complete or partial success. Details of the outcome criteria have been described previously by Williams et al. [12].
E. Assay Methods
PAC and PRA were measured using commercial kits. PAC was determined by radioimmunoassay (SPAC-S Aldosterone kit; Fuji Rebio, Co., Ltd, Tokyo, Japan) at all centers. The reference range of PAC in the supine position was 3.0 to 15.9 ng/dL. PRA was measured by radioimmunoassay or enzyme immunoassay. The reference range of PRA in the supine position was 0.3 to 2.9 ng/mL/h (PRA-FR RIA kit; Fuji Rebio, Co., Ltd.) at 16 of the study centers, 0.2 to 2.3 ng/mL/h (PRA EIA kit; Yamasa Co., Ltd., Choshi, Japan) at 8 centers, and 0.2 to 2.7 ng/mL/h (PRA RIA kit) at 3 centers. The plasma active renin concentration (ARC) was measured by immunoradiometric assay (Renin IRMA-FR kit; Fuji Rebio, Co., Ltd.) at one center. The ARC was converted to a PRA value and divided by 5 according to the Japan Endocrine Society guideline [16]; the PRA value was used for the analyses. The reference range of ARC in the supine position was 2.5 to 21.4 pg/mL.
F. Measurements and Statistical Analysis
Clinical and biochemical outcome was calculated with respect to the LI. We compared clinical characteristics between patients with and those without a surgical benefit. The LI was categorized into three groups: 2 to 4, 4 to 10, and >10. The reason for this classification is that, although an LI >4 is the most commonly used cutoff as a criterion for positivity, one-half of the centers involved in this study use more permissive cutoffs (LI 2 to 4) [19]. An LI of 10 was derived from the area under the receiver operating characteristic curve for evaluating a clinical benefit (Supplemental Fig. 1). The cutoff was determined as the value affording the optimal sensitivity and specificity (Youden index). For clinical utility, the LI value of 9.9 calculated from the analysis was changed to 10. We compared the baseline clinical characteristics among the three LI groups. We also compared the proportion of patients with surgical outcomes in terms of complete biochemical success and clinical benefit among the three LI groups.
A logistic regression analysis was performed to evaluate the effect of the AVS results on surgical outcome. The adjusted variables evaluated in the analysis (age, sex, body mass index, systolic blood pressure, antihypertensive medication use, estimated glomerular filtration rate, serum potassium concentration, plasma aldosterone concentration, and presence of diabetes mellitus) were selected according to previous reports [11, 12].
Statistical analyses were performed using EZR statistical software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [20]. Continuous variables were expressed as mean with the SD. Categorical variables were expressed as numbers and percentages. Continuous variables were analyzed using the Student t test and categorical variables using the Fisher exact test. Analysis of group difference used one-way ANOVA and the Fisher exact test with a post hoc Bonferroni analysis. All tests were two-tailed, with P < 0.05 indicating a significant difference.
2. Results
Between January 2006 and December 2016, 481 patients with PA who had been adrenalectomized based on cosyntropin-stimulated AVS findings and who had available follow-up data regarding clinical and/or biochemical outcomes 6 months after surgery were recruited. Of these, 104 patients were excluded from the current study for the following reasons: 71 with unsuccessful cosyntropin-stimulated AVS, 10 with Cushing syndrome, and 23 with bilateral aldosterone suppression during AVS. In total, 377 patients (351 with a clinical outcome, 273 with a biochemical outcome, and 247 with both outcomes) were analyzed in the current study. Of these, 52 patients had an LI between 2 and 4, 106 had an LI between 4 and 10, and 219 had an LI > 10 (Supplemental Fig. 2).
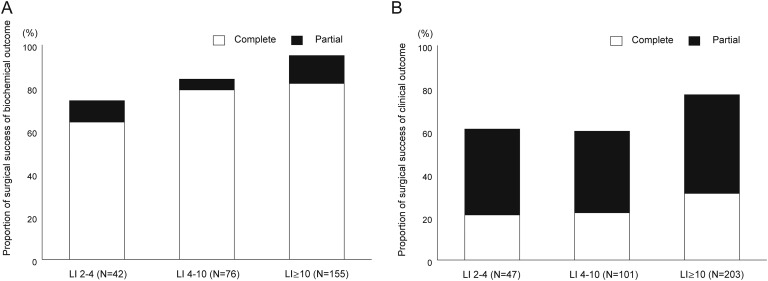
The clinical characteristics included a mean age of 53 years, nearly equivalent sex prevalence, moderate doses of antihypertensive medications used, and frequent hypokalemia (Table 1). Complete biochemical success was found in 214 (78.4%) and partial biochemical success in 31 (11.4%) of the 273 patients with biochemical outcome data (Fig. 1A). On the other hand, complete clinical success was observed in 95 (27.1%) and partial clinical success in 152 (43.3%) of the 351 patients with clinical outcome data; thus, 70% of patients achieved a clinical benefit (Fig. 1B).
Table 1.
Comparison of Baseline Clinical Characteristics in Patients With Respect to Adrenal Venous Sampling LI
| Characteristics | Total (N = 377) | LI 2–4 (n = 52) | LI 4–10 (n = 106) | LI > 10 (n = 219) | Overall P Value | Pairwise Comparison (P Values) |
||
|---|---|---|---|---|---|---|---|---|
| LI 2–4 vs LI 4–10 | LI 2–4 vs LI > 10 | LI 4–10 vs LI > 10 | ||||||
| Age, y | 52.0 ± 11.4 | 50.3 ± 10.2 | 52.6 ± 10.7 | 52.1 ± 12.0 | 0.47 | NA | NA | NA |
| Female, % | 46.9 | 53.8 | 34.9 | 51.1 | 0.01 | 0.079 | 1.0 | 0.019 |
| Body mass index | 24.4 ± 4.2 | 25.1 ± 3.7 | 25.0 ± 3.8 | 23.9 ± 4.4 | 0.028 | 1.00 | 0.19 | 0.06 |
| Systolic blood pressure, mm Hg | 141 ± 18 | 137 ± 18 | 142 ± 19 | 142 ± 18 | 0.22 | NA | NA | NA |
| Diastolic blood pressure, mm Hg | 86 ± 12 | 84 ± 12 | 88 ± 12 | 86 ± 12 | 0.078 | NA | NA | NA |
| Duration of hypertension, y | 10.6 ± 9.0 | 7.5 ± 7.1 | 10.9 ± 9.1 | 11.2 ± 9.2 | 0.031 | 0.092 | 0.027 | 1.0 |
| Defined daily dose of antihypertensive medications | 2.0 ± 1.3 | 1.7 ± 1.1 | 1.9 ± 1.1 | 2.1 ± 1.4 | 0.11 | NA | NA | NA |
| Diabetes mellitus, % | 16.4 | 13.4 | 22.1 | 14.4 | 0.22 | NA | NA | NA |
| Serum potassium, mEq/L | 3.3 ± 0.6 | 3.6 ± 0.5 | 3.5 ± 0.5 | 3.2 ± 0.5 | <0.01 | 0.49 | <0.01 | <0.01 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 78 ± 22 | 87 ± 23 | 78 ± 20 | 77 ± 22 | <0.01 | 0.036 | <0.01 | 1.0 |
| Plasma aldosterone concentration, ng/dL | 35.7 ± 24.7 | 22.7 ± 15.6 | 25.8 ± 11.8 | 43.5 ± 27.8 | <0.01 | 1.0 | <0.01 | <0.01 |
| Plasma renin activity, ng/mL/h | 0.37 ± 0.42 | 0.42 ± 0.32 | 0.40 ± 0.30 | 0.34 ± 0.48 | 0.3 | NA | NA | NA |
| Ratio of plasma aldosterone concentration to plasma renin activity | 181 ± 224 | 85.1 ± 103 | 109 ± 117 | 239 ± 264 | <0.01 | 1.0 | <0.01 | <0.01 |
Data are presented as mean ± SD unless otherwise indicated.
Abbreviation: NA, not available.
Figure 1.
Biochemical and clinical outcomes of adrenalectomized patients with primary aldosteronism. The proportion of patients with outcomes in terms of biochemical and clinical outcomes was assessed with respect to the LI, which was categorized into three groups (2 to 4, 4 to 10, and >10). (A) Complete biochemical success was in nearly 80% of the patients and (B) clinical benefit was achieved in nearly 70%. There was a tendency for favorable biochemical and clinical outcomes with an increasing LI value [(A) P < 0.01 for trend for biochemical outcome, (B) P < 0.01 for clinical outcome]. Using LI > 4 as a criterion for positivity, (A) 187 of 231 (81%) patients showed complete biochemical success and (B) 218 of 304 (72%) patients showed a clinical benefit.
The baseline characteristics of the participating patients evaluated with respect to the LI are summarized in Table 1. Substantial differences in 7 of 13 variables were found among 3 LI groups. A biochemical complete success was achieved in 27 of 42 (64.3%) with an LI between 2 and 4, in 60 of 76 (78.9%) with an LI between 4 and 10, and in 127 of 155 (81.9%) with an LI > 10 (P = 0.024 for trend) (Fig. 1A). A clinical cure was achieved in 10 of 47 (21.3%) patients with an LI between 2 and 4, in 22 of 101 (21.8%) with an LI between 4 and 10, and in 63/203 (31.0%) with an LI > 10 (P = 0.071 for trend); a clinical benefit was achieved in 29 of 47 (61.7%) patients with an LI between 2 and 4, in 60 of 101 (59.4%) with an LI between 4 and 10, and in 158 of 203 (77.8%) with an LI > 10 (P < 0.01 for trend) (Fig. 1B). Using a criterion for positivity of LI > 4, 187 of 231 (81%) showed a biochemical cure, whereas 85 of 304 (27.9%) showed a clinical cure and 218 of 304 (71.7%) patients showed a clinical benefit.
We performed a logistic regression analysis to determine the effect of AVS lateralization cutoffs on surgical outcome. In a univariate analysis, using the patients with an LI between 2 and 4 as the reference group, biochemical complete success and clinical benefits in terms of surgical outcomes were associated only those with an LI > 10 but not those with an LI between 4 and 10 (Table 2). After adjustment for confounders, a complete biochemical success was associated with both an LI between 4 and 10 (OR, 2.83; 95% CI, 1.14 to 7.01) and LI > 10 (OR, 3.55; 95% CI, 1.47 to 8.55) (Table 2), whereas a clinical benefit was associated with an LI > 10 (OR, 2.30; 95% CI, 1.03 to 5.16) but not with an LI between 4 and 10 (OR, 0.97; 95% CI, 0.45 to 2.10). A criterion for positivity of LI > 4 was associated with surgical outcomes in terms of the complete biochemical success, but not clinical benefit (Table 2). Additionally, we evaluated a criterion of CLR < 1 for surgical outcomes in terms of complete biochemical success and clinical benefit. Although a criterion for positivity of CLR < 1 showed a high proportion of complete success of biochemical outcome with equal to that of LI > 10, no substantial differences were found in patients with and without meeting a criterion of CLR < 1 for biochemical and clinical outcomes (Table 2).
Table 2.
Surgical Outcomes in Terms of Biochemical and Clinical Outcomes as Determined by AVS Findings in Unadjusted and Adjusted Analyses
| Biochemical Outcome | Patients | Complete Success (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|---|
| LI category | ||||
| 2–4 | 42 | 27 (64) | 1.00 (reference) | 1.00 (reference) |
| 4–10 | 76 | 60 (79) | 2.08 (0.90–4.82) | 2.83 (1.14–7.01) |
| >10 | 155 | 127 (82) | 2.52 (1.19–5.35) | 3.55 (1.47–8.55) |
| AVS criterion | ||||
| LI < 4 | 42 | 27 (64) | 1.00 (reference) | 1.00 (reference) |
| LI > 4 | 231 | 187 (81) | 2.36 (1.16–4.81) | 3.21 (1.44–7.18) |
| CLR > 1 | 29 | 22 (76) | 1.00 (reference) | 1.00 (reference) |
| CLR < 1 | 244 | 192 (79) | 1.17 (0.48–2.90) | 1.40 (0.54–3.66) |
| Clinical outcome | Patients | Benefits (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| LI category | ||||
| 2–4 | 47 | 29 (61) | 1.00 (reference) | 1.00 (reference) |
| 4–10 | 101 | 60 (59) | 0.91 (0.45–1.85) | 0.97 (0.45–2.10) |
| >10 | 203 | 158 (77) | 2.18 (1.11–4.28) | 2.30 (1.03–5.16) |
| AVS criterion | ||||
| LI < 4 | 47 | 29 (61) | 1.00 (reference) | 1.00 (reference) |
| LI > 4 | 304 | 218 (72) | 1.57 (0.83–2.98) | 1.44 (0.70–2.99) |
| CLR > 1 | 37 | 26 (70) | 1.00 (reference) | 1.00 (reference) |
| CLR < 1 | 314 | 221 (70) | 1.01 (0.48–2.12) | 0.61 (0.27–1.40) |
Adjusted for age, sex, body mass index, systolic blood pressure, defined daily dose of antihypertensive medications, estimated glomerular filtration rate, serum potassium concentration, plasma aldosterone concentration, and diabetes mellitus.
3. Discussion
Our results demonstrated an association between AVS aldosterone lateralization cutoffs and surgical outcomes based on recent international criteria in unilateral adrenalectomized patients with PA. The major difference of the current study was to correlate the LI cutoff and clinical and biochemical outcomes after adrenal surgery using the PASO criteria [12]. The results provided evidence that LI cutoff of 4 is an adequate cutoff to determine unilateral disease and to achieve good biochemical outcome, whereas the difference of clinical benefit for LI between 4 and 10 and that for LI > 10 was relatively small, although it was statistically significant. Our study by standardized outcome measure validated that an LI > 4 recommended by clinical guidelines [5] may be appropriate for determining unilateral disease in PA.
Before the recent international standardized criteria on surgical outcome of patients with PA were established [12], the postoperative outcomes of patients with PA were usually determined by a combination of blood pressure, hormone profiles, serum potassium levels, and occasionally pathological findings based on local reference criteria [11]. This is one of the reasons that resolution or improvement of PA has differed widely across studies that used different lateralization cutoffs of AVS. Although the receiver operating characteristic analysis showed an LI of 9.9 as the optimal cutoff (Youden index) for evaluating clinical outcome (Supplemental Fig. 1), the AUC (0.59; 95% CI, 0.53 to 0.66), which afforded a sensitivity of 65% and a specificity of 57%, was not sufficient because a tendency, rather than a clear association, for clinical and biochemical outcomes in patients with this LI value was detected (Fig. 1). The best cutoff for the AVS LI was therefore difficult to establish. Our results suggest that unilateral hyperaldosteronism may consist of heterogeneous etiologies.
An international survey of AVS (Adrenal Vein Sampling International Study) [19] reported that an LI > 4 is the most common criterion associated with cosyntropin-stimulated AVS. Considering that > 80% of patients can achieve complete success in terms of biochemical outcome determined by recent international standardized criteria with a commonly used criterion of LI > 4 (Fig. 1; Table 2), this cutoff is preferable when biochemical success is the goal. In the PASO study [12], PA patients with an LI > 4 showed preferable surgical outcomes in terms of biochemical than those with an LI < 4 in univariate analysis. In contrast, a higher LI value (>10) was needed to obtain an independent surgical benefit in terms of clinical outcome (Table 2). We used nine variables for a logistic regression analysis; these variables were reported by the associated factor for surgical outcome of PA [11, 12]. In addition, similar important differences of variables, especially in clinical outcome, between patients with and those without surgical benefit were seen in present study (Supplemental Table 1). In these confounders, age, body mass index, and systolic blood pressure were predictors of clinical benefit, whereas but no clinical factors were associated with the outcome of biochemical benefit (data not shown), suggesting that clinical outcome depended on preoperative clinical findings, whereas biochemical outcome was determined by only AVS LI.
Notably, 60% of patients with available clinical and biochemical outcome data, respectively, showed a surgical benefit in clinical and complete success in biochemical among the patients with an LI of 2 to 4 (Fig. 1). Although an LI > 4 is the most commonly used AVS criterion, approximately one-half of the reference centers in the Adrenal Vein Sampling International Study used more permissive LI values, ranging from 2 to 4 [19]. We found a favorable tendency, albeit not a substantial association, for a clinical benefit in patients with these LI values who also had higher systolic blood pressure, higher defined daily dose of antihypertensive medications, or lower serum potassium levels. We also found that younger patients achieved a good biochemical outcome more frequently than did older patients (P < 0.01) (data not shown); however, on AVS finding, contralateral aldosterone suppression was not associated with surgical benefits in terms of clinical and biochemical outcomes (data not shown). Additionally, we have reported that LI between 2 and 4 can be found even in patients with negative confirmatory testing for PA [21]. Therefore, clinicians could send patients in these LI zones to surgery with careful consideration for patient characteristics. However, the results should be carefully interpreted because of the small number of patients and that only selected patients underwent surgery in this subgroup.
Contralateral aldosterone suppression has been secondarily used by a decision criterion for laterality of hyperaldosteronism followed in LI [5, 22]. In the present study, a criterion of CLR < 1 was not associated with outcomes in terms of complete biochemical success and clinical benefit. However, a criterion for positivity of CLR < 1 showed high proportion of a surgical benefit equal to that of LI > 10, suggesting that a certain number of patients with CLR > 1 showed complete success of biochemical outcome. Therefore, a more permissive cutoff of CLR for a decision criterion of aldosterone laterality during non-ACTH stimulated AVS might be adopted.
When comparing our results with those of PASO study [12], the proportion of patients who achieved a surgical benefit in terms of clinical outcome was smaller in our study (70% vs 84%). This may be because of the slightly older age and lower systolic blood pressure of our participants (Table 1). Additionally, biochemical outcome was smaller in our study compared with the PASO study [12]. This may have occurred because an ARR cutoff of 20 for screening in Japan’s guideline [16] has been set, aiming at higher sensitivity to avoid false negatives and to not to overlook PA patients. The cutoff, however, may affect the biochemical outcome after surgery. Furthermore, our patients showed wide SD of ARR (mean, 103; interquartile range, 55.1 to 212; range, 8.2 to 1890), indicating an overlap with the normal range. Routine confirmatory testing may be needed when the ARR is positive after surgery, as suggested by the PASO study. Additionally, a high prevalence of positive KCNJ5 mutation has been reported in Japan [23]. Because KCNJ5-mutated aldosterone-producing adenoma have the specific features of high CYP11B1 and low CYP11B2 [24], this may affect the lateralization of PA by AVS. Finally, racial difference and high salt intake may be related to poor biochemical outcomes by forming bilateral hyperplasia with asymmetrical aldosterone production. In patients who had available data for pathological findings and assessment of biochemical outcome after surgery, 70% were diagnosed with aldosterone-producing adenomas based on the histological presence of adenoma and complete biochemical success after surgery. Some of the remaining patients might have aldosterone-producing lesions; histological details wait for further analysis.
4. Limitations
Our study has several limitations, with the main one being its retrospective nature. A second limitation is that, although a surgical indication is based essentially on the Japan Endocrine Society guidelines, details of the indication criteria used for adrenal surgery have not been completely standardized among centers. For this reason, our study included a heterogeneous patient population. A prospective standardized protocol study is needed to validate our results. A third limitation is the protocol used for cosyntropin administration. Cosyntropin administration is debated because it improves the AVS [25], whereas it lost lateralization of aldosterone excess in some cases. There are many reports of the usefulness of noncosyntropin-stimulated AVS in expert centers worldwide [19]; hence, our results apply only to patients with cosyntropin-stimulated AVS. A fourth limitation is the small number of patients in the group with no biochemical success.
5. Conclusion
Our study demonstrated the correlation between LI of AVS and postoperative outcomes using the standardized criteria. The results provided evidence that LI cutoff of 4 is to determine unilateral disease and to achieve good biochemical outcome, whereas the difference of clinical benefit for LI between 4 and 10 and that for LI > 10 was relatively small. Our study validated by standardized outcome measure that an LI > 4 recommended by clinical guidelines may be appropriate for determining unilateral disease in PA.
Supplementary Material
Acknowledgments
Financial Support: This study was supported in part by Grants JP17ek0109122 and JP18ek0109352 for the Japan Primary Aldosteronism Study from the Practical Research Project for Rare/Intractable Disease from the Japan Agency for Medical Research and Development (to M.N.), by Grants 27-1402 and 30-1008 from the National Center for Global Health and Medicine, Japan (to M.N.), and Grant 17K16173 Grant-in-Aid for Young Scientists (B) (to H.U.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ARC
active renin concentration
- ARR
aldosterone to renin ratio
- AVS
adrenal venous sampling
- CLR
contralateral aldosterone suppression ratio
- LI
lateralization index
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PRA
plasma renin activity
- PASO
Primary Aldosteronism Surgical Outcome
References and Notes
- 1. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F, Investigators PS; PAPY Study Investigators . A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–2300. [DOI] [PubMed] [Google Scholar]
- 2. Käyser SC, Dekkers T, Groenewoud HJ, van der Wilt GJ, Carel Bakx J, van der Wel MC, Hermus AR, Lenders JW, Deinum J. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826–2835. [DOI] [PubMed] [Google Scholar]
- 3. Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826–4833. [DOI] [PubMed] [Google Scholar]
- 4. Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M, Endres S; German Conn’s Registry-Else Kröner-Fresenius-Hyperaldosteronism Registry . Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. 2012;60(3):618–624. [DOI] [PubMed] [Google Scholar]
- 5. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 6. Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62(1):62–69. [DOI] [PubMed] [Google Scholar]
- 7. Auchus RJ, Wians FH Jr, Anderson ME, Dolmatch BL, Trimmer CK, Josephs SC, Chan D, Toomay S, Nwariaku FE. What we still do not know about adrenal vein sampling for primary aldosteronism. Horm Metab Res. 2010;42(6):411–415. [DOI] [PubMed] [Google Scholar]
- 8. Webb R, Mathur A, Chang R, Baid S, Nilubol N, Libutti SK, Stratakis CA, Kebebew E. What is the best criterion for the interpretation of adrenal vein sample results in patients with primary hyperaldosteronism? Ann Surg Oncol. 2012;19(6):1881–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136(6):1227–1235. [DOI] [PubMed] [Google Scholar]
- 10. Mulatero P, Bertello C, Sukor N, Gordon R, Rossato D, Daunt N, Leggett D, Mengozzi G, Veglio F, Stowasser M. Impact of different diagnostic criteria during adrenal vein sampling on reproducibility of subtype diagnosis in patients with primary aldosteronism. Hypertension. 2010;55(3):667–673. [DOI] [PubMed] [Google Scholar]
- 11. Steichen O, Zinzindohoué F, Plouin PF, Amar L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm Metab Res. 2012;44(3):221–227. [DOI] [PubMed] [Google Scholar]
- 12. Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF Jr, Gomez-Sanchez CE, Funder JW, Reincke M; Primary Aldosteronism Surgery Outcome (PASO) investigators . Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Umakoshi H, Tsuiki M, Takeda Y, Kurihara I, Itoh H, Katabami T, Ichijo T, Wada T, Yoshimoto T, Ogawa Y, Kawashima T, Sone T, Inagaki N, Takahashi K, Watanabe M, Matsuda Y, Kobayashi H, Shibata H, Kamemura K, Otsuki M, Fujii Y, Yamamto K, Ogo A, Yanase T, Suzuki T, Naruse M. Significance of computed tomography and serum potassium in predicting subtype diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103(3):900–908. [DOI] [PubMed] [Google Scholar]
- 14. Wolley M, Gordon RD, Pimenta E, Daunt N, Slater GJ, Ahmed AH, Stowasser M. Repeating adrenal vein sampling when neither aldosterone/cortisol ratio exceeds peripheral yields a high incidence of aldosterone-producing adenoma. J Hypertens. 2013;31(10):2005–2009. [DOI] [PubMed] [Google Scholar]
- 15. Shibayama Y, Wada N, Umakoshi H, Ichijo T, Fujii Y, Kamemura K, Kai T, Sakamoto R, Ogo A, Matsuda Y, Fukuoka T, Tsuiki M, Suzuki T, Naruse M. Bilateral aldosterone suppression and its resolution in adrenal vein sampling of patients with primary aldosteronism: analysis of data from the WAVES-J study. Clin Endocrinol (Oxf). 2016;85(5):696–702. [DOI] [PubMed] [Google Scholar]
- 16. Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A; Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society . Guidelines for the diagnosis and treatment of primary aldosteronism--the Japan Endocrine Society 2009. Endocr J. 2011;58(9):711–721. [DOI] [PubMed] [Google Scholar]
- 17. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension . The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37(4):253–390. [DOI] [PubMed] [Google Scholar]
- 18. Umakoshi H, Wada N, Ichijo T, Kamemura K, Matsuda Y, Fuji Y, Kai T, Fukuoka T, Sakamoto R, Ogo A, Suzuki T, Tsuiki M, Naruse M; WAVES-J Study Group . Optimum position of left adrenal vein sampling for subtype diagnosis in primary aldosteronism. Clin Endocrinol (Oxf). 2015;83(6):768–773. [DOI] [PubMed] [Google Scholar]
- 19. Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, Degenhart C, Deinum J, Fischer E, Gordon R, Kickuth R, Kline G, Lacroix A, Magill S, Miotto D, Naruse M, Nishikawa T, Omura M, Pimenta E, Plouin PF, Quinkler M, Reincke M, Rossi E, Rump LC, Satoh F, Schultze Kool L, Seccia TM, Stowasser M, Tanabe A, Trerotola S, Vonend O, Widimsky J Jr, Wu KD, Wu VC, Pessina AC. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97(5):1606–1614. [DOI] [PubMed] [Google Scholar]
- 20. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Umakoshi H, Naruse M, Wada N, Ichijo T, Kamemura K, Matsuda Y, Fujii Y, Kai T, Fukuoka T, Sakamoto R, Ogo A, Suzuki T, Nanba K, Tsuiki M; WAVES-J Study Group . Adrenal venous sampling in patients with positive screening but negative confirmatory testing for primary aldosteronism. Hypertension. 2016;67(5):1014–1019. [DOI] [PubMed] [Google Scholar]
- 22. Wolley MJ, Gordon RD, Ahmed AH, Stowasser M. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism? A retrospective study. J Clin Endocrinol Metab. 2015;100:1477–1484. [DOI] [PubMed] [Google Scholar]
- 23. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. 2015;22(2):191–200. [DOI] [PubMed] [Google Scholar]
- 24. Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, Williams TA, Mulatero P. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monticone S, Satoh F, Giacchetti G, Viola A, Morimoto R, Kudo M, Iwakura Y, Ono Y, Turchi F, Paci E, Veglio F, Boscaro M, Rainey W, Ito S, Mulatero P. Effect of adrenocorticotropic hormone stimulation during adrenal vein sampling in primary aldosteronism. Hypertension. 2012;59(4):840–846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.