Abstract
Hormones and endocrine-disrupting chemicals are generally thought to have permanent “organizational” effects when exposures occur during development but not adulthood. Yet, an increasing number of studies have shown that pregnant females are disrupted by endocrine-disrupting chemical exposures, with some effects that are permanent. Here, we examined the long-term effects of exposure to oxybenzone, an estrogenic chemical found in sunscreen and personal care products, on the morphology of the mammary gland in mice exposed during pregnancy and lactation. Female mice were exposed to vehicle or 30, 212, or 3000 µg oxybenzone/kg/d, from pregnancy day 0 until weaning. A nulliparous group, receiving vehicle treatment, was also evaluated. Mammary glands were collected 5 weeks after involution for whole-mount, histological, immunohistochemical, and molecular analyses. Exposure to 3000 µg oxybenzone/kg/d induced permanent changes to ductal density that was significantly different from both the nulliparous and vehicle groups. The two highest doses of oxybenzone similarly induced an intermediate phenotype for expression of progesterone receptor. A monotonic, dose-dependent increase in cell proliferation was also observed in the oxybenzone-treated females, becoming statistically significant at the highest dose. Finally, oxybenzone exposure induced an intermediate phenotype for Esr1 expression in all oxybenzone-treated groups. These data suggest that oxybenzone, at doses relevant to human exposures, produces long-lasting alterations to mammary gland morphology and function. Further studies are needed to determine if exposure to this chemical during pregnancy and lactation will interfere with the known protection that pregnancy provides against breast cancer.
Keywords: benzophenone, estrogen receptor, involution, parity, vulnerable period, xenoestrogen
Exposure to oxybenzone, an estrogen receptor agonist, during pregnancy and lactation had long-lasting effects on morphology and expression of hormone receptors in the mouse mammary gland.
The mammary gland is a unique organ that grows and changes rapidly throughout a female’s lifetime, including during puberty when the ductal tree reaches its full size and then again during pregnancy when differentiation and growth support lactation. After the initial development of the mammary gland in the embryonic stage, much of the growth and reorganization of the mammary gland that occurs at or after puberty is hormone dependent [1]. During early pregnancy, estrogen produced by the corpus luteum induces ductal morphogenesis and is important for the induction of the progesterone receptor (PR) in mammary epithelial cells [2], which is mediated by estrogen receptor (ER)α [3]. Although ERα is highly expressed in adult mammary epithelial cells, ERα expression diminishes in pregnancy and is again highly expressed during lactation [4]. The hormones of pregnancy induce rapid ductal side-branching, followed by development of lobuloalveolar units, e.g., the differentiated epithelial structures that develop during pregnancy and are milk-secreting compartments. These structures ultimately fill the fat pad and produce milk [5]. Progesterone and prolactin induce alveolar and lobuloalveolar development by stimulating epithelial proliferation, causing branching and reorganization of ducts [1, 6]. Late in pregnancy, PR expression decreases and is absent entirely in lactation [2, 7, 8]. Proliferation is absent during mid and late lactation, because the mammary gland has already grown to accommodate milk production [7, 9].
As the pups grow and begin to eat solid foods, they consume less milk, eventually leading to natural weaning. With the loss of suckling, milk accumulates in the lumen of alveolar structures, inducing mechanical strain; the presence of milk in the alveoli causes systemic concentrations of lactogenic hormones to fall [10]. Once this occurs, the mammary gland undergoes involution, which progresses in two distinct phases. In the first phase, massive numbers of secretory cells undergo apoptosis; if suckling occurs, this phase is reversible. In the second phase, the mammary gland undergoes extensive remodeling and reorganization to return to a prepregnant appearance; this phase is irreversible [11]. The process of involution requires macrophages, expression of a number of genes [e.g., signal transducer and activator of transcription (Stat)3, Fas ligand, and TGF-β/Wnt5a] [12], and the inactivation of Stat5a and Stat5b, which halt prolactin signaling and reduce cell survival [13]. After involution, the mammary gland returns to a state similar to a nulliparous adult female, but some distinguishing features remain. For example, the differentiated cells produced during pregnancy comprise a new mammary epithelial cell population that is molecularly and physiologically distinct from the mammary epithelial cells present before pregnancy; these parity-induced epithelial cells serve as progenitors of lobuloalveolar cells [14] and persist at the terminal ends of ducts [15], allowing the gland to respond more rapidly during subsequent pregnancies.
A woman’s lifetime exposure to estrogen is a risk factor for breast cancer; early menarche and late menopause increase risk [16]. Furthermore, exposures to heightened levels of estrogens in utero, such as those experienced by twins or after pharmaceutical treatment with estrogens, such as diethylstilbestrol, also increase breast cancer risk in females exposed in the womb [17–21]. Yet, pregnancy with high levels of circulating estrogens is known to reduce breast cancer risk in adult women [22]; women with an early age of first pregnancy [23, 24] and women who breastfeed for >12 months experience the greatest reductions in breast cancer risk, suggesting that involution involves protective events for the mammary gland [6, 25–27]. It has been hypothesized that pregnancy-associated protection from breast cancer arises as a result of the higher degree of differentiation of mammary tissues induced by pregnancy hormones and the diminished epithelial cell proliferation after involution [23, 28, 29]. Over 200 genes differ in their expression between breast cells collected from nulliparous and parous women, indicative of the long-term, permanent effects that pregnancy has on the mammary gland [30].
Because of the important role for lifelong estrogen exposure in determining risk for mammary cancer, recent studies have evaluated the hypothesis that exposures to estrogenic environmental chemicals could disrupt mammary gland development and ultimately influence mammary cancer risk [31, 32]. Most of these studies have evaluated “organizational” exposures, e.g., those occurring during gestation, which induce changes that persist even when the exposure ends [33–35]. Exposures during adulthood are generally deemed “activational,” as the changes are reversible; when the exposures cease, the effects are expected to as well. Despite these common assumptions, xenoestrogen exposures during pregnancy and lactation can induce permanent, organizational effects. For example, women that were prescribed diethylstilbestrol during pregnancy have an increased risk of breast cancer compared with unexposed women [36]. Xenoestrogen exposures are therefore important to consider, not only during gestation but also in adults as well, as they may contribute to lifelong estrogen exposure.
For many decades, scientists believed that the high levels of sex hormones present during pregnancy meant that xenoestrogens in the environment were not relevant to the health of the mammary gland; this presumption was based on an expectation that “low-level” exposures to “weak” estrogens could not produce effects when the concentrations of endogenous hormones were dramatically higher. Recent work has shown that the mammary gland is, in fact, susceptible to xenoestrogen exposures during pregnancy and lactation. Mammary glands collected from female mice exposed, during pregnancy and lactation, to low doses of bisphenol S, an estrogenic compound, exhibited morphological deficits consistent with early involution [7]. Other studies have similarly demonstrated that adult exposures to xenoestrogens permanently affect the health of the mother. For example, female mice exposed to bisphenol A (BPA) during pregnancy gave birth to male offspring that developed glucose intolerance, heightened insulin resistance, and altered pancreatic islets of Langerhans in adulthood [37]. The mothers that were only exposed to BPA during pregnancy also developed similar effects, months after exposures ended; the dams weighed more and had higher insulin, leptin, triglyceride, and glycerol levels in the blood and greater insulin resistance, 4 months postpregnancy [37].
Oxybenzone (benzophenone-3), a common ingredient in consumer products as a result of its use as an ultraviolet filter [38], is an ERα agonist [39, 40]; it does not appear to be an agonist for ERβ [41]. Several metabolites of oxybenzone are also estrogenic [42]. Oxybenzone is also antiestrogenic and antiandrogenic in some contexts [38]. The main source of human exposure is through sunscreen [43] and other personal care products, including hair spray, cosmetics (often from products with sunscreen agents in them, such as lotions, lipsticks, and shampoo), fragrances, and skin care products; it is also found as a UV protectant in carpets, furniture, and clothing [43–45]. Oxybenzone is absorbed dermally and orally from these products before it is metabolized and excreted in urine [43, 44]. Human exposure to oxybenzone is widespread if not ubiquitous. Data from the US Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey biomonitoring study, revealed detectable levels of oxybenzone in the urine of 100% of pregnant and 98% of nonpregnant women [46]. Oxybenzone had one of the highest concentrations of nonpersistent chemicals measured in urine in pregnant women, increasing concerns about exposures to this population. Importantly, oxybenzone is present in human urine year round, as evidenced by a Danish children’s cohort, where it was measured in urine samples collected during the winter with minimal sun and short days [45]. Finally, several studies have evaluated associations between human exposure to oxybenzone and adverse health outcomes. Prenatal oxybenzone exposure was positively associated with body weight and head circumference in boys but negatively associated with body weight in girls at birth [43, 47].
In this study, we evaluated the effects of oxybenzone on mice exposed during pregnancy and lactation. Because it is an ERα agonist, we hypothesized that exposure to oxybenzone during pregnancy and lactation would disrupt morphology, cell proliferation, gene expression, and hormone receptor expression in the mammary gland and that these effects would persist long after exposures ceased. One prior study—a National Toxicology Program carcinogenesis and toxicology study—examining the effects of oxybenzone on the mammary gland reported that 2 years of exposure to oxybenzone (at doses ranging from 15 to 65 mg/kg/d) decreased the incidence of fibroadenomas in the female F344/N rat, possibly as a result of inhibition of steroid sulfatase (which regulates the formation of estrone and its conversion to estradiol) [48]. Here, we present evidence that the mammary gland is a sensitive target to oxybenzone when exposures occur during pregnancy and lactation, a window of susceptibility that is poorly studied.
1. Methods
A. Animals—Acute Exposure Study
Six- to 8-week-old female Balb/C mice were ovariectomized, given 1 week to recover from surgery and for ovarian hormone levels to drop. Females were then randomly assigned to one of three treatment groups. For 4 days, females were weighed and orally dosed daily by pipet with corn oil, 17β-estradiol dissolved in corn oil or oxybenzone dissolved in corn oil. Doses were 250 µg 17β-estradiol/kg/d and 3000 µg oxybenzone/kg/d. On the day after the final oral treatment, mice were euthanized by CO2 inhalation. The uterus was removed, trimmed of fat, and weighed with an analytical balance. It was then snap frozen for quantitative RT-PCR (qRT-PCR) analysis as described later.
B. Animals—Chronic Study (Pregnancy, Lactation, and Postinvolution)
Six- to 8-week-old female Balb/C mice were mated with males and housed in polysulfone cages with food (LabDiet Chow 5058; LabDiet, St. Louis, MO) and water provided ad libitum. The animals were maintained at the University of Massachusetts Amherst, Amherst Animal Facility, in temperature- and light-controlled conditions (12 hours light, 12 hours dark). All experimental procedures were approved by the University of Massachusetts Institutional Animal Care and Use Committee.
Before mating, females were randomly assigned to one of five treatment groups using statistical software to give a normal distribution of body weight to each treatment group. From pregnancy day 0 until the day before weaning [on lactational day (LD) 21], females were weighed and orally dosed daily by pipet with either corn oil or oxybenzone dissolved in corn oil. Three doses of oxybenzone were used. One group of pregnant female mice received the lowest dose (30 µg/kg/d), which was chosen because it is the tolerable daily intake (TDI) of oxybenzone [49]. The middle dose (212 µg/kg/d) was chosen because it approximates exposures to oxybenzone in pregnant women in the 95th percentile (95P) of exposure. This was calculated by multiplication of the concentrations reported in the 95P of pregnant women in the United States with the average daily urinary output during pregnancy (2.2 L) and division of 70 kg body weight [46]. The highest dose (3000 µg/kg/d) was chosen because it is the toxicological no-observed adverse-effect level (NOAEL) dose for oxybenzone [49, 50] based on developmental and reproductive toxicity assays. Doses ~10 times higher are needed to produce urine concentrations in rodents that replicate human urine concentrations, suggesting that all three doses are environmentally relevant (see [48] and unpublished data). The vehicle group received oil instead of oxybenzone for the same dosing period. A second control group of nulliparous female mice was housed under the same conditions and was treated the same, receiving oil instead of oxybenzone, for the same length of time. The oxybenzone dose (and volume of vehicle, equivalent to 1 µL/g body weight) was adjusted for dam body weight daily. The oxybenzone used was >99% pure.
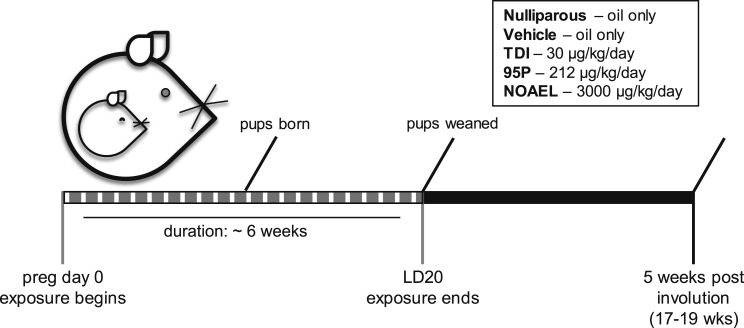
Dams delivered naturally (birth designated LD0) and were allowed to nurse normally. On LD21, the pups were weaned, and the dams were moved to new cages. Dams were group housed (two females per cage) with other animals from the same treatment group for 5 weeks after weaning to allow full involution of the mammary gland. At ~17 to 20 weeks of age (depending on efficiency of pregnancy), the females were euthanized for tissue collection. A schematic summarizing the exposure period and experimental design is shown in Fig. 1.
Figure 1.
Schematic of exposure period and experimental design. Dotted gray bar indicates the exposure period for the adult female mice (pregnancy day 0 through LD20). Animals in the nulliparous treatment group were treated with vehicle for the same length of time as other groups but were not mated. Samples were collected from all parous females, 5 weeks after weaning, and from age-matched nulliparous females. All females were unexposed (to either vehicle or oxybenzone) for the 5-week period.
C. Euthanasia and Tissue Collection
At 5 weeks postweaning, female mice were euthanized via CO2 inhalation. From every dam, the left and right third (pectoral/thoracic) mammary glands were dissected from the skin, spread on a glass slide (Thermo Fisher Scientific, Waltham, MA), and fixed in neutral-buffered formalin (10%; Thermo Fisher Scientific) overnight (standard whole-mount preparation). The right fourth inguinal mammary gland was fixed in neutral-buffered formalin (10%) overnight for paraffin embedding and histology. The left fourth inguinal mammary gland was dissected from the skin, the lymph node was removed, and it was then flash frozen on dry ice and stored at −80°C for qRT-PCR analyses.
D. Whole-Mount Preparation and Analysis
After fixation, whole-mounted mammary glands were processed through an alcohol series, defatted with toluene, stained with carmine alum, dehydrated in an alcohol and xylene series, and preserved in k-pax heat-sealed bags (Thermo Fisher Scientific) with methyl salicylate (Acros Organics, Morris Plains, NJ) [51]. Two digital images of whole-mount mammary glands (one from the left; one from the right third pectoral glands) were obtained using an AxioImager dissection microscope (Carl Zeiss Microscopy, Jena, Germany) at ×30 magnification and a Zeiss high-resolution color camera. To evaluate mammary gland morphology, ZEN software (Carl Zeiss Microscopy) was used to measure ductal density [51]. A 13 × 16 grid (180 crosshairs, 0.3 mm apart) was placed on each image, and the fraction of crosshairs that fell on ducts was quantified and averaged for the two images for each sample.
E. Tissue Processing and Histological Staining
To prepare mammary tissue for paraffin sections, fixed excised tissue was washed in PBS, dehydrated through a series of alcohols, and embedded with paraffin (Leica Biosystems, Richmond, IL) under vacuum. Sections (5 μm) were cut on a Fisher rotary microtome and mounted on positively charged slides (Thermo Fisher Scientific). These sections were used for histological and immunohistochemical analyses.
For histological evaluations, slides were deparaffinized with xylene and a series of alcohols, stained with Harris’ hematoxylin and eosin (H&E; Thermo Fisher Scientific), dehydrated, and mounted with permanent mounting media (Thermo Fisher Scientific). Digital images were collected using a Zeiss Axio Oberserver.Z1 inverted microscope, a ×10 objective, and a high-resolution color camera (Carl Zeiss Microscopy). To quantify the fraction of the mammary gland comprised of epithelium, one entire longitudinal section of mammary gland was imaged. Three images were randomly selected and an identical 10 × 13 grid (108 crosshairs, 100 µm apart) was placed on each image, and the number of crosshairs on epithelial tissue was recorded, as well as the crosshairs that did not fall on mammary tissue at all. The values for the three images were averaged for each sample.
F. Mammary Gland Immunohistochemistry
Expression of four markers was evaluated using standard methods for immunohistochemistry and commercial antibodies, including rabbit anti-ERα (Cat. #06-935; MilliporeSigma, St. Louis, MO [52]); rabbit anti-Ki67 (Cat. #RM-9106-S1; Thermo Fisher Scientific [53]), a marker of proliferation; rabbit anti-PR (Cat. #ab131486; Abcam, Cambridge, MA [54]); and rabbit anti-Wnt5a (Cat. #ab174963; Abcam [55]). See Table 1 for a summary of these antibodies. In brief, sections were deparaffinized, hydrated through a series of alcohols, microwaved in 10 mM citrate buffer (pH 6) for antigen retrieval, and treated with hydrogen peroxide to quench endogenous peroxidases. Nonspecific binding was blocked with 1% milk protein in 5% normal goal serum (Cell Signaling Technology, Danvers, MA). Sections were incubated with primary antibodies (see Table 1 for concentrations) at 4°C for 14 to 16 hours and washed and incubated with secondary antibody (goat anti-rabbit, Cat. #ab64256; Abcam [56]), followed by streptavidin peroxidase complex (Abcam, Cat# ab64269). Diaminobenzidene chromogen (Cat. #ab64238; Abcam) was used to visualize reactions. Sections were counterstained with Harris hematoxylin (Thermo Fisher Scientific). Each immunohistochemical run included a negative control in which the primary antibody was replaced with 5% normal goat serum.
Table 1.
Information About Antibodies
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species; Raised in Polyclonal or Monoclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| ERα | Anti-ERα (C1355) | EMD Millipore, 06-935 | Rabbit; polyclonal | 1:1000 | AB_31035 [52] | |
| Ki67 | Ki67 | Thermo Fisher Scientific, RM-9106-S1 | Rabbit; monoclonal | 1:1000 | AB_149792 [53] | |
| Progesterone receptor | Anti-PR | Abcam, ab131486 | Rabbit; polyclonal | 1:500 | AB_11156044 [54] | |
| Wnt5a | Anti-Wnt5a | Abcam, ab174963 | Rabbit; polyclonal | 1:500 | AB_2725803 [55] | |
| Secondary | Biotinylated goat anti-rabbit IgG | Abcam, ab64256 | Goat; polyclonal | Ready to use (5 µg/mL) | AB_2661852 [56] |
Nonoverlapping images were taken of each sample for ERα, Ki67, and PR at ×40 magnification with a Zeiss Axio Observer.Z1 inverted microscope and ×20 magnification for Wnt5a. Expression of ERα and PR was evaluated by counting at least 500 epithelial cells in two to four separate fields. Expression of Ki67 was evaluated by counting at least 800 epithelial cells in four separate fields. Expression of ERα, Ki67, and PR was expressed as a percent ratio of the total number of epithelial cells evaluated.
Wnt5a expression was assessed qualitatively. For both epithelium and stroma, a representative image was chosen for low, medium, and high expression of Wnt5a and assigned a number (low = 1, medium = 2, and high = 3). Three images of epithelium and three images of stroma were assessed per sample for Wnt5a expression. For each image of mammary tissue incubated with antibodies for Wnt5a, a number was assigned from 0 (no expression, resembling the negative control) to 3 by comparing it with the representative images. The scores for each image were averaged (to get average scores for epithelium and stroma) to characterize Wnt5a expression for each mammary gland.
G. qRT-PCR
Total RNA was extracted from uterine tissue (in the acute exposure study) or mammary glands (in the pregnancy/lactation/involution study) of individual mice using Trizol reagent (Thermo Fisher Scientific) and a BeadBug microtube homogenizer (MilliporeSigma), according to the manufacturers’ instructions. RNA integrity was evaluated using a bioanalyzer, and total RNA was quantified by UV spectrophotometry (Nanodrop 1000; Thermo Fisher Scientific). RNA (1 μg) from each sample was reverse transcribed to cDNA using reverse transcription (Thermo Fisher Scientific). The FastStart Universal SYBR Green Master kit (Roche Diagnostics, Indianapolis, IN) was used for the qRT-PCRs, along with 1 μL cDNA and 300 nM forward and 300 nM reverse primers for each target gene. β-Actin was used as a housekeeping gene. Every sample was run in duplicate for each gene target. The thermal profile was as follows: 10 minutes at 95°C and 40 cycles of 15 seconds at 95°C, 30 seconds at 60°C, and 15 seconds at 72°C; a melting-curve analysis was conducted to identify nonspecific products. Relative quantification was determined using the ΔΔCt method to correct for differences in β-actin [57]. Primer sequences are reported in Table 2.
Table 2.
Information About Primers
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| ERα (Esr1) | TGC AAT GAC TAT GCC TCT GG (782-801) | CTC CGG TTC TTG TCA ATG GT (921-902) |
| ERβ (Esr2) | TGT GTG TGA AGG CCA TGA TT | TCT TCG AAA TCA CCC AGA CC |
| PR (ProgR) total | AAA GGA TCC GCA GGT TCT C | GTT CCA TCT TCC AGC GGA TA |
| Wnt5a | GAA TCC CAT TTG CAA CCC CTC ACC | GCT CCT CGT GTA CAT TTT CTG CCC |
| β-Actin | CAC ACC CGC CAC CAG TTC GC (89-108) | TTG CAC ATG CCG GAG CCG TT (162-143) |
Abbreviation: ProgR, progesterone receptor.
H. Statistical Analysis
Mouse experiments were run in two separate batches. All treatment groups were included in both batches. After running two-way ANOVA to determine if batches significantly influenced outcomes, the batches were combined. For some outcomes, only dams from batch 1 were evaluated (e.g., qRT-PCR and immunohistochemistry). Additional outcomes evaluated in batch 2 animals will be presented in future manuscripts.
Morphological, histological, and immunohistochemical analyses were conducted by observers blind to the treatment groups. Data were analyzed using SPSS Version 24 (IBM, Inc., Armonk, NY). Continuous variable data were analyzed using one-way ANOVA General Linear Model analyses with treatment as the independent variable, followed by Fisher least significant difference (LSD) post hoc tests. Data were considered statistically significant at P < 0.05. Graphs illustrate means ± SE unless otherwise stated. For whole-mount evaluations, sample sizes were the following: vehicle (n = 14), nulliparous (n = 12), 30 µg oxybenzone/kg/d (TDI; n = 13), 212 μg oxybenzone/kg/d (95P; n = 14), and 3000 µg oxybenzone/kg/d (NOAEL; n = 14). For histological, immunohistochemical, and qRT-PCR analyses, sample sizes were the following: vehicle (n = 7), nulliparous (n = 5), 30 µg oxybenzone/kg/d (TDI; n = 6), 212 μg oxybenzone/kg/d (95P; n = 7), and 3000 µg oxybenzone/kg/d (NOAEL; n = 7). For acute exposure studies, sample sizes were the following: vehicle (n = 6), 3000 µg oxybenzone/kg/d (n = 6), and 250 µg 17β-estradiol/kg/d (n = 4).
2. Results
A. Acute Exposures to Oxybenzone
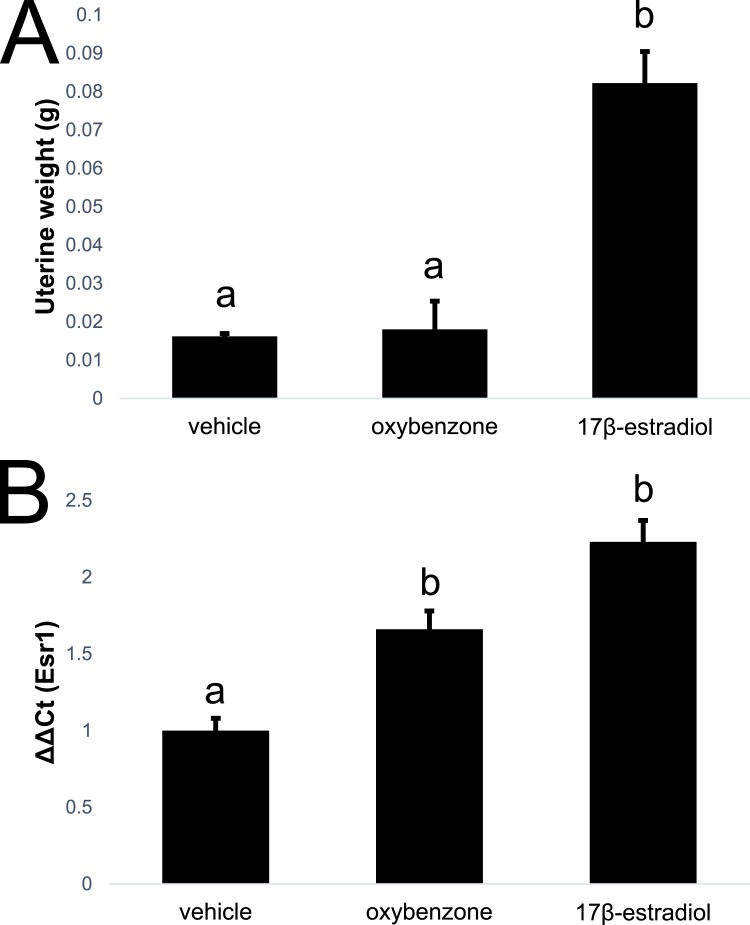
Prior studies have demonstrated that oxybenzone and its metabolites are estrogenic, antiestrogenic, and antiandrogenic in different in vitro contexts [38]. Additional studies in immature rats have shown that high doses of oxybenzone (>1000 mg/kg/d) are needed to alter uterine weight, a classical read-out of estrogenic activity [58]. Effects of acute (4-day) exposures to oxybenzone (3000 µg/kg/d, the high dose tested in the remainder of this study) were examined in ovariectomized adult females. Unlike 17β-estradiol, oxybenzone did not alter the weight of the uterus (Fig. 2A). It did, however, produce modest but substantial effects on expression of ERα (Esr1) in the uterus, consistent with an estrogenic response (Fig. 2B). In contrast to acute exposures to 17β-estradiol, acute oxybenzone exposures did not alter morphology or proliferation in the mammary gland (data not shown).
Figure 2.
Acute effects of oxybenzone on ER-mediated endpoints in vivo. (A) Unlike acute exposure to 17β-estradiol, acute exposure to 3000 µg oxybenzone/kg/d did not induce an increase in uterine weight. (B) Acute exposure to 17β-estradiol or oxybenzone increased expression of ERα (Esr1) in the uterus. Different letters indicate significant differences among groups, P < 0.05, Fisher LSD post hoc after significant ANOVA.
B. Pregnancy Outcomes and Body Weight
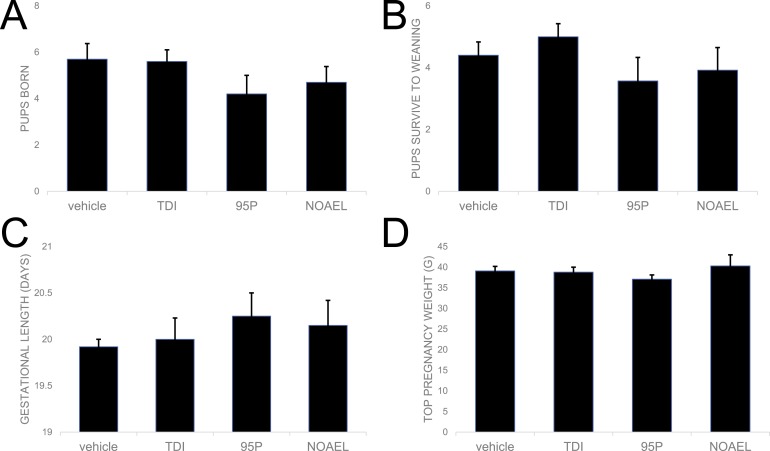
Pregnancy outcomes were evaluated in all parous females from the four treatment groups (vehicle, oxybenzone-TDI, oxybenzone-95P, and oxybenzone-NOAEL). There were no substantial effects of treatment on the number of pups that were born (present on the day of parturition) or the number of pups that survived to weaning (Fig. 3A and 3B). Oxybenzone exposure induced modest changes to the length of gestation, measured as the time between when a sperm plug was observed and when the pups were born, although these differences were not statistically significant (Fig. 3C).
Figure 3.
Oxybenzone has only minor effects on pup weight and survival and no effects on dam weight during pregnancy. (A) Oxybenzone produced no substantial differences in the number of pups born or (B) the number of pups surviving to weaning. (C) No substantial effects of oxybenzone on gestational length were observed. (D) By design, no differences in dam body weight were observed at the start of the experiment (data not shown) or at the heaviest point during pregnancy.
Dams had similar body weights in all treatment groups at the start of the study by design, as body weight was used to distribute females equally between treatment groups. Body weights were also indistinguishable between groups just before parturition (Fig. 3D).
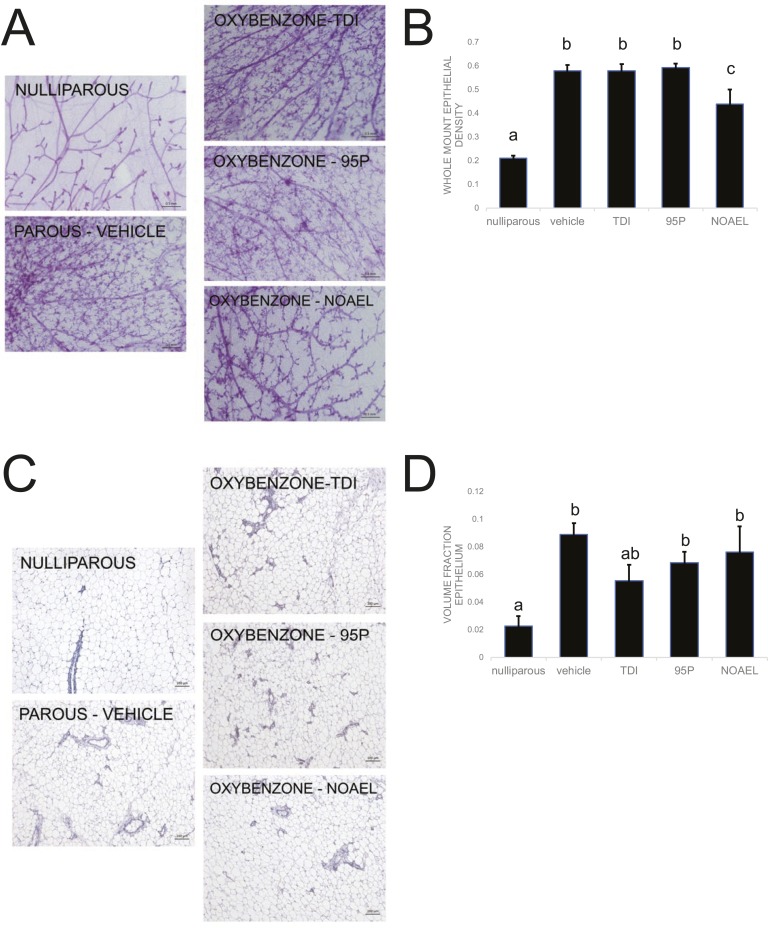
C. Oxybenzone Alters Mammary Gland Morphology and Histomorphology
To determine whether exposure to oxybenzone during pregnancy and lactation could affect the long-term morphology of the mammary gland, we examined whole-mount mammary glands collected from female mice, 5 weeks after weaning, once mammary gland involution was complete (Fig. 4A). With the use of unbiased stereology methods, we calculated the density of epithelial tissue in each mammary gland. As expected, nulliparous females had significantly lower ductal density compared with the parous vehicle-exposed females (P < 0.001, Fisher LSD; Fig. 4B). The nulliparous females were also significantly different from all three oxybenzone treatment groups (P < 0.001, Fisher LSD; Fig. 4B). Neither the oxybenzone-TDI nor the oxybenzone-95P groups were statistically different from the vehicle-exposed group. However, the oxybenzone-NOAEL-treated females had mammary glands with significantly lower ductal densities than the vehicle group (P < 0.001, Fisher LSD), consistent with an intermediate phenotype between nulliparous and parous mammary glands (Fig. 4B).
Figure 4.
Oxybenzone alters mammary gland morphology and histomorphology. (A) Representative whole-mount mammary gland images collected from females in each treatment group. Original scale bars, 0.5 mm. (B) Quantification of ductal density reveals an intermediate phenotype in the oxybenzone-NOAEL treatment group. (C) Representative H&E sections of mammary glands collected from females of each treatment group. Original scale bars, 100 µm. (D) Quantification of volume fraction epithelium reveals an intermediate phenotype in the TDI-treated group. In all panels, different letters indicate significant differences among groups, P < 0.05, Fisher LSD post hoc after significant ANOVA.
We further investigated this finding by observing whether oxybenzone exposure could affect the histology of the mammary gland. We examined paraffin sections after H&E staining to quantify the volume fraction of epithelium in a single longitudinal section through the gland (Fig. 4C). Nulliparous mammary glands had significantly less epithelium compared with the vehicle parous mammary glands, as expected (P = 0.001, Fisher LSD; Fig. 4D). The nulliparous glands also had significantly less epithelium compared with the oxybenzone-95P and oxybenzone-NOAEL groups (P < 0.05, Fisher LSD; Fig. 4D). The oxybenzone-TDI mammary glands were not statistically different from either the nulliparous or the vehicle parous mammary glands, consistent with an intermediate phenotype (Fig. 4D).
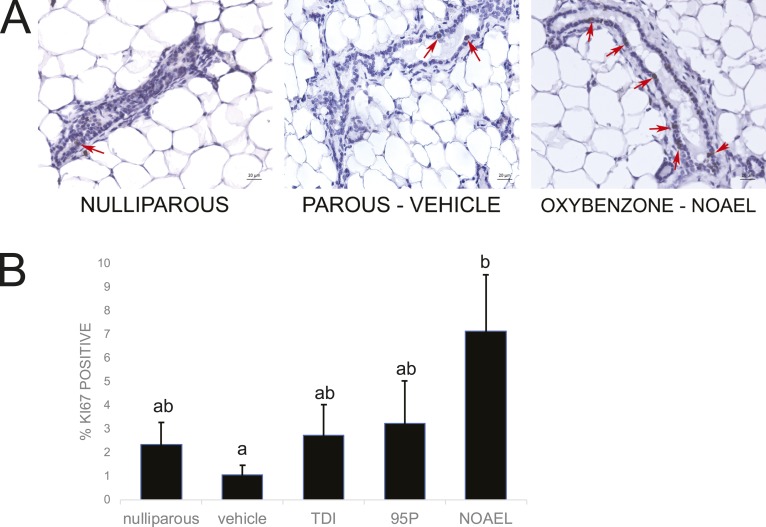
D. Oxybenzone Alters Proliferation in Mammary Epithelium
Proliferation is high in the mammary gland epithelium during pregnancy and then low during both lactation and involution when the gland is either producing milk or when the epithelial cells are rapidly dying [2]. To evaluate whether maternal exposures to oxybenzone affect proliferation in the mammary gland after involution, we quantified expression of Ki67, a marker of proliferation, in the mammary epithelium (Fig. 5A). Although parity decreased Ki67 expression (parous vehicle-exposed vs nulliparous females), this difference was not statistically significant (Fig. 5B). Interestingly, there was a dose-dependent increase in Ki67 expression in the oxybenzone-treated females, with a statistically significant increase in the oxybenzone-NOAEL-treated females compared with the vehicle parous females (P = 0.01, Fisher LSD; Fig. 5A and 5B).
Figure 5.
Oxybenzone alters mammary gland cell proliferation. (A) Immunohistochemistry for Ki67, a marker of proliferation. Original scale bars, 20 µm; red arrows indicate positive cells. (B) Quantification of Ki67 expression in the mammary gland reveals a monotonic dose-dependent response that becomes significant in the oxybenzone-NOAEL treatment group. Different letters indicate significant differences among groups, P < 0.05, Fisher LSD post hoc after significant ANOVA.
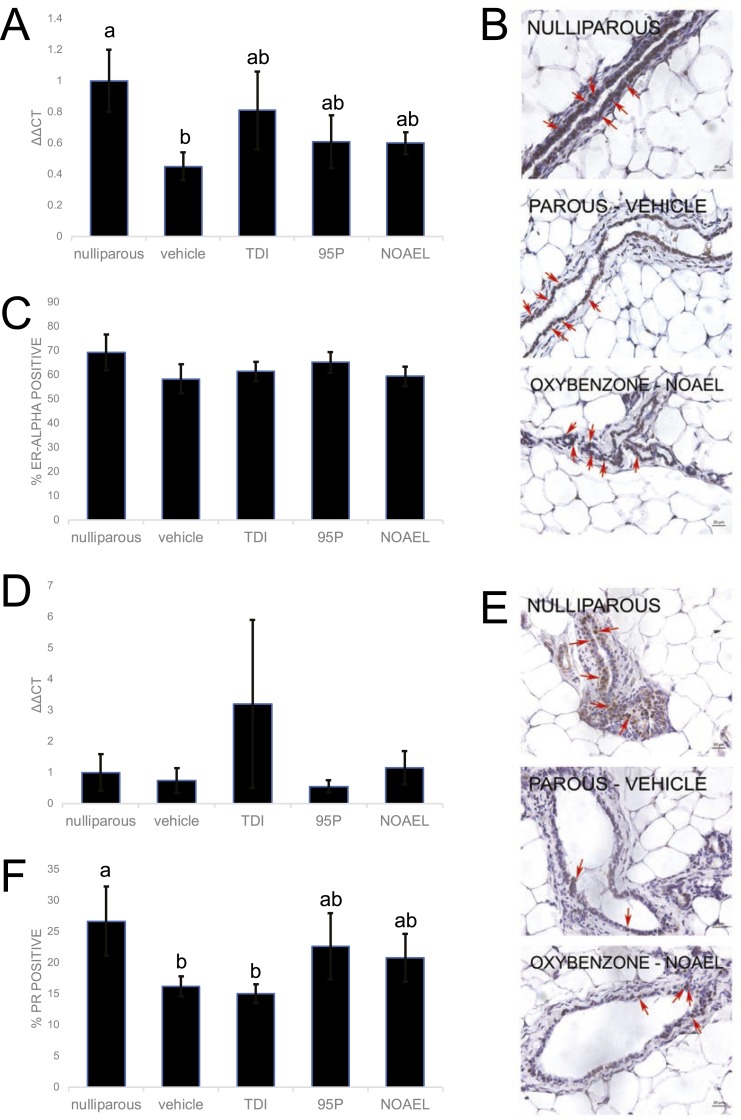
E. Oxybenzone Alters Expression of Esr1 in Mammary Epithelium
ERα is highly expressed in mammary epithelium during adulthood; it diminishes during pregnancy and then returns to higher expression levels during lactation [4]. We first evaluated whether maternal exposure to oxybenzone alters expression of Esr1, the gene encoding ERα, in the mammary gland after involution. qRT-PCR analyses revealed significant differences in Esr1 expression as a result of parity; expression of Esr1 was significantly diminished in mammary epithelium from parous vehicle-exposed females compared with nulliparous females (P < 0.05, Fisher LSD; Fig. 6A). Yet, expression of Esr1 in all three oxybenzone-treated groups was indistinguishable from both the nulliparous and the parous vehicle-exposed animals, consistent with an intermediate phenotype (Fig. 6A).
Figure 6.
Oxybenzone alters Esr1 and PR in the mammary gland. (A) Expression of Esr1, the gene encoding ERα, in the mammary gland reveals an intermediate phenotype for all three treated groups. (B) Mammary gland sample after an immunohistochemistry for ERα expression. Original scale bars, 20 µm; red arrows indicate positive cells. (C) Quantification of ERα expression in mammary gland samples reveals no effects of parity or oxybenzone treatment. (D) mRNA expression of PR (total) in the mammary gland reveals no differences among treatment groups. (E) Immunohistochemistry for PR. Original scale bars, 20 µm; red arrows indicate positive cells. (F) Quantification of PR expression in mammary tissue reveals an intermediate phenotype in the oxybenzone-95P and oxybenzone-NOAEL treatment groups. In all panels, different letters indicate significant differences among groups, P < 0.05, Fisher LSD post hoc after significant ANOVA.
We next evaluated expression of ERα protein using immunohistochemistry (Fig. 6B). There was no effect of parity on the percentage of epithelial cells expressing ERα (Fig. 6C). Furthermore, the percentage of epithelial cells expressing ERα was not affected in any oxybenzone-treated group (Fig. 6C).
F. Oxybenzone Alters Expression of Progesterone Receptor in Mammary Epithelium
PR expression in the mammary gland is mediated by ERα [3], and thus, PR is often seen as a measure of ERα functionality [59]. In the adult mammary gland, PR expression is scattered throughout the epithelium; by late pregnancy, PR expression drops [60, 61]. It is absent in the mammary epithelium during lactation, and expression returns during involution [2, 6, 62]. Because we observed that parity altered expression of Esr1, and oxybenzone produced an intermediate phenotype, we next evaluated the effects of these factors on expression of PR. PR mRNA was not affected by either oxybenzone or parity (Fig. 6D). However, analysis of PR protein expression revealed an effect of both parity and oxybenzone on PR-positive epithelial cells (Fig. 6E). Similar to what was seen with Esr1 expression, parity significantly decreased the fraction of PR-positive cells compared with the nulliparous glands (P < 0.05, Fisher LSD; Fig. 6F). This decreased percentage of PR-positive cells was also observed for the oxybenzone-TDI mammary glands, (P < 0.05, Fisher LSD). Conversely, in the oxybenzone-95P- and oxybenzone-NOAEL-treated females, the diminished expression of PR associated with parity was no longer observed, consistent with an intermediate phenotype.
G. Oxybenzone Does Not Affect ERβ-Mediated Gene Expression
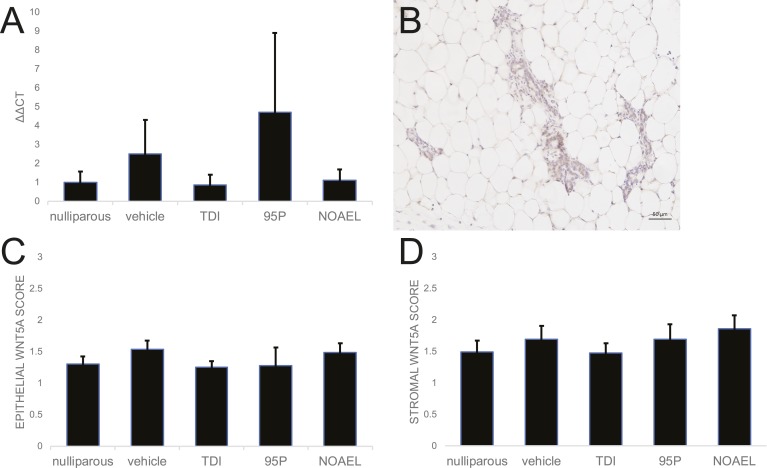
The role of ERβ in the mammary gland at different stages of development has been debated, largely as a result of difficulty identifying antibodies that are specific to this isoform [63]. Although some studies have suggested that ERβ is highly expressed in mammary epithelium in adulthood, pregnancy, and lactation [64], others fail to detect Esr2, the gene encoding ERβ, in this tissue [65]. With the use of qRT-PCR, no Ct values were observed for Esr2 in any mammary sample; Esr2 was detected in ovary, suggesting that ERβ was not expressed in any mammary glands, regardless of parity (data not shown). To determine if oxybenzone alters ERβ-mediated gene expression, we evaluated the expression of Wnt5a, a gene that is upregulated when ERβ is expressed in mammary cells [66]. No substantial differences were observed between any groups for Wnt5a gene expression (Fig. 7A). Likewise, qualitative evaluations of Wnt5a protein expression in both the stromal and epithelial compartments of the mammary gland did not reveal any differences in expression based on parity or oxybenzone exposure (Fig. 7B–7D). Expression of other ERβ-mediated genes, including CCAAT/enhancer-binding protein delta and TGF-β2 [66], was also not affected by oxybenzone exposures (data not shown).
Figure 7.
Oxybenzone does not alter Wnt5a gene or protein expression. (A) Expression of Wnt5a, a gene downstream of ERβ, is unaffected by parity or oxybenzone treatment. (Esr2, the gene encoding ERβ, produced no CT values using qRT-PCR.) (B) Immunohistochemistry for Wnt5a, a gene downstream of ERβ. Original scale bar, 50 µm. (C) Quantification of Wnt5a expression in the epithelium of the mammary gland using qualitative scores from 0 to 3 for relative expression reveals no significant differences among treatment groups. (D) Quantification of Wnt5a expression in the mammary stroma using a qualitative scale reveals no substantial differences associated with parity or oxybenzone exposure.
3. Discussion
In this study, we examined mammary glands from female mice exposed to three doses of oxybenzone during pregnancy and lactation and observed long-lasting effects of exposure on the morphology, histology, and molecular profiles of the mammary glands. We compared the oxybenzone-exposed animals with parous vehicle-exposed animals, as well as nulliparous females, to evaluate whether oxybenzone exposure interfered with the reorganization of the mammary gland that is characteristic of pregnancy, lactation, and involution. As expected, ductal density was significantly increased in the parous vehicle-exposed females compared with the nulliparous females. We found that the highest dose of oxybenzone permanently altered the morphology of the mammary gland, as measured in the whole mount, indicative of changes in the three-dimensional organization of this tissue. The substantial reduction in ductal density in these females produced an intermediate phenotype resembling a mix between the nulliparous and vehicle mammary glands (Fig. 4A). Additional studies are needed to determine if this intermediate phenotype is a result of oxybenzone-induced reorganization of the mammary gland in pregnancy (e.g., limited proliferation), in lactation (e.g., incomplete differentiation of lobuloalveolar structures), or during the processes of involution (e.g., excessive apoptosis and clearing of epithelial structures).
We also observed changes to the histomorphology of the mammary glands; similar to ductal density evaluated in whole mounts, the amount of epithelium quantified in H&E sections increased with parity in vehicle-exposed animals. In these two-dimensional views of the mammary gland, females from the oxybenzone-TDI group were not significantly different from either the nulliparous or the parous vehicle-exposed females, again consistent with an intermediate phenotype (Fig. 4C). Collectively, these results suggest that the effects of exposures to this compound during pregnancy and lactation can have long-lasting effects on the morphology of the mammary gland. Importantly, these results do not indicate whether the mammary gland is the direct target of oxybenzone exposure or if the effects that we have observed are a result of long-term alterations to the hypothalamic-pituitary-ovarian axis.
Full-term pregnancies in early adulthood can decrease breast cancer risk by up to 50% [25, 67–69]. In nulliparous animals given estrogen and progesterone, this same protection is also induced, suggesting that the protection from pregnancy is hormonally controlled [67]. It is suspected that this protection comes from the activity of tumor-suppressor genes, as well as the interplay between ERα and ERβ. ERα, when bound to estrogen, can activate transcription of downstream targets, inducing growth and differentiation of epithelium. In contrast, ERβ is considered to have inhibitory behavior on proliferation, increasing genomic surveillance and increasing the expression of tumor suppressor pathways, such as p53 [66, 67, 70–75]. In fact, parous animals have a stronger response to DNA damage and more p53 tumor suppressor action to enhance genomic surveillance [76–78]. This suggests that a chemical that binds ERα, such as oxybenzone, may induce different, and perhaps even contradictory, effects compared with a chemical that binds to ERβ. Pregnancy is also protective against carcinogen-induced mammary cancer [76]. One study in mice demonstrated that pregnancy induced downregulation of growth factor genes and upregulation of growth-inhibitory molecules, such as TGF-β3 [76]. Pregnancy also induces more differentiation of the mammary gland and persistent changes in hematopoietic cells in the mammary gland [76], leading some researchers to suggest that differentiation of mammary tissues may play a role in the protection from breast cancer [22]. Even when controlling for age of first pregnancy, a protective effect of lactation on breast cancer has been observed in women [79], with increased protection associated with longer duration of lactation [80]. Future studies will examine if exposures to oxybenzone during pregnancy and lactation alter cancer risk in mice. If diminished differentiation of mammary epithelium associated with some doses of oxybenzone suggest a loss of some of the protective effect of pregnancy, then it is also possible that these females will be more sensitive to chemical-induced carcinogenesis. Additional studies are needed to evaluate this hypothesis.
In parous rats and mice, ERα-positive epithelial cells are reduced in the mammary gland, and less proliferation is observed [81–84]. Here, we observed no effects of either parity or oxybenzone exposure on the fraction of ERα-positive cells in mammary epithelium, although parity did decrease the expression of Esr1 expression in the mammary gland (Fig. 6). Proliferation was also affected by oxybenzone exposure, with a substantial increase in the highest dose group (Fig. 5). Because oxybenzone is an ERα agonist, and ERα promotes mammary epithelial proliferation, it is possible that this finding is a result of downstream ERα action, although it is surprising that this effect persisted for 5 weeks after exposures ceased. This, again, might indicate a direct effect of oxybenzone on the mammary gland (e.g., by permanently changing the number of parity-induced epithelial cells [14]), or it might indicate an indirect effect on the mammary gland (e.g., by altering the release of gonadotropin hormones and disrupting the hypothalamic-pituitary-ovarian axis). Additional studies are needed to evaluate these possibilities.
Finally, we examined gene and protein expression downstream from ERα and ERβ. Parity decreased the number of epithelial cells expressing PR, and an intermediate phenotype of expression was observed in the two higher doses of oxybenzone (Fig. 6E); no changes were observed for PR gene expression. Because PR is downstream of ERα [3], it is possible that this effect may have been induced by alterations in ERα action that occurred during exposure. We also evaluated the effects of oxybenzone exposure on expression of Wnt5a, which is downstream of ERβ. Although we were not able to detect Esr2 in mammary tissue, ERβ agonists alter parity-associated mammary gland phenotypes, consistent with indirect effects of ERβ-mediated pathways on mammary gland health (unpublished data). Qualitative expression of Wnt5a protein, as well as Wnt5a gene expression, was unaffected by either parity or oxybenzone exposure. These results are not particularly surprising considering oxybenzone’s actions as an ERα agonist.
It is important to note that the doses chosen for this study are not high doses. In all three oxybenzone-treated groups, no overt signs of toxicity were observed (e.g., no changes to the number of pups, pup survival, or dam weight gain; Fig. 2). All three doses are at or below the toxicological NOAEL dose, and reproductive toxicity is reported at much higher doses (25 to 45 mg/kg) [50]. We selected doses to replicate exposures at which no adverse effects were previously reported (the NOAEL dose), a dose that reflects exposures in pregnant women (the 95P dose), and the TDI dose. Other groups have found that applied doses, ~10-fold higher than the highest dose administered here, are needed to replicate human urinary concentrations (see [48] and unpublished data). Thus, the results that we observed are concerning, as they are occurring at doses that are relevant for regulation of this compound and/or for exposed humans [85]. Importantly, acute exposures to the NOAEL dose did not induce a uterotrophic response, although they did alter expression of Esr1 in the uterus (Fig. 2). These results are consistent with prior studies indicating that oxybenzone is estrogenic but with lower potency than other known estrogenic endocrine disruptors [58]. However, it cannot be discounted that the effects of oxybenzone on the mammary gland when exposures occur during pregnancy and lactation could be a result of mechanisms distinct from ERα. Additional studies are needed to evaluate this possibility. Another potential limitation of the study is the route of exposure; humans are largely exposed to oxybenzone dermally via personal care products. Our animals were exposed orally, which may allow the chemical to be metabolized by the liver before entering circulation, unlike uptake via dermal exposures, where oxybenzone can enter circulation and reach organs before metabolism [45]; thus, our exposures may underestimate those experienced by pregnant women.
Studies by Alonso-Magdalena et al. [37] showed that low-dose BPA exposures induced metabolic disruptions in male offspring exposed during gestation but also in their mothers exposed during pregnancy; these effects manifested long after exposures ended. This study challenged the long-held belief that low-dose xenoestrogens would not affect the health of the mother as a result of high concentrations of circulating estrogens. Our study similarly suggests that oxybenzone exposure during pregnancy and lactation can affect the maternal mammary gland after involution, and these effects can be permanent, consistent with the organizational role of hormones during critical windows of development. Collectively, these results indicate that pregnancy and lactation should be considered a vulnerable period of susceptibility to xenoestrogens, not just for the developing fetus and neonate but also for her mother as well.
Acknowledgments
The authors thank the other members of the Vandenberg and Jerry laboratories who provided helpful feedback and assistance with this project. We specifically thank Durga Kolla, Mary Morcos, Danny McSweeney, Aastha Pokharel, Amy Roberts, Lynn Chuong, Mary Hagen, and Libby Daniele for assistance with animal handling, chemical exposures, and necropsies.
Financial Support: This work was supported by funding from the University of Massachusetts Commonwealth Honors College Grant (to C.D.L.), Endocrine Society Summer Research Fellowship (to C.D.L.), and National Institutes of Health Grant U01ES026140 (to D.J.J., K.A.D., and L.N.V.). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Endocrine Society, or University of Massachusetts.
Disclosure Summary: L.N.V. has received travel reimbursement from universities, governments, nongovernmental organizations, and industry to speak about endocrine-disrupting chemicals. C.D.L., R.B., K.A.D., and D.J.J. have nothing to disclose.
Glossary
Abbreviations:
- 95P
95th percentile
- BPA
bisphenol A
- ER
estrogen receptor
- H&E
hematoxylin and eosin
- LD
lactational day
- LSD
least significant difference
- NOAEL
no-observed adverse-effect level
- PR
progesterone receptor
- qRT-PCR
quantitative RT-PCR
- Stat
signal transducer and activator of transcription
- TDI
tolerable daily intake
References and Notes
- 1. Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1(4):467–475. [DOI] [PubMed] [Google Scholar]
- 2. Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7(1):49–66. [DOI] [PubMed] [Google Scholar]
- 3. Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci USA. 2007;104(37):14718–14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gustafsson JA, Warner M. Estrogen receptor beta in the breast: role in estrogen responsiveness and development of breast cancer. J Steroid Biochem Mol Biol. 2000;74(5):245–248. [DOI] [PubMed] [Google Scholar]
- 5. Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95(9):5076–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1(4):533–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LaPlante CD, Catanese MC, Bansal R, Vandenberg LN, Bisphenol S. Bisphenol S alters the lactating mammary gland and nursing behaviors in mice exposed during pregnancy and lactation. Endocrinology. 2017;158(10):3448–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;80(2):137–148. [DOI] [PubMed] [Google Scholar]
- 9. Traurig HH. A radioautographic study of cell proliferation in the mammary gland of the pregnant mouse. Anat Rec. 1967;159(2):239–247. [DOI] [PubMed] [Google Scholar]
- 10. Feng Z, Marti A, Jehn B, Altermatt HJ, Chicaiza G, Jaggi R. Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J Cell Biol. 1995;131(4):1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sutherland KD, Lindeman GJ, Visvader JE. The molecular culprits underlying precocious mammary gland involution. J Mammary Gland Biol Neoplasia. 2007;12(1):15–23. [DOI] [PubMed] [Google Scholar]
- 12. Meier-Abt F, Bentires-Alj M. How pregnancy at early age protects against breast cancer. Trends Mol Med. 2014;20(3):143–153. [DOI] [PubMed] [Google Scholar]
- 13. Baxter FO, Neoh K, Tevendale MC. The beginning of the end: death signaling in early involution. J Mammary Gland Biol Neoplasia. 2007;12(1):3–13. [DOI] [PubMed] [Google Scholar]
- 14. Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129(6):1377–1386. [DOI] [PubMed] [Google Scholar]
- 15. Wagner KU, Smith GH. Pregnancy and stem cell behavior. J Mammary Gland Biol Neoplasia. 2005;10(1):25–36. [DOI] [PubMed] [Google Scholar]
- 16. Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. [DOI] [PubMed] [Google Scholar]
- 17. Ekbom A, Trichopoulos D, Adami HO, Hsieh CC, Lan SJ. Evidence of prenatal influences on breast cancer risk. Lancet. 1992;340(8826):1015–1018. [DOI] [PubMed] [Google Scholar]
- 18. Trichopoulos D. Is breast cancer initiated in utero? Epidemiology. 1990;1(2):95–96. [PubMed] [Google Scholar]
- 19. Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, Karlan BY, Kaufman R, Noller KL, Palmer JR, Robboy SJ, Saal RC, Strohsnitter W, Titus-Ernstoff L, Troisi R. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365(14):1304–1314. [DOI] [PubMed] [Google Scholar]
- 20. Palmer JR, Wise LA, Hatch EE, Troisi R, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Herbst AL, Noller KL, Hyer M, Hoover RN. Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1509–1514. [DOI] [PubMed] [Google Scholar]
- 21. Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C. Does breast cancer start in the womb? Basic Clin Pharmacol Toxicol. 2008;102(2):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo IH, Russo J. Primary prevention of breast cancer by hormone-induced differentiation. Recent Results Cancer Res. 2007;174:111–130. [DOI] [PubMed] [Google Scholar]
- 23. Dall GV, Britt KL. Estrogen effects on the mammary gland in early and late life and breast cancer risk. Front Oncol. 2017;7:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sivaraman L, Medina D. Hormone-induced protection against breast cancer. J Mammary Gland Biol Neoplasia. 2002;7(1):77–92. [DOI] [PubMed] [Google Scholar]
- 25. MacMahon B, Cole P, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Valaoras VG, Yuasa S. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43(2):209–221. [PMC free article] [PubMed] [Google Scholar]
- 26. Faupel-Badger JM, Arcaro KF, Balkam JJ, Eliassen AH, Hassiotou F, Lebrilla CB, Michels KB, Palmer JR, Schedin P, Stuebe AM, Watson CJ, Sherman ME. Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst. 2013;105(3):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, Bahl R, Martines J. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):96–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Russo J, Russo IH. Breast development, hormones and cancer. Adv Exp Med Biol. 2008;630:52–56. [DOI] [PubMed] [Google Scholar]
- 29. Medina D, Sivaraman L, Hilsenbeck SG, Conneely O, Ginger M, Rosen J, Omalle BW. Mechanisms of hormonal prevention of breast cancer. Ann N Y Acad Sci. 2001;952(1):23–35. [DOI] [PubMed] [Google Scholar]
- 30. Belitskaya-Lévy I, Zeleniuch-Jacquotte A, Russo J, Russo IH, Bordás P, Ahman J, Afanasyeva Y, Johansson R, Lenner P, Li X, de Cicco RL, Peri S, Ross E, Russo PA, Santucci-Pereira J, Sheriff FS, Slifker M, Hallmans G, Toniolo P, Arslan AA. Characterization of a genomic signature of pregnancy identified in the breast. Cancer Prev Res (Phila). 2011;4(9):1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soto AM, Brisken C, Schaeberle C, Sonnenschein C. Does cancer start in the womb? Altered mammary gland development and predisposition to breast cancer due to in utero exposure to endocrine disruptors. J Mammary Gland Biol Neoplasia. 2013;18(2):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwarzman MR, Ackerman JM, Dairkee SH, Fenton SE, Johnson D, Navarro KM, Osborne G, Rudel RA, Solomon GM, Zeise L, Janssen S. Screening for chemical contributions to breast cancer risk: a case study for chemical safety evaluation. Environ Health Perspect. 2015;123(12):1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24(2):199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Macon MB, Fenton SE. Endocrine disruptors and the breast: early life effects and later life disease. J Mammary Gland Biol Neoplasia. 2013;18(1):43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rudel RA, Fenton SE, Ackerman JM, Euling SY, Makris SL. Environmental exposures and mammary gland development: state of the science, public health implications, and research recommendations. Environ Health Perspect. 2011;119(8):1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hilakivi-Clarke L. Maternal exposure to diethylstilbestrol during pregnancy and increased breast cancer risk in daughters. Breast Cancer Res. 2014;16(2):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim S, Choi K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: a mini-review. Environ Int. 2014;70:143–157. [DOI] [PubMed] [Google Scholar]
- 39. Schlumpf M, Schmid P, Durrer S, Conscience M, Maerkel K, Henseler M, Gruetter M, Herzog I, Reolon S, Ceccatelli R, Faass O, Stutz E, Jarry H, Wuttke W, Lichtensteiger W. Endocrine activity and developmental toxicity of cosmetic UV filters--an update. Toxicology. 2004;205(1-2):113–122. [DOI] [PubMed] [Google Scholar]
- 40. Suzuki T, Kitamura S, Khota R, Sugihara K, Fujimoto N, Ohta S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol Appl Pharmacol. 2005;203(1):9–17. [DOI] [PubMed] [Google Scholar]
- 41. Molina-Molina JM, Escande A, Pillon A, Gomez E, Pakdel F, Cavaillès V, Olea N, Aït-Aïssa S, Balaguer P. Profiling of benzophenone derivatives using fish and human estrogen receptor-specific in vitro bioassays. Toxicol Appl Pharmacol. 2008;232(3):384–395. [DOI] [PubMed] [Google Scholar]
- 42. Wang L, Kannan K. Characteristic profiles of benzonphenone-3 and its derivatives in urine of children and adults from the United States and China. Environ Sci Technol. 2013;47(21):12532–12538. [DOI] [PubMed] [Google Scholar]
- 43. Han C, Lim YH, Hong YC. Ten-year trends in urinary concentrations of triclosan and benzophenone-3 in the general U.S. population from 2003 to 2012. Environ Pollut. 2016;208(Pt B):803–810. [DOI] [PubMed] [Google Scholar]
- 44. Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003--2004. Environ Health Perspect. 2008;116(7):893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krause M, Klit A, Blomberg Jensen M, Søeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewiecki KT. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35(3):424–436. [DOI] [PubMed] [Google Scholar]
- 46. Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119(6):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, Silva MJ, Brambilla C, Pin I, Charles MA, Cordier S, Slama R. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120(3):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toxicology and carcinogenesis studies of benzophenone (CAS No. 119-61-9) in F344/N rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser. 2006;(533):1–264. [PubMed]
- 49. Careghini A, Mastorgio AF, Saponaro S, Sezenna E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res Int. 2015;22(8):5711–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anadón A, Bell D, Binderup ML, Bursch W, Castle L, Crebelli R, Engel KH, Franz R, Gontard N, Haertlé T, Husøy T, Jany KD, Leclercq C, Lhuguenot JC, Mennes W, Milana MR, Pfaff K, Svensson K, Toldrá F, Waring R, Wölfle D. Toxicological evaluation of benzophenone. EFSA J. 2009;7(6):1104. [Google Scholar]
- 51. Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, Soto AM. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26(3-4):210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.RRID:AB_31035.
- 53.RRID:AB_149792.
- 54.RRID:AB_11156044.
- 55.RRID:AB_2725803.
- 56.RRID:AB_2661852.
- 57. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 58. Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109(3):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cho H, Aronica SM, Katzenellenbogen BS. Regulation of progesterone receptor gene expression in MCF-7 breast cancer cells: a comparison of the effects of cyclic adenosine 3′,5′-monophosphate, estradiol, insulin-like growth factor-I, and serum factors. Endocrinology. 1994;134(2):658–664. [DOI] [PubMed] [Google Scholar]
- 60. Kariagina A, Aupperlee MD, Haslam SZ. Progesterone receptor isoform functions in normal breast development and breast cancer. Crit Rev Eukaryot Gene Expr. 2008;18(1):11–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haslam SZ, Shyamala G. Progesterone receptors in normal mammary glands of mice: characterization and relationship to development. Endocrinology. 1979;105(3):786–795. [DOI] [PubMed] [Google Scholar]
- 62. LaPlante CD, Vandenberg LN. Data describing lack of effects of 17α-ethinyl estradiol on mammary gland morphology in female mice exposed during pregnancy and lactation. Data Brief. 2017;14:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nelson AW, Groen AJ, Miller JL, Warren AY, Holmes KA, Tarulli GA, Tilley WD, Katzenellenbogen BS, Hawse JR, Gnanapragasam VJ, Carroll JS. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity [published correcton appears in Mol Cell Endocrinol 2017;443(5):175]. Mol Cell Endocrinol. 2017;440:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saji S, Jensen EV, Nilsson S, Rylander T, Warner M, Gustafsson JA. Estrogen receptors alpha and beta in the rodent mammary gland. Proc Natl Acad Sci USA. 2000;97(1):337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138(11):4613–4621. [DOI] [PubMed] [Google Scholar]
- 66. Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147(10):4831–4842. [DOI] [PubMed] [Google Scholar]
- 67. Jerry DJ, Dunphy KA, Hagen MJ. Estrogens, regulation of p53 and breast cancer risk: a balancing act. Cell Mol Life Sci. 2010;67(7):1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rosner B, Colditz GA, Willett WC. Reproductive risk factors in a prospective study of breast cancer: the Nurses’ Health Study. Am J Epidemiol. 1994;139(8):819–835. [DOI] [PubMed] [Google Scholar]
- 70. Lewandowski SA, Thiery J, Jalil A, Leclercq G, Szczylik C, Chouaib S. Opposite effects of estrogen receptors alpha and beta on MCF-7 sensitivity to the cytotoxic action of TNF and p53 activity. Oncogene. 2005;24(30):4789–4798. [DOI] [PubMed] [Google Scholar]
- 71. Behrens D, Gill JH, Fichtner I. Loss of tumourigenicity of stably ERbeta-transfected MCF-7 breast cancer cells. Mol Cell Endocrinol. 2007;274(1-2):19–29. [DOI] [PubMed] [Google Scholar]
- 72. Hartman J, Lindberg K, Morani A, Inzunza J, Ström A, Gustafsson JA. Estrogen receptor beta inhibits angiogenesis and growth of T47D breast cancer xenografts. Cancer Res. 2006;66(23):11207–11213. [DOI] [PubMed] [Google Scholar]
- 73. Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 2004;64(1):423–428. [DOI] [PubMed] [Google Scholar]
- 74. Hodges-Gallagher L, Valentine CD, El Bader S, Kushner PJ. Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res Treat. 2008;109(2):241–250. [DOI] [PubMed] [Google Scholar]
- 75. Sotoca AM, van den Berg H, Vervoort J, van der Saag P, Ström A, Gustafsson JA, Rietjens I, Murk AJ. Influence of cellular ERalpha/ERbeta ratio on the ERalpha-agonist induced proliferation of human T47D breast cancer cells. Toxicol Sci. 2008;105(2):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. D’Cruz CM, Moody SE, Master SR, Hartman JL, Keiper EA, Imielinski MB, Cox JD, Wang JY, Ha SI, Keister BA, Chodosh LA. Persistent parity-induced changes in growth factors, TGF-beta3, and differentiation in the rodent mammary gland. Mol Endocrinol. 2002;16(9):2034–2051. [DOI] [PubMed] [Google Scholar]
- 77. Becker KA, Lu S, Dickinson ES, Dunphy KA, Mathews L, Schneider SS, Jerry DJ. Estrogen and progesterone regulate radiation-induced p53 activity in mammary epithelium through TGF-beta-dependent pathways. Oncogene. 2005;24(42):6345–6353. [DOI] [PubMed] [Google Scholar]
- 78. Ewan KB, Henshall-Powell RL, Ravani SA, Pajares MJ, Arteaga C, Warters R, Akhurst RJ, Barcellos-Hoff MH. Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res. 2002;62(20):5627–5631. [PubMed] [Google Scholar]
- 79. McTiernan A, Thomas DB. Evidence for a protective effect of lactation on risk of breast cancer in young women. Results from a case-control study. Am J Epidemiol. 1986;124(3):353–358. [DOI] [PubMed] [Google Scholar]
- 80. Zhou Y, Chen J, Li Q, Huang W, Lan H, Jiang H. Association between breastfeeding and breast cancer risk: evidence from a meta-analysis. Breastfeed Med. 2015;10(3):175–182. [DOI] [PubMed] [Google Scholar]
- 81. Rajkumar L, Kittrell FS, Guzman RC, Brown PH, Nandi S, Medina D. Hormone-induced protection of mammary tumorigenesis in genetically engineered mouse models. Breast Cancer Res. 2007;9(1):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sivaraman L, Hilsenbeck SG, Zhong L, Gay J, Conneely OM, Medina D, O’Malley BW. Early exposure of the rat mammary gland to estrogen and progesterone blocks co-localization of estrogen receptor expression and proliferation. J Endocrinol. 2001;171(1):75–83. [DOI] [PubMed] [Google Scholar]
- 83. Yang J, Yoshizawa K, Nandi S, Tsubura A. Protective effects of pregnancy and lactation against N-methyl-N-nitrosourea-induced mammary carcinomas in female Lewis rats. Carcinogenesis. 1999;20(4):623–628. [DOI] [PubMed] [Google Scholar]
- 84. Sivaraman L, Gay J, Hilsenbeck SG, Shine HD, Conneely OM, Medina D, O’Malley BW. Effect of selective ablation of proliferating mammary epithelial cells on MNU induced rat mammary tumorigenesis. Breast Cancer Res Treat. 2002;73(1):75–83. [DOI] [PubMed] [Google Scholar]
- 85. Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitam Horm. 2014;94:129–165. [DOI] [PubMed] [Google Scholar]