Abstract
Staphylococcus aureus is a predominant cause of fatal pneumonia following influenza A virus (IAV) infection. Herein we investigate the influence of antecedent IAV infection on S. aureus virulence gene expression. Using a murine model, comparing the USA300 and USA300ΔsaeR/S strains, we demonstrate that S. aureus pathogenesis following IAV infection is SaeR/S dependent. Furthermore, we show that IAV modulates the lung environment to rapidly up-regulate S. aureus virulence factors containing the SaeR-binding domain. Data demonstrate that the pathogen response to IAV infection impacts host outcome and provides evidence that the ability of S. aureus to sense and respond to the lung environment determines severity of pneumonia.
Keywords: Staphylococcus aureus, SaeR/S, influenza A, coinfection, superinfection, pneumonia
Influenza A virus (IAV) infections are associated with increased susceptibility to secondary bacterial infections, wherein morbidity and mortality increase significantly. Retroactive studies of samples from the 4 pandemic influenza outbreaks of the last century identified that secondary bacterial infections were associated with 40%–95% of fatal cases [1]. In the previous decade, influenza coinfections have displayed mortality rates ranging from 11- to 15-fold higher than those of influenza alone, and have consistently ranked within the top 10 leading causes of death in the United States [2, 3].
In the early 20th century, Streptococcus pneumoniae was the major cause of bacterial superinfection following IAV. However, since the 1950s, a shift toward Staphylococcus aureus as a causative agent in secondary infections was observed [1, 4]. Staphylococcus aureus is now recognized as one of the most frequent causes of severe bacterial coinfections [1]. Interestingly, S. aureus colonizes the nares of approximately 50% of the general population [1, 5]. Despite this close proximity to the lower respiratory tract, S. aureus acting as a primary infectious agent of community-associated pneumonia in healthy individuals occurs infrequently. Recent work describing the interactions of upper respiratory tract commensals has demonstrated that members of the upper respiratory tract transiently encounter the lower respiratory tract [6]. Taken together, these observations imply that S. aureus frequently encounters the lung environment in a significant portion of the population without leading to disease.
The innate ability of S. aureus to sense and respond to environments within a human host is dependent on tightly controlled gene regulatory systems [5]. SaeR/S is a 2-component gene regulatory system that controls transcription of virulence genes essential in neutrophil evasion and S. aureus pathogenesis [5]. In the current study, we use a murine model of secondary pneumonia to demonstrate that S. aureus pathogenesis following antecedent IAV infection is SaeR/S dependent. Furthermore, we provide evidence that IAV modulates the host lung environment to rapidly up-regulate S. aureus virulence factors containing the SaeR-binding domain.
MATERIALS AND METHODS
Bacterial Cultures
Staphylococcus aureus strains USA300, USA400, and isogenic saeR/S deletion mutant strains (∆saeR/S) were grown and harvested as described previously [7]. Bacterial inocula were resuspended in sterile Dulbecco’s phosphate-buffered saline.
IAV Strains
IAV/WSN/1933(H1N1) (hereafter “WSN”) was propagated in the Obar laboratory (Geisel School of Medicine at Dartmouth), and IAV/PR/8/1934 (PR8) was purchased from Charles River Laboratories. IAV infections were preformed using a total volume of 50 µL sterile phosphate-buffered saline (PBS) containing 750 plaque-forming units (PFU) for WSN or 100 PFU for PR8.
Mouse Models of Pneumonia and In Vivo S. aureus Gene Expression Analysis
All studies conformed to National Institutes of Health guidelines and were approved by the Animal Care and Use Committee at Montana State University, Bozeman (MSU). Male and female BALB/c mice (8–10 weeks old) were either bred at the MSU Animal Resources Center or were commercially acquired.
All challenges with S. aureus were performed on day 6 post-IAV infection. WSN-infected mice were challenged via intranasal administration of S. aureus with 5 × 108 for gene expression assays and for morbidity and mortality studies. PR8-infected mice were challenged via intratracheal administration of S. aureus at 5 × 108 colony-forming units (CFU)/50 µL for gene expression, or 2.5 × 108 for morbidity and mortality assays. Doses were determined by pilot studies and are in accordance with trends observed in Miller et al [8].
Mouse models of intranasal infections were adapted from Lee et al [3]. Mice were slightly anesthetized with isoflurane and scruffed in an upright position while a 50-µL volume was administered directly to mouse nares. After aspiration, mice were held upright for an additional 60 seconds to ensure distribution into the lower respiratory tract. For intratracheal infection, mice were deeply anesthetized with isoflurane and suspended from front incisors at a 60°–70° angle. A blunt-tipped, bent 18-g needle was inserted through the trachea and suspended above the carina to administer 50 µL directly into the lungs. Mouse nostrils were then blocked until a large gasp was observed. Mice were euthanized using a morbidity scale based on activity, feeding, and respiratory distress in combination with weight loss exceeding 20% of initial body mass.
Mice were euthanized at 4, 6, and 8 hours post–S. aureus challenge and lungs were homogenized in 2 mL of PBS. Aliquots (100 µL) were used for CFU determination and the remaining sample was centrifuged at 4000 rpm (3724 × g) for 10 minutes at 4°C. Supernatant was removed and pelleted lung tissue was resuspended in buffer RLT (Qiagen). Staphylococcus aureus RNA was purified as described previously [9]. Purified RNA from individual mice within each treatment group (3–4 per experiment) was pooled and adjusted to a final concentration of 5 ng/µL and subjected to Quantigene 2.0 assays (Affymetrix) or TaqMan reverse-transcription polymerase chain reaction (RT-PCR) (Supplementary Table 1) [7].
RESULTS AND DISCUSSION
Previous studies have suggested that dysregulated lung physiology, including impairment of mucociliary clearance as well as modulation of the host immune system in response to IAV, is a major contributor to increased host susceptibility to secondary S. aureus infections [1]. Specifically, IAV has been demonstrated to influence type 1 interferon responses, nicotinamide adenine dinucleotide phosphate oxidase activity, and antimicrobial peptide production [1].
These efforts have provided insight into disease progression by identifying a role for IAV infection in the dissemination of S. aureus from the upper respiratory tract to the lower respiratory tract [10]. Additionally, the expression of specific S. aureus toxins has been linked to the severity of pneumonia [3]. However, the contribution of IAV infection on S. aureus virulence regulation remains incompletely defined. To that end, we investigated the impact of an antecedent IAV infection on S. aureus pathogenesis during secondary bacterial pneumonia.
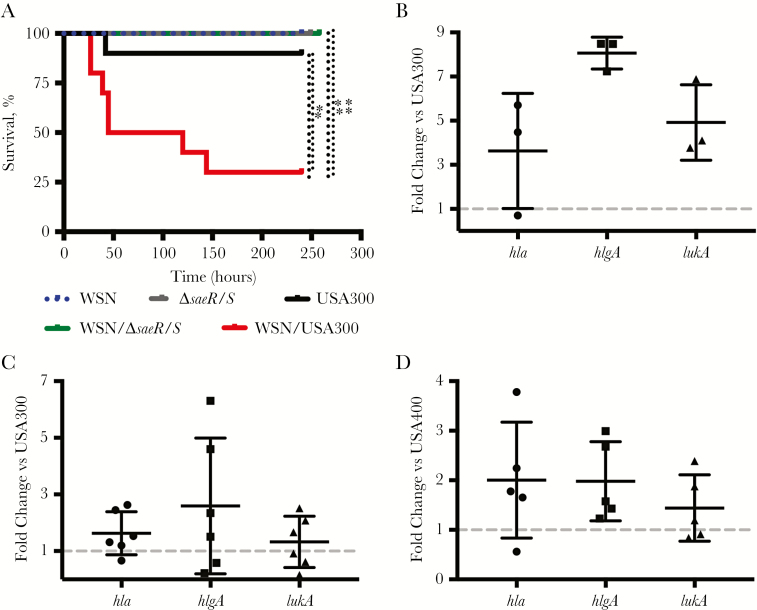
Mice were infected intranasally with WSN followed by subsequent challenge with USA300 or USA300∆saeR/S (∆saeR/S) at 6 days post–primary IAV infection [3]. Consistent with published observations, infection with IAV or S. aureus only had minimal impact on survival. However, coinfection with USA300 led to a significant increase in murine mortality (Figure 1A) [1, 4]. In contrast, mice infected with WSN but challenged with ∆saeR/S retained a survival rate of 100%, suggesting that SaeR/S is a key contributor to S. aureus secondary pneumonia (Figure 1A).
Figure 1.
Staphylococcus aureus bacterial pneumonia following antecedent influenza A virus (IAV) infection is saeR/S dependent. Mice were intranasally mock-infected with phosphate-buffered saline or infected with IAV. Six days postinfection, mice were challenged with S. aureus. A, Survival assay of mice infected with IAV/WSN/1933(H1N1) (WSN) (750 plaque-forming units/50 µL) and challenged with USA300 (5 × 108 colony-forming units [CFU]/50 µL), n = 10 mice per group. *P < .05; **P < .01, log-rank (Mantel-Cox) test. B–D, TaqMan reverse-transcription polymerase chain reaction quantification of select SaeR-target genes. Four hours after S. aureus challenge (5 × 108 CFU/50 µL), RNA was isolated from 3–6 mice per treatment group. Data displayed are the mean fold change of S. aureus expression levels from coinfected lungs compared to lungs infected with S. aureus alone. Gene transcripts were normalized to gyrB expression and calibrated to expression in S. aureus–only infection. B, WSN and USA300. C, PR8 and USA300. D, PR8 and USA400.
To confirm that the increased susceptibility to USA300 following IAV infection was not due to responses specific to WSN or route of infection, we infected mice with IAV PR8 via an intratracheal route (Supplementary Figure 1) [8]. Similar to the results of mice coinfected with WSN and S. aureus, PR8-infected mice challenged with USA300 had significant increases in mortality compared with mice infected with USA300 alone (Supplementary Figure 1).
Given the relative infrequency of S. aureus acting as a primary infectious agent in the lung environment, in combination with the observed absence of mortality in mice challenged with IAV and ∆saeR/S, we hypothesized that SaeR/S-regulated virulence factors were induced during coinfection with IAV. Thus, we investigated the influence of antecedent IAV infection on the expression of several SaeR-regulated virulence genes with described roles in lung infections (hlgA, hla, and lukA) [11]. TaqMan quantitative RT-PCR was used to measure the transcript abundance in mice infected with S. aureus alone or coinfected with WSN or PR8 (Figure 1B and 1C) at 4 hours following bacterial challenge. Indeed, antecedent infection with IAV led to increased expression of all virulence genes assayed compared to infection with S. aureus alone. To ensure the observed upregulation of SaeR-target genes was not specific to coinfection with USA300, we repeated the experiment using USA400. Challenge with USA400 following IAV demonstrated similar increased expression of saeR target genes compared to challenge with USA400 alone (Figure 1D).
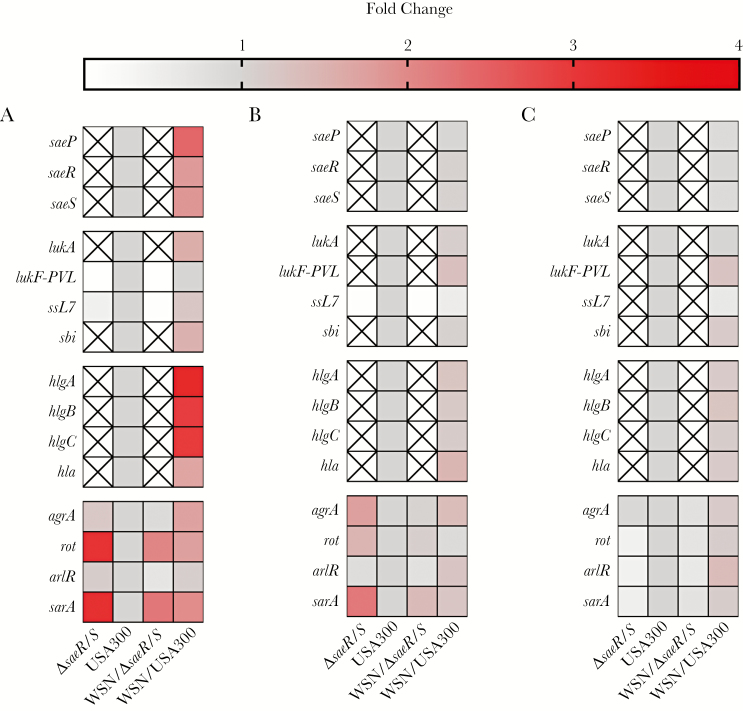
To substantiate the impact of antecedent IAV infection on the regulation of saeS and saeR, we evaluated S. aureus gene expression in vivo at 4, 6, and 8 hours post–S. aureus challenge (Figure 2). Expression of select S. aureus virulence genes and regulatory systems were determined (Figure 2A–C; Supplementary Table 2) [9]. Quantigene 2.0 analyses of S. aureus gene expression as a primary or secondary infectious agent during lung infection demonstrated that antecedent IAV infection rapidly increased expression of saeR, saeS, and SaeR-regulated virulence genes in mice coinfected with USA300 compared to expression of these genes during pneumonia caused by USA300 alone. Specifically, at 4 hours post–S. aureus challenge, virulence genes containing the SaeR-binding domain including lukA, ssL7, sbi, hlgA, hlgB, hlgC, and hla were up-regulated in coinfected mice (Figure 2A). The bicomponent γ-hemolysins hlgA, hlgB, and hlgC displayed the greatest fold-increase in expression compared to USA300 infection alone and expression decreased by 6 hours, suggesting that early activation of these toxins may be critical for S. aureus to establish a productive infection within the lung environment. While no comprehensive studies of S. aureus gene expression from patients following IAV have been conducted, efforts using rabbit models of pneumonia—where S. aureus toxin production is believed to be more indicative of a human infection—have demonstrated that active immunization against secreted γ-toxins resulted in protection of >97% of all subjects tested compared to survival of <1% in those unvaccinated [12, 13], suggesting that our observation of early production of γ-toxins may correlate with secondary S. aureus pneumonia in humans.
Figure 2.
Influenza A virus (IAV) infection promotes rapid up-regulation of Staphylococcus aureus SaeR-regulated genes. Mice were infected intranasally with IAV/WSN/1933(H1N1). Six days post-IAV infection, mice were challenged with USA300 or ∆saeR/S. Four (A), 6 (B), or 8 (C) hours post–S. aureus challenge, mouse lungs were harvested. RNA was purified from harvested lungs and treatment groups were pooled (n = 4–5 mice/treatment group/experiment). Gene transcripts were normalized to gyrB and calibrated to the expression levels of USA300-only infection at corresponding time points. Differential regulation of SaeR target genes was assessed by Quantigene 2.0 assays. “X” indicates transcript levels below the limit of detection. Data are reported as mean fold change of 2 separate experiments.
Mice infected with ΔsaeR/S showed no differences in expression of these virulence genes due to preceding IAV infection and were below the level of detection at all time points (Figure 2A–C), demonstrating a predominant role for SaeR/S in regulating lukA, sbi, hlgA, hlgB, hlgC, and hla in vivo and implicating these factors in disease outcome. Expression of agrA and sarA (genes that do not contain the SaeR-recognition sequence) were increased in USA300 following IAV infection. In our studies, bacterial CFUs were equal between USA300- and ΔsaeR/S-infected mice at 4 hours postchallenge and, therefore, differences in pathogen burden did not impact gene expression results (Supplementary Figure 2A and 2B). Bacterial burdens recovered at 8 hours postchallenge were lower in ΔsaeR/S- compared with USA300-infected mice; however, CFUs were equal between both coinfected treatment groups. Interestingly, agrA and sarA were increased during infection with ΔsaeR/S even without antecedent IAV infection. Bloes et al demonstrated the importance of Arg-regulated phenol-soluble modulins in secondary S. aureus pneumonia by challenging mice with USA300- and PSM-deficient mutant 2 days post–IAV infection [14]. However, our data suggest that SaeR/S is the predominant regulator initiating virulence factor expression following IAV.
Over time, the impact of IAV on S. aureus virulence gene expression in USA300-infected mice was reduced (Figure 2B and 2C; Supplementary Figure 3; Supplementary Table 3). As previous studies have demonstrated that SaeR/S influences primary S. aureus pneumonia, we analyzed S. aureus gene expression in USA300 over time to determine if SaeR-regulated genes were differentially regulated (Supplementary Figure 3; Supplementary Table 3) [15]. Indeed, SaeR/S-regulated genes increased in expression over time in USA300-infected mice even without antecedent IAV infection. In addition, unlike the increased expression of the bicomponent γ-hemolysins at 4 hours, increased expression of lukF-PVL, a toxin linked to the severity of S. aureus lung infections, was not observed until 6 hours after S. aureus challenge in coinfected mice (Figure 2B and 2C; Supplementary Table 2). These observations, combined with the mortality data (Figure 1A; Supplementary Figure 1) and data from other studies, suggest that the timing of induction of virulence genes regulated by SaeR is critical to initiation of infection [1, 10, 14]. Based on these data, we hypothesize that components of the IAV-infected lung environment are recognized by SaeS, leading to early up-regulation of SaeR-regulated toxins. Conversely, when S. aureus encounters a healthy lung environment, no immediate activation of SaeR/S occurs. The delay in toxin production in absence of IAV may provide sufficient time for detection by innate responses, leading to clearance of S. aureus from the lung environment.
In conclusion, these data provide new understanding of S. aureus pathogenesis following antecedent IAV infection and suggest that the initiation and severity of secondary infection by S. aureus in the lung environment are saeR/S dependent. We acknowledge that our choice of IAVs represents seasonal influenza strains, and additional studies are necessary to determine if our results are applicable to pandemic IAVs. Further efforts focused on S. aureus sensing and responding to the surrounding lung environment during IAV infection will provide insight into environmental changes due to IAV that up-regulate S. aureus virulence during secondary pneumonia.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the US National Institutes of Health (grant numbers R01A1090046, R01AI103353, GM110732, and U54GM115371); funds from the Montana University System Research Initiative (51040-MUSRI2015-03) and Montana State University Agriculture Experiment Station; and an equipment grant from the Murdock Charitable Trust.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 48th Annual Meeting of the Society of Leukocyte Biology, Raleigh, North Carolina, 2015; Northwest Branch Meeting of the American Society for Microbiology, Seattle, Washington, 2015; International Society for Microbial Ecology General Meeting, Montreal, Canada, 2016; and 50th Annual Meeting of the Society for Leukocyte Biology, Vancouver, Canada, 2017.
References
- 1. Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol 2017; 8:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu J, Murphy SL, Kochanek KD, Bastian BA. National vital statistics reports deaths: final data for 2013. Natl Cent Heal Stat 2016; 64:1–119. [PubMed] [Google Scholar]
- 3. Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis 2010; 201:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 2014; 12:252–62. [DOI] [PubMed] [Google Scholar]
- 5. Guerra FE, Borgogna TR, Patel DM, Sward EW, Voyich JM. Epic immune battles of history: neutrophils vs. Staphylococcus aureus. Front Cell Infect Microbiol 2017; 7:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dickson RP, Huffnagle GB. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog 2015; 11:e1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis 2010; 201:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller MA, Stabenow JM, Parvathareddy J, et al. . Visualization of murine intranasal dosing efficiency using luminescent Francisella tularensis: effect of instillation volume and form of anesthesia. PLoS One 2012; 7:e31359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zurek OW, Nygaard TK, Watkins RL, et al. . The role of innate immunity in promoting SaeR/S-mediated virulence in Staphylococcus aureus. J Innate Immun 2014; 6:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddinger RM, Luke-Marshall NR, Hakansson AP, Campagnari AA. Host physiologic changes induced by influenza a virus lead to Staphylococcus aureus biofilm dispersion and transition from asymptomatic colonization to invasive disease. MBio 2016; 7. doi:10.1128/mBio.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitur K, Parker D, Nieto P, et al. . Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. In: Miller LS, ed. PLoS Pathog, vol 11 San Francisco, CA: Public Library of Science, 2015:e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spaulding AR, Salgado-Pabón W, Merriman JA, et al. . Vaccination against Staphylococcus aureus pneumonia. J Infect Dis 2014; 209:1955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diep BA, Chan L, Tattevin P, et al. . Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A 2010; 107:5587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bloes DA, Haasbach E, Hartmayer C, et al. . Phenol-soluble modulin peptides contribute to influenza A virus-associated Staphylococcus aureus pneumonia. Infect Immun. American Society for Microbiology 2017; 85:e00620–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montgomery CP, Boyle-Vavra S, Daum RS. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One 2010; 5:e15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.