Zrzavy et al. analyse the phenotype of microglia in evolving lesions from patients with multiple sclerosis. Microglia lose their homeostatic phenotype in active lesions and express activation markers functionally related to tissue injury. Macrophages in the lesions are derived in part from resident microglia and in part from recruited myeloid cells.
Keywords: multiple sclerosis, microglia, macrophages, demyelination, neurodegeneration
Abstract
Microglia and macrophages accumulate at the sites of active demyelination and neurodegeneration in the multiple sclerosis brain and are thought to play a central role in the disease process. We used recently described markers to characterize the origin and functional states of microglia/macrophages in acute, relapsing and progressive multiple sclerosis. We found microglia activation in normal white matter of controls and that the degree of activation increased with age. This microglia activation was more pronounced in the normal-appearing white matter of patients in comparison to controls and increased with disease duration. In contrast to controls, the normal-appearing white matter of patients with multiple sclerosis showed a significant reduction of P2RY12, a marker expressed in homeostatic microglia in rodents, which was completely lost in active and slowly expanding lesions. Early stages of demyelination and neurodegeneration in active lesions contained microglia with a pro-inflammatory phenotype, which expressed molecules involved in phagocytosis, oxidative injury, antigen presentation and T cell co-stimulation. In later stages, the microglia and macrophages in active lesions changed to a phenotype that was intermediate between pro- and anti-inflammatory activation. In inactive lesions, the density of microglia/macrophages was significantly reduced and microglia in part converted to a P2RY12+ phenotype. Analysis of TMEM119, which is expressed on microglia but not on recruited macrophages, demonstrated that on average 45% of the macrophage-like cells in active lesions were derived from the resident microglia pool. Our study demonstrates the loss of the homeostatic microglial signature in active multiple sclerosis with restoration associated with disease inactivity.
Introduction
Multiple sclerosis is a chronic inflammatory demyelinating disease of the CNS, which, with time, results in profound neurodegeneration in the brain and spinal cord (Lassmann et al., 2007). Active demyelination and axonal or neuronal injury are associated with the focal accumulation of microglia and macrophages (Babinski, 1885; Esiri and Reading, 1987; Ferguson et al., 1997; Prineas et al., 2001), but an understanding of the functional phenotype and origin of these cells in different stages of lesion formation is limited (Bogie et al., 2014). Microglia and macrophages have been classified as pro-inflammatory (M1) or anti-inflammatory (M2) (Martinez and Gordon, 2014; Michell-Robinson et al., 2015; Walker and Lue, 2015), though this strict classification is no longer considered valid (Ransohoff, 2016). Previous studies in multiple sclerosis demonstrated the expression of molecules involved in phagocytosis, antigen presentation and cytotoxicity in active lesions (Brück et al., 1995; Höftberger et al., 2004; Haider et al., 2011; Fischer et al., 2012). Macrophages and microglia have been shown to have an intermediate phenotype (Zhang et al., 2011; Vogel et al., 2013; Peferoen et al., 2015) and no clear lesion stage-dependent polarization patterns have been identified, which may be due both to lack of well-defined multiple sclerosis lesion types included in these studies and the absence of unique markers that define microglia (Bogie et al., 2014).
Microglia were originally defined as the resident phagocytic cells in the brain by their morphological appearance (small cell with elongated nucleus and slender, ramified cell processes), but it has already been noted in these earliest studies and confirmed later that these cells under pathological conditions can transform through an activated state into cells with classical round to oval macrophage phenotype (Kettenmann et al., 2011). On the other hand, radiation bone marrow chimeric animals showed that myeloid cells recruited from the circulation can invade the brain and transform to a morphological phenotype resembling resident microglia (Lassmann et al., 1993; Mildner et al., 2007; Prinz et al., 2014). Recently, it was shown that resident microglia populate the brain during early development from the yolk sac (Ginhoux et al., 2010; Kierdorf et al., 2013), but that under pathological conditions additional macrophages are recruited into the lesions from the blood (Hickey and Kimura, 1988). Thus, in descriptive studies on phagocytes within brain lesions, microglia and macrophages should be defined by a combination of their morphological and molecular phenotypes. However, when discussing their origin, the terms yolk sac-derived (resident) microglia versus recruited monocytes/macrophages should be used.
We performed a comprehensive investigation of microglia and macrophage phenotypes using new cell-specific markers in conjunction with a spectrum of functional markers (Walker and Lue, 2015) on carefully staged lesions in acute, relapsing and progressive forms of multiple sclerosis. We were able to determine the contribution of resident microglia versus recruited macrophages in multiple sclerosis lesions and the functional state of microglia and macrophages during the evolution of inflammatory demyelinating lesions at well-defined disease stages.
Materials and methods
Sample characterization
Our study was performed on an archival collection of brain autopsy tissue from 31 patients with multiple sclerosis and 18 age-matched controls collected during the past decades in the Center for Brain Research of the Medical University of Vienna. Patient demographics, clinical course and the spectrum of lesions included in this study are summarized in Table 1. The multiple sclerosis sample contained 12 cases of acute or relapsing multiple sclerosis, seven with primary and 12 with secondary progressive disease. The study was approved by the ethics committee of the Medical University of Vienna (EK. Nr.: 535/2004/2016).
Table 1.
Clinical demographics
| Case | Details | Sex | Age | Lesion type | Region of interest | Disease duration, months | |
|---|---|---|---|---|---|---|---|
| C 1 | Controls | F | 30 | NWM | |||
| C 2 | Controls | F | 36 | NWM | |||
| C 3 | Controls | M | 37 | NWM | |||
| C 4 | Controls | F | 39 | NWM | |||
| C 5 | Controls | F | 42 | NWM | |||
| C 6 | Controls | F | 45 | NWM | |||
| C 7 | Controls | M | 46 | NWM | |||
| C 8 | Controls | F | 47 | NWM | |||
| C 9 | Controls | M | 65 | NWM | |||
| C 10 | Controls | M | 70 | NWM | |||
| C 11 | Controls | F | 71 | NWM | |||
| C 12 | Controls | F | 71 | NWM | |||
| C 13 | Controls | M | 72 | NWM | |||
| C 14 | Controls | F | 80 | NWM | |||
| C 15 | Controls | M | 83 | NWM | |||
| C 16 | Controls | F | 84 | NWM | |||
| C 17 | Controls | F | 88 | NWM | |||
| C 18 | Controls | F | 97 | NWM | |||
| MS 1 | AMS | F | 34 | Act. lesion III | NAWM, initial, EA, act. centre | 4 | |
| MS 2 | AMS | M | 35 | Act. lesion III | NAWM, initial, EA, act. centre | 1.5 | |
| MS 3 | AMS | F | 45 | Act. lesion III, SEL | NAWM, initial, EA, LA, SEL:edge, SEL:core | 0.2 | |
| MS 4 | AMS | M | 45 | 2× Act. lesion III | NAWM, initial, EA, LA, act. centre | 0.6 | |
| MS 5 | AMS | F | 46 | Act. lesion II | NAWM, EA | 0.5 | |
| MS 6 | AMS | F | 46 | Act. lesion II | NAWM, EA, act. centre | 7 | |
| MS 7 | AMS | F | 46 | Act. lesion II | NAWM, EA, act. centre | 3 | |
| MS 8 | AMS | M | 52 | Act. lesion II | NAWM, EA, act. centre | 1.5 | |
| MS 9 | AMS | M | 59 | Act. lesion II | NAWM, EA, act. centre | 5 | |
| MS 10 | AMS | F | 69 | Act. lesion III | NAWM, initial, EA,LA, | 2 | |
| MS 11 | AMS | M | 78 | Act. lesion III | NAWM, EA, LA | 2 | |
| MS 12 | RRMS | F | 40 | Act. lesion III | NAWM, initial, EA, act. centre | 120 | |
| MS 13 | PPMS | F | 34 | 3× inactive lesion | NAWM, inactive core | 204 | |
| MS 14 | PPMS | M | 36 | Act. lesion II | NAWM, EA, act. centre | 61 | |
| MS 15 | PPMS | M | 53 | SEL | NAWM, SEL:edge, SEL:core | 168 | |
| MS 16 | PPMS | F | 54 | Act. lesion I | NAWM, EA, LA, | 72 | |
| MS 17 | PPMS | F | 55 | Act. lesion I | NAWM, EA, act. centre | 168 | |
| MS 18 | PPMS | M | 67 | SEL | NAWM, SEL:edge, SEL:core | 87 | |
| MS 19 | PPMS | F | 77 | SEL | NAWM, SEL:edge, SEL:core | 168 | |
| MS 20 | SPMS | M | 34 | SEL | NAWM, SEL:edge, SEL:core | 120 | |
| MS 21 | SPMS | M | 41 | SEL | NAWM, SEL:edge, SEL:core | 137 | |
| MS 22 | SPMS | F | 42 | Act. lesion I | NAWM, EA, LA, | 216 | |
| MS 23 | SPMS | F | 46 | SEL | NAWM, SEL:edge, SEL:core | 444 | |
| MS 24 | SPMS | F | 48 | Act. lesion I | NAWM, EA, act. centre | 410 | |
| MS 25 | SPMS | F | 53 | SEL | NAWM, SEL:edge, SEL:core | 241 | |
| MS 26 | SPMS | F | 53 | Inactive lesion | NAWM, inactive core | 360 | |
| MS 27 | SPMS | M | 56 | Act. lesion II | NAWM, EA, act. centre | 372 | |
| MS 28 | SPMS | F | 59 | SEL | NAWM, SEL:edge, SEL:core | 492 | |
| MS 29 | SPMS | F | 61 | Inactive lesion | NAWM, inactive core | 288 | |
| MS 30 | SPMS | F | 62 | SEL, inactive lesion | NAWM, SEL:edge, SEL:core | 144 | |
| MS 31 | SPMS | F | 81 | Inactive lesion | NAWM, inactive core | 432 | |
AMS = acute multiple sclerosis; RRMS = relapsing/remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis; PPMS = primary progressive multiple sclerosis; NWM = normal white matter of controls; NAWM = normal-appearing white matter of multiple sclerosis patients. Act. Lesion = classical active lesions; SEL = slowly expanding lesions; EA = early active lesion; LA = late active lesion; M = male; F = female.
We analysed pathological changes in the white matter of multiple sclerosis and focused on the following regions of interest (Figs 1 and 2): (i) normal-appearing white matter, at least 1 cm distant from any lesions (Fig. 1A and B); (ii) initial lesions, defined as ‘pre-phagocytic’ lesions by Barnett and Prineas (2004) (Fig. 1A and C), which are only present in cases with a pattern III of demyelination according to Lucchinetti et al. (2000); (iii) the early active lesion edge of classical active lesions following pattern I, II or III type of demyelination (Lucchinetti et al., 2000), defined by the presence of macrophages or microglia with myelin degradation products reactive for myelin oligodendrocyte glycoprotein (MOG) (Fig. 1A, D, F, G and H) (Brück et al., 1995); (iv) the late active lesion stage with macrophages containing degradation products reactive for major myelin proteins, such as proteolipid protein (PLP) or myelin basic protein (MBP); (v) the macrophage-rich inactive centre of classical active lesions, with foamy macrophages containing empty vacuoles as a footprint of neutral lipid degradation products (Fig. 1A, E and F); (vi) the active edge of slowly expanding (‘smouldering’) lesions present in the progressive stage of multiple sclerosis (Fig. 1J); (vii) the inactive centre of slowly expanding lesions (Fig. 1J); and (viii) the inactive centre of inactive lesions. The respective lesions were defined according to previously published criteria (Brück et al., 1995; Lassmann, 2011; Frischer et al., 2015). The respective cases and lesions were selected from a much larger sample of multiple sclerosis cases (Frischer et al., 2015) based on the presence of the respective lesion types or lesion areas and their sufficient size for quantitative analysis of the respective markers. The normal white matter of age-matched controls was analysed as a reference background for microglia activation in multiple sclerosis.
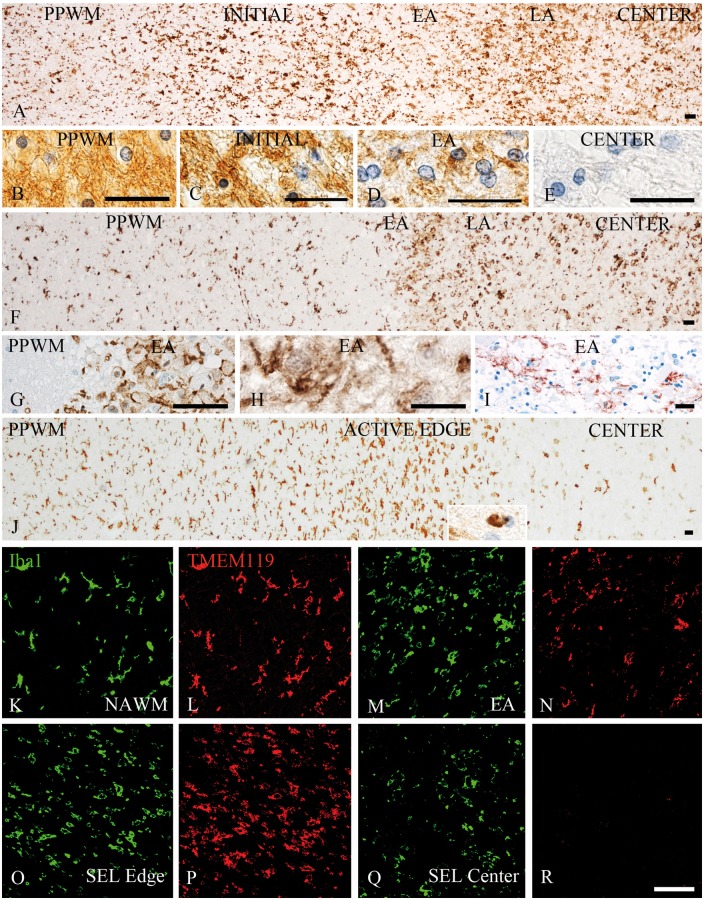
Figure 1.
Patterns of microglia and macrophage reaction in different types of multiple sclerosis lesions. (A–E) Active lesion following pattern III demyelination as defined by Lucchinetti et al. (2000) in a patient with acute multiple sclerosis; (A) low magnification image depicting the distribution and morphology of Iba1-positive cells in different zones of the active lesions including the peri-plaque white matter (PPWM), the initial ‘pre-phagocytic’ lesion area (INITIAL), the early active (EA) and the late active (LA) lesion zones and the macrophage-containing inactive lesion centre (CENTER). There is already profound microglia activation in the initial lesion areas and these cells are transformed into or replaced by macrophage-like cells in the areas, where myelin has been destroyed (early active, late active and centre); the myelin pathology in these different lesion areas are shown in B–E; normal myelin and glia are seen in the PPWM (B). In the initial area myelin is still preserved, but there is some oedema and many oligodendrocytes show nuclear condensation and chromatin margination reflecting apoptosis (C). In the early active zone, myelin is lost, but there are many macrophages with intracytoplasmic myelin degradation products reactive for MOG (D). No myelin or MOG reactivity is seen in the demyelinated lesion centre, but there are still many macrophages with empty vacuoles reflecting the neutral lipid stage of myelin degradation (E). (F–I) Active lesion following pattern II demyelination as defined by Lucchinetti et al. (2000) in a patient with acute multiple sclerosis. (F) Low magnification image depicting the distribution and morphology of Iba1-positive cells in different zones of the active lesions, including the peri-plaque white matter, the early active and the late active lesion zones and the macrophage-containing inactive lesion centre. In contrast to pattern III lesions, there is no zone of initial demyelination with oligodendrocyte apoptosis; in contrast, microglia density is reduced in a small zone surrounding the actively demyelinating lesion area (F and G) possibly due to recruitment of peri-plaque microglia to the site of active demyelination (early active and late active zones), the actively demyelinating area is characterized by a high density of cells with macrophage phenotype (F), which contain early myelin degradation products (H). In addition, there is deposition of activated complement (C9neo antigen) at the sites of active demyelination in these lesions (I). (J) Slowly expanding lesion in a patient with secondary progressive multiple sclerosis; low magnification image depicting the distribution and morphology of Iba1-positive cells in different zones of the active lesions including the peri-plaque white matter, the active lesion edge and the inactive lesion centre. An increased density of Iba1-positive cells with a phenotype of activated microglia is seen at the active edge; in contrast, there are only very few Iba1-positive microglia-like cells in the inactive lesion centre; the insert shows a macrophage with early myelin degradation products. (K–R) Double staining for Iba1 (green) and TMEM119 (red) shows co-expression of these molecules in most cells in the normal-appearing white matter (K and L) and the active edge of slowly expanding lesions (O and P), while TMEM119 is expressed only in a subset of cells with macrophage or microglia phenotype in early active multiple sclerosis lesions (M and N). In the centre of classical active lesions and slowly expanding lesions (SEL) Iba1-positive macrophages can be present, which are negative for TMEM119 (Q and R). Scale bars = 100 µm.
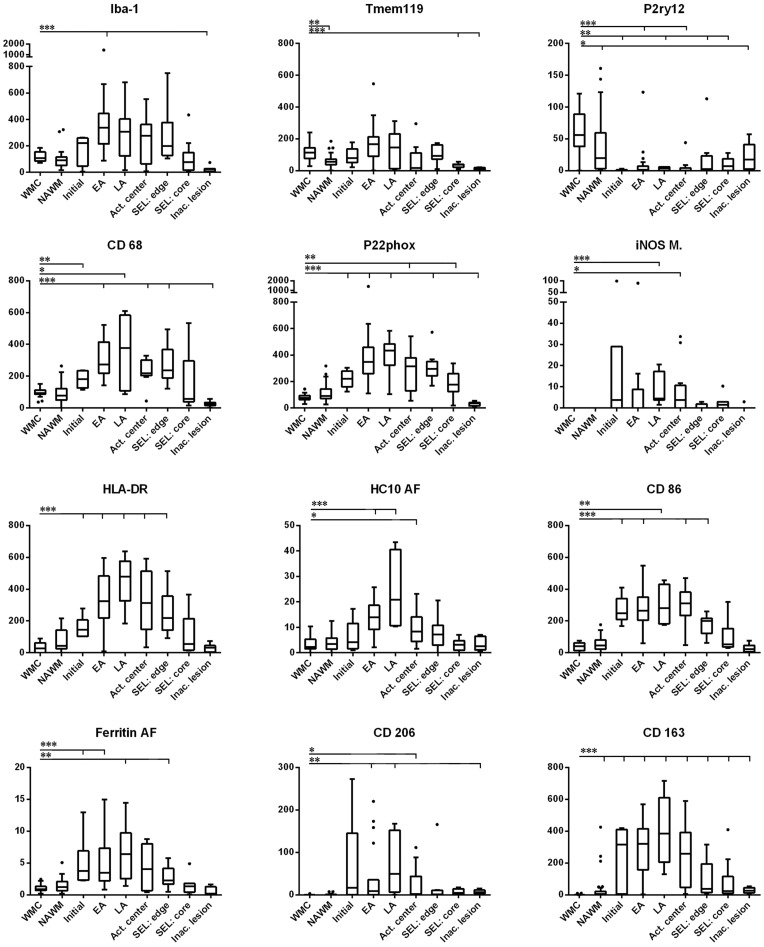
Figure 2.
Quantitative evaluation of microglia/macrophages expressing different phenotypic markers in multiple sclerosis lesions. Following immunohistochemistry for the respective microglia/macrophage markers, the numbers of positive cells were quantified as described in the ‘Materials and methods’ section. Overall, Iba1-positive macrophages and microglia cells are similar in numbers in the normal white matter of controls and in the normal-appearing white matter of patients with multiple sclerosis. In active lesions, these cells increase already in initial lesion stages (when present in pattern III lesions) and reach their peak in early/late active lesion areas. Numerous macrophages are still present in the inactive lesion centre of these plaques. Slowly expanding lesions are surrounded by a rim of microglia cells with some intermingled macrophages and have only very few microglia or macrophages in the inactive lesion centre. A similar quantitative profile is seen for the pro-inflammatory markers CD68 (phagocytosis), p22phox (oxidative burst) and the molecules involved in antigen presentation and co-stimulation (HLA-D, HC10 and CD86). P2RY12, the marker associated with the homeostatic state of microglia in rodents is completely lost in active lesion stages, but reappears on microglia in the centre of inactive lesions. Putative M2 markers (CD163 and CD206) have their peak of expression in late active/ inactive lesions, although their expression patterns were highly variable from case to case. TMEM119 is expressed in about half of microglia and macrophages in active lesions and further decreases in the inactive lesion centres. The values for Iba1, TMEM119, P2RY12, CD68, p22phox, iNOS, HLADR, CD86, CD206 and CD163 are cells per mm2 and thus the numbers in the y-axis are directly comparable. Due to the expression of the respective antigens in other cells in addition to microglia, the values for HC10 (MHC Class I) and ferritin were obtained by quantitative densitometry and are, thus, not directly comparable with the numerical counts for the other markers. WMC = white matter of controls; NAWM = normal-appearing white matter of multiple sclerosis patients; Initial = initial lesions [‘pre-phagocytic’ lesions (Barnett and Prineas, 2004)]; EA = early active lesions; LA = late active lesions; Act. center = macrophage-rich centre of active lesions; SEL: edge = active edge of smouldering lesions; SEL: core = lesion centre of smouldering lesions; Inac. lesions = inactive lesions.
Immunohistochemistry
To define microglia and macrophage phenotypes, we used Iba1 as a general marker for both microglia and macrophages. For microglia, we used TMEM119 and P2RY12. In the brain, TMEM119 is expressed on microglia-derived cells but not on recruited blood-derived macrophages (Butovsky et al., 2014; Bennett et al., 2016; Satoh et al., 2016). P2RY12 is expressed in homeostatic microglia defined in experimental animals (Butovsky et al., 2014). Other markers were used for phagocytosis (CD68), for antigen presentation, processing and co-stimulation (HLA-D, HC10/MHC I, and CD86), for oxygen and nitric oxide radical production (p22phox and iNOS), for microglia activation and iron uptake or storage (ferritin and ferritin light). The mannose receptor (CD206) and the haptoglobin/haemoglobin receptor (CD163) were used as paradigmatic markers for an M2 anti-inflammatory phenotype. Immunohistochemistry for these markers was first standardized by analysis of lymph node tissue and normal brain samples before being used on multiple sclerosis brain tissue. Primary antibodies used for immunohistochemistry and specific staining conditions are summarized in Table 2.
Table 2.
Antibodies used in immunohistochemistry
| Antibody | Origin | Target | Dilution | Antigen retrieval | Source |
|---|---|---|---|---|---|
| PLP | Mouse (mAB, IgG2a) | Proteolipid protein | 1:1000 | St (E) | MCA839G; Serotec |
| MOG | Mouse (mAB, IgG1) | Myelin oligodendrocyte glycoprotein | 1: 1000 | St (C) | Piddlesden et al., 1993 |
| IBA-1 | Rabbit (pAB) | Ionized calcium binding adaptor molecule 1 | 1: 3000 | St (E) | 019‐19741; Wako |
| CD68 | Mouse (mAB; IgG1) | CD68 110-kD transmembrane glycoprotein in macrophages | 1 : 100 | St (E) | M0814; Dako |
| HLA-DR | Mouse(mAB; IgG1) | MHC Class II antigen | 1:100 | St (C) | M0775; Dako |
| p22phox | Rabbit (pAB) | NADPH oxidase protein | 1 : 100 | St (C) | sc-20781; Santa Cruz |
| Tmem119 | Rabbit (pAB) | Transmembrane protein 119 | 1:100 | ‐ | HPA051870; Sigma-Aldrich |
| P2ry12 | Rabbit (pAB) | Purinergic receptor | 1:2500 | St (E) | Harvard, Dr. Butovsky |
| HC10 | Mouse (mAB; IgG2a) | Heavy chain of MHC Class I | 1:2000 | St (E) | Stam et al., 1990 |
| Ferritin | Rabbit (pAB) | Iron storage protein | 1:1000 | St (E) | MO (F5012); Sigma-Aldrich |
| FTL | Rabbit (pAB) | Ferritin light chain | 1:100 | ‐ | 10727‐1-AP; Proteintech |
| CD206 | Mouse (mAB; IgG1) | Mannose receptor | 1:100 | St (E) | ab117644; Abcam |
| CD163 | Mouse (mAB; IgG1) | Haemoglobin-haptoglobin scavenger receptor | 1:1,000 | St (C) | NCL-CD163; Novocastra |
| iNOS | Rabbit (pAB) | Inducible nitric oxide synthase I | 1:200 | St (E) | PA1‐37925; Thermo Scientific |
| CD86 | Goat (pAB) | Co-stimulatory T cell signal | 1:250 | St (C) | AF-141-NA; R&D Systems |
mAB = monoclonal antibody; pAB polyclonal antibody; ST (C) = antigen retrieval with citrate buffer; ST (E) = antigen retrieval with EDTA buffer; MHC = major histocompatibility complex.
We found that most of the markers used in this study were expressed on different macrophage subtypes in the lymph node (Supplementary Fig. 1). The only exception was P2RY12, which was not expressed at all in lymphatic tissue. TMEM119, which is unique to microglia in the brain and absent in recruited macrophages, was present on follicular dendritic cells (Satoh et al., 2016). The macrophage populations stained with the other markers and their location within the lymph node tissue varied, apparently reflecting their functional polarization. In addition, other cells were stained with some of the markers; MHC II antigens being prominently expressed also on dendritic cells, MHC I antigen on a large variety of different cell types and p22phox on granulocytes. Only few macrophages within follicles expressed CD163 or CD206, while these antigens were mainly seen on medullary macrophages (Supplementary Fig. 1).
Immunohistochemistry was performed using a biotin-streptavidin technique (Fischer et al., 2013). Sections were deparaffinized and antigen retrieval was done as outlined in Table 2. Non-specific protein binding was blocked by incubation with 10% foetal calf serum. Primary antibodies were applied overnight at the dilutions indicated in Table 2. Immunohistochemistry was completed by using species-specific biotinylated secondary antibodies against mouse, rabbit or goat immunoglobulins and incubation of sections with streptavidin/peroxidase complex and the reaction product was developed with diaminobenzidine. For control, immunohistochemistry was performed in the absence of the primary antibodies and by using normal rat and goat serum or isotype-matched monoclonal antibodies, directed against non-microglia/macrophage targets.
For double staining with primary antibodies derived from different species, the same antigen retrieval techniques and incubation with primary antibodies was used as described above. Antibody binding was visualized with either alkaline phosphatase-conjugated secondary antibodies or with biotinylated secondary antibodies and peroxidase-conjugated streptavidin. Alkaline phosphatase or peroxidase reaction products were visualized by development with fast blue BB salt (blue) or amino ethyl carbazole (AEC; red), respectively.
As both primary antibodies come from the same species (rabbit), double staining for P2RY12 or Iba1 and TMEM119 was performed with a different protocol (Bauer and Lassmann, 2016) by using extensive heat-induced epitope retrieval between the subsequent immunohistochemical reactions. Details of the protocol and the respective control experiments are provided in Supplementary Fig. 3.
Quantitative evaluation
All sections were screened for white matter lesions after staining for myelin (Luxol® fast blue or immunohistochemistry for MOG or PLP). The selection of the proper areas for quantification was performed prior to immunohistochemistry for macrophage/microglia antigens on the basis of lesion staging as described above. Following immunohistochemistry with macrophage/microglia markers on respective serial sections the previously defined regions of interest were manually outlined. Each lesion type and randomly sampled areas of normal-appearing white matter were quantitatively analysed. In normal controls, randomly sampled areas of white matter were quantified in the same way. For the quantitative evaluation, sections stained by immunohistochemistry were overlaid by a morphometric grid (0.2256 mm2) placed within the ocular lens and two to three fields per region were quantified. For each case, average counts per square millimetre were calculated for each region of interest and compared by statistical analysis.
Digital optical densitometry was performed for markers, which were not exclusively expressed in macrophages and microglia, such as the MHC Class I marker HC10 and ferritin, according to a previously published protocol (Hametner et al., 2013). Expression of these markers was quantified by calculating the positive DAB signal area fraction using ImageJ. One to two images per region of interest were taken with the 10× objective (0.43 mm2). Images were saved as TIFFs. For digitally removing haematoxylin counterstaining, a colour deconvolution plugin (freeware kindly provided by A.C. Ruifrok, NIH) was run. Further RGB images were converted into 8-bit greyscale images and inverted. A threshold was set in resulting images and the area fraction was calculated.
Statistical analysis
Statistical analysis was performed with IBM SPSS and GraphPad Prism. Due to uneven distribution of our data, statistical analysis was performed with non-parametric tests. Descriptive analysis included median value and range. Differences between two groups were assessed with Wilcoxon Mann-Whitney U-test. In case of multiple testing (comparison of more than two groups), significant values were corrected with the Bonferroni-Holm procedure. The reported P-values are results of two-sided tests. A P-value ≤ 0.05 was considered statistically significant.
Gene expression analysis
For the analysis of microglia/macrophage signature genes and genes related to phagocytosis, antigen processing/presentation, co-stimulation and oxidative stress, we re-evaluated whole-genome microarray data from multiple sclerosis white matter lesions and controls established previously in our laboratory (Fischer et al., 2012). For this analysis we selected genes that were described to be microglia-specific in comparison to other brain cells (Chiu et al., 2013; Hickman et al., 2013) or in comparison to peripheral myeloid cells (Gautier et al., 2012; Hickman et al., 2013; Butovsky et al., 2014) or to be involved in pro- or anti-inflammatory microglia/macrophage function such as phagocytosis, oxygen radical production, M1 or M2 phenotype or iron metabolism (Fig. 3). The data pool was derived from micro-dissected formalin-fixed paraffin-embedded tissue containing either normal-appearing white matter, the actively demyelinating lesion edge (containing parts of initial and early active lesions), or the inactive, macrophage-containing centre of highly active lesions from four patients with acute multiple sclerosis (Marburg, 1906) or normal white matter of age-matched control subjects. Detailed information on sample selection/dissection, mRNA isolation and microarray technologies has been published (Fischer et al., 2012). All microarray data have been deposited in NCBI’s Gene Expression Omnibus (accession number GSE32915). Gene expression data derived from normal-appearing white matter, initial and active lesions were compared to normal white matter of controls and presented as fold-changes.
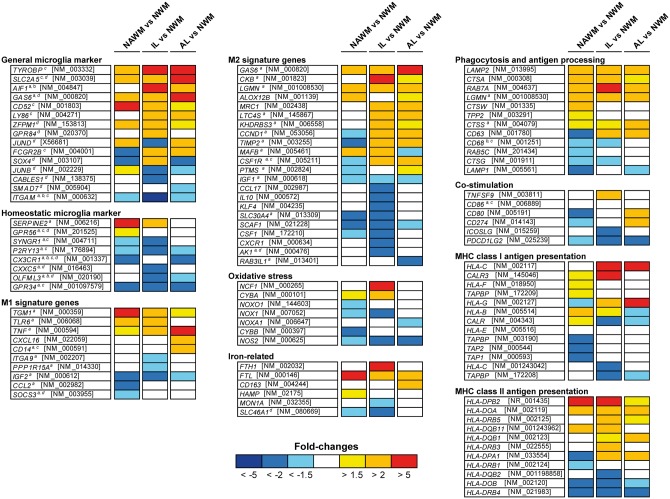
Figure 3.
Expression of microglia genes in normal-appearing white matter, initial ‘pre-phagocytic’ lesion areas and the macrophage-rich centre of classical active multiple sclerosis lesions in comparison to normal white matter of controls. The respective lesion areas were microdissected from active lesions from four patients with acute multiple sclerosis following pattern III of demyelination according to Lucchinetti et al. (2000). Expression levels are provided as fold changes in comparison to those in normal white matter of controls. The microglia genes were grouped according to their functional designation provided by aButovsky et al. (2014); bChiu et al. (2013); cHickman et al. (2013); and dGautier et al. (2012). NWM = normal white matter of controls; NAWM = normal-appearing white matter of multiple sclerosis patients; IL = lesion edge containing initial (pre-phagocytic) and early active lesion areas; AL = centre of active lesions highly infiltrated by foamy macrophages.
Results
Global dynamics of microglia/macrophage infiltration in multiple sclerosis lesions
Active lesion areas contained on average three times higher numbers of Iba1-positive cells in comparison to the number of microglia cells and perivascular macrophages in the normal white matter of controls and the normal-appearing white matter of multiple sclerosis patients (Figs 1A, F and 2). The highest numbers of these cells were seen in early active stages of classical active lesions and their density declined at later stages of lesion maturation. In slowly expanding lesions (Fig. 1J), high densities of microglia/macrophages were restricted to the active lesion edge, while in the inactive lesion centre their numbers were low. Only few microglia cells or macrophages were seen in inactive lesions and their density in the tissue was significantly reduced in comparison to the microglia density in the normal or normal-appearing white matter of control and multiple sclerosis brains, respectively. The macrophage/microglia profile was related to the lesion type (classical active versus slowly expanding; Fig. 1A, F and J). No significant differences in microglia/macrophage numbers were seen when active lesions in acute and relapsing multiple sclerosis were compared with those that were formed in primary or secondary progressive multiple sclerosis. However, as described before (Frischer et al., 2015), classical active plaques were mainly seen in early multiple sclerosis, while slowly expanding lesions were most prominent in patients with primary or secondary progressive multiple sclerosis. Thus, when active and slowly expanding lesions were combined, overall a lower degree of microglia/macrophage infiltration was seen in progressive compared to acute/relapsing multiple sclerosis. No significant differences in microglia/macrophage density and activation status in multiple sclerosis lesions were seen in relation to age or gender of the patients.
The contribution of resident microglia versus recruited macrophages in multiple sclerosis brain
Overall, the markers used in our study did not discriminate between cells with a microglia (slender cell process bearing cells) or macrophage phenotype (round to oval cells without cell processes; Supplementary Fig. 2). An exception was P2RY12, which was never observed on cells with a macrophage phenotype in multiple sclerosis lesions and the normal white matter of controls. The morphological phenotype of these cells does not allow conclusions whether they originated from the yolk sac-derived microglia pool or from recruited myeloid cells. Thus far, the only marker that may allow for such differentiation is TMEM119, which in experimental models and human brain tissue has been shown to be only expressed in microglia, but not on recruited myeloid cells (Bennett et al., 2016; Satoh et al., 2016). We found that the numbers of Iba1+ and TMEM119+ cells with microglia phenotype in the normal white matter of controls and the normal-appearing white matter of multiple sclerosis patients were similar and these cells were co-labelled with these markers (Figs 1K, L and 2). In contrast, in early stages of active lesions in multiple sclerosis, on average 43% of Iba1+ cells were TMEM119+ and there was a decreasing gradient of TMEM119+ cells from initial to advanced active lesions (Fig. 2). Similarly, the majority of activated microglia-like cells at the lesion edge of slowly expanding lesions were double-stained for Iba1 and TMEM119 (Fig. 1O and P), while the number of TMEM119+ cells decreased towards the inactive lesion centre (Fig. 1Q and R). In addition, we found a gradual transition of TMEM119+ cells with a morphological microglia phenotype into macrophages at the initial and active lesion edge (Figs 1 and 4). Double staining of TMEM119 and the microglia/macrophage markers CD68, p22phox and HLA-D showed co-expression of the markers in most microglia in the normal white matter of controls (Fig. 4) and the normal-appearing white matter of patients with multiple sclerosis. Our data indicate that in active multiple sclerosis lesions a major subset of cells, which ingest myelin debris and become phenotypically macrophages, are derived from the yolk sac-derived microglia pool, but that with maturation of the lesions yolk sac-derived microglia are lost in part and additional myeloid cells are recruited from peripheral monocytes.
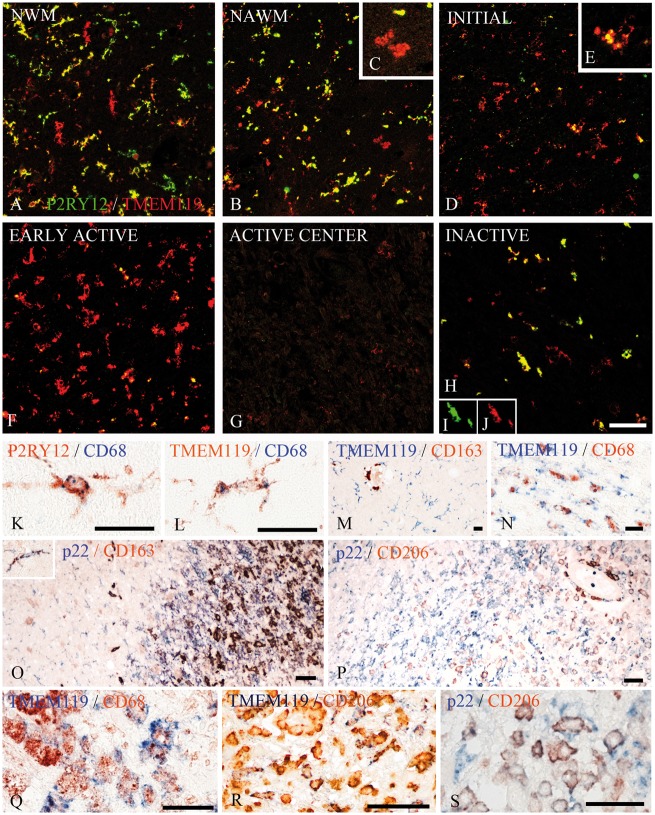
Figure 4.
Co-expression of microglia/macrophage markers in multiple sclerosis lesions. (A–J) Double staining for P2RY12 (green) and TMEM119 (red) shows that in the normal white matter of controls most microglia co-express P2RY12, although there are some, which are single positive for TMEM119 (A). In the normal-appearing white matter of multiple sclerosis a similar pattern as in control normal white matter is seen, but some of the TMEM119 single positive cells have a macrophage-like phenotype (B and insetC). P2RY12 is massively reduced in TMEM119+ cells in initial lesions (D and insetE) and there is a further reduction of P2RY12 expression in early active lesions, which still contain high numbers of TMEM119+ cells (F) and in the active centre of active lesions, which contain only few TMEM119+ cells (G). In inactive lesions, only few microglia-like cells are seen, but the majority of these cells co-express P2RY12 and TMEM119 (H). The insetsI and J show the expression of both antigens in a single double stained cell. (K–S) In the normal white matter of controls, the majority of microglia identified by the expression of P2RY12 (K) or TMEM119 (L) were activated and also expressed the phagocytosis-associated marker CD68. (M) An example of the normal-appearing white matter of an multiple sclerosis patient with numerous TMEM119+ microglia cells, while CD163 expression is restricted to perivascular macrophages. In N, activated microglia in initial lesion areas of a pattern III lesion are shown, which co-express TMEM119 and CD68. The edge of an active pattern III lesion is shown in O. In the initial lesion areas, most microglia cells express p22phox (NADPH oxidase) but are negative for CD163, although some microglia co-express both antigens (inset). In the early active lesion edge, there is still a dominant expression of p22phox, while co-expression of p22phox with CD163 increases towards the more advanced lesion parts. A similar profile is shown in P, which documents that CD206 appears in p22phox-positive macrophages predominately in advanced lesion stages. (Q–S) The expression profile of the markers in macrophages in the inactive lesion centre of active plaques. Some of the CD68+ (Q) or CD206+ macrophages (R) are co-labelled with TMEM119. In addition, some pro-inflammatory activated macrophages positive for p22phox co-express the anti-inflammatory M2 marker CD206 (S). Scale bars = 100 µm.
Gene expression profile of macrophage/microglia markers in different multiple sclerosis lesion stages
To determine gene expression, microarray data from microdissected tissue areas containing normal-appearing white matter, the active lesion edge (containing parts of initial and early active lesions) and the macrophage-rich inactive lesion centre from active lesions of patients who died in the course of acute multiple sclerosis (Marburg, 1906) were compared to the normal white matter of age-matched controls.
In comparison to the normal white matter of controls, we found a profound downregulation of genes associated with homeostatic microglia function in active lesion areas with initial and active demyelination, whereas the changes were less pronounced in the normal-appearing white matter and in the lesion centre (Fig. 3). Genes dominantly expressed in active lesion areas were pro-inflammatory molecules related to phagocytosis, antigen presentation and oxidative injury. Genes associated with anti-inflammatory (M2) molecules were up- and downregulated.
Immunohistochemical patterns of microglia/macrophage activation
Based on the differential gene expression profiles described above, we analysed key microglia/macrophage molecules by quantitative immunohistochemistry in relation to the activity of the demyelinating and neurodegenerative process in multiple sclerosis lesions.
Microglia in normal white matter of controls have a pre-activated phenotype
We found that in age-matched controls, similar numbers of Iba1-positive microglia expressed the microglia marker TMEM119, whereas P2RY12 was only present on average on 52% of microglia (Figs 2 and 4A). In addition, profound expression of the activation markers CD68 and p22phox was seen in 87% and 74% of Iba1+ microglia cells, respectively. Double staining with p22phox or CD68 revealed that the vast majority of P2RY12+ and TMEM119+ cells also expressed the activation markers (Fig. 4K and L). The expression of p22phox (NADPH oxidase) in microglia correlated significantly with the age of the patients (P = 0.029), but we did not find gender-dependent differences in microglia marker expression. In comparison, the expression of MHC II or CD86 was only seen in 26% and 38% of Iba1+ microglia-like cells, respectively (Fig. 2). The expression of iNOS and the M2 markers CD163 or CD206 was sparse and restricted to perivascular macrophages. Ferritin and ferritin light were found in oligodendrocytes and to a variable extent in microglia or perivascular cells (Hametner et al., 2013). Overall, in the normal white matter of controls, microglia showed an intermediate phenotype between a homeostatic and pro-inflammatory state.
Loss of P2RY12+ microglia in the normal-appearing white matter of patients with multiple sclerosis
The expression profile of microglia markers in the normal-appearing white matter of patients with multiple sclerosis was similar to that seen in the normal white matter of controls, though the total numbers of microglia stained for P2RY12 and TMEM119 was reduced (Fig. 2; P = 0.025 for P2RY12 and P = 0.001 for TMEM119). In sections double-stained for P2RY12 a substantial number of cells were single positive for TMEM119 and some of these cells revealed a macrophage-like phenotype (Fig. 4B). Pro-inflammatory markers were moderately increased and this was significant for p22phox (P < 0.01), when calculated as percentage of total microglia. The expression of the phagocytosis-associated marker CD68 was significantly increased in the normal-appearing white matter of patients with progressive multiple sclerosis in comparison to the acute and relapsing multiple sclerosis (P = 0.003), but no difference was seen between patients with primary or secondary progressive multiple sclerosis.
Classical active lesions
Classical active lesions contained high numbers of macrophage- and microglia-like cells; most of them in a state of activation and without expression of P2RY12 (Figs 2, 4D and F). When different stages of active lesions were compared, initial ‘pre-phagocytic’ lesions (according to Barnett and Prineas, 2004) primarily contained cells with a microglia phenotype and were in part positive for TMEM119, p22phox, CD68, CD86 and Class II MHC antigens (Figs 2, 4D and N). This pro-inflammatory activation state reached its peak in lesions containing macrophage-like cells with TMEM119 reactivity with early or late myelin degradation products (early or late active lesion stage; Figs 2 and 4F). In contrast, expression of iNOS was very low in macrophage- and microglia-like cells throughout all lesion stages. Expression of the M2 markers CD206 and CD163 was highly variable between initial and early active lesions of different cases. In some, these antigens were seen already on microglia-like cells in addition to perivascular and tissue macrophages, while in the majority of cases and lesions, their presence was restricted to perivascular cells and some tissue macrophages (Fig. 4O and P). In macrophage-containing inactive lesion sites TMEM119+ cells became rare despite the abundance of Iba1+ cells (Fig. 1Q and R). The expression of CD68 in macrophages remained, while the expression of pro-inflammatory molecules (p22phox and MHC antigen) declined. Expression of CD206, CD163 and ferritin peaked in the late active and inactive macrophage-containing core of active lesions (Figs 2 and 4O, P, R and S).
Comparison between active lesions following different patterns of demyelination
Classical active lesions with early active demyelination have previously been classified according to their patterns of demyelination (Lucchinetti et al., 2000) (Fig. 1A and F). Besides the presence and absence of complement activation, they differ in their lesion architecture. Pattern III lesions have an ill-defined border and are surrounded by areas of variable size, which show the features of initial (‘pre-phagocytic’) lesions characterized by microglia activation, loss of distal oligodendrocyte processes and oligodendrocyte apoptosis (Lucchinetti et al., 2000; Barnett and Prineas, 2004). In contrast, pattern I and II lesions are well demarcated from the surrounding normal-appearing white matter and initial stages of demyelination are seen within a dense rim of activated macrophages at the lesion edge (Lucchinetti et al., 2000). When we compared the overall macrophage/microglia density and their activation phenotype in the active and inactive portions of these lesions, we found no differences between pattern I, II and III lesions. However, we did find that pattern II lesions had significantly lower expression of TMEM119 (P = 0.038) in comparison to pattern III lesions.
Slowly expanding (‘smouldering’) lesions
These lesions were characterized by a narrow rim of microglia with some intermingled macrophages containing early and late myelin degradation products (Figs 1J and 2). A large proportion of macrophages and microglia at the active lesion edge expressed TMEM119 (Fig. 1O and P) and were also stained by CD68, p22phox, MHC antigen and ferritin, while expression of M2 macrophage-related molecules was very low. Only few phagocytes were present in the inactive lesion core (Fig. 2).
Inactive lesions
Completely inactive lesions contained only very few macrophage-like or microglia-like cells (Figs 1J and 2). The density of microglial cells was significantly lower compared to the normal-appearing white matter. Such lesions were distinguished from other multiple sclerosis lesions by the re-appearance of P2RY12 on the majority of the remaining microglia (Figs 2, 4H, I and J). Nonetheless, despite the reappearance of P2RY12, the majority of these cells also expressed pro-inflammatory activation markers. These results suggest that loss of the P2RY12 homeostatic marker is related to lesion activity.
Discussion
We investigated the innate immune response in the brain of subjects with multiple sclerosis using two unique markers: TMEM119, which differentiates microglia from recruited monocytes and P2RY12, which identifies a homeostatic microglia phenotype in rodent CNS tissue. We found that microglia cells in the control human brain have a pro-inflammatory activation state. Expression of some activation markers in microglia of the normal human brain has been described previously (Vogel et al., 2013). This differs from rodents where microglia in normal brain display a homeostatic phenotype (Butovsky et al., 2014), with little or no expression of pro-inflammatory markers (Schuh et al., 2014). It is not clear whether co-morbidities, such as the systemic exposure to infections and environmental toxins or vascular and neurodegenerative events related to brain ageing (Conde and Streit, 2006; Perry et al., 2010; Haider et al., 2016) account for the pro-inflammatory microglia activation in the normal CNS or whether such background activation is an inherent property of microglia in the human CNS.
We found that P2RY12 reactive microglia are reduced in the normal-appearing white matter in multiple sclerosis in comparison to the normal white matter of controls and that this phenotype is completely lost in active lesions. Loss of the microglia homeostatic phenotype is seen in other neurodegenerative conditions of the CNS (Butovsky et al., 2014, 2015). The expression of P2RY12 on microglia in the normal human brain has recently been confirmed and its loss on microglia has been shown in a single multiple sclerosis case and lesion of undefined activity stage (Mildner et al., 2016). In our study in multiple sclerosis, loss of P2RY12 reactivity on microglia was not restricted to the actual lesions, but also seen in the normal-appearing white matter, distant from focal plaques. This is true not only for chronic disease, but also in acute multiple sclerosis with a disease duration of weeks to months. Loss of the homeostatic microglia phenotype has been reported in active EAE lesions (Butovsky et al., 2014). The chronic inflammatory environment in the multiple sclerosis brain may contribute to its loss not only in the lesions, but also in the normal-appearing white matter. This is supported by our observation that in completely inactive lesions, in which inflammation has subsided, P2RY12 is re-expressed on the remaining microglia cells.
Multiple sclerosis is a chronic inflammatory disease of the CNS and it would thus be expected that active demyelination and neurodegeneration are associated with a pro-inflammatory state of microglia or macrophages. In early lesions, the number of microglia and macrophages increase and the cells dominantly express markers, which are associated with phagocytosis, oxidative injury and antigen presentation or co-stimulation in the course of T cell activation. Several studies report active tissue injury with the various expression of M1 versus M2 markers (Zhang et al., 2011; Vogel et al., 2013; Peferoen et al., 2015) and we also found that macrophage- and microglia-like cells in active multiple sclerosis lesions express both markers for M1 and M2 activation. This is not surprising, since M1 and M2 phenotypes are seen in macrophages in vitro after stimulation with defined mixtures of cytokines (Moore et al., 2015; Healy et al., 2017), while the situation may be more complicated in inflammatory brain lesions in vivo, where gene expression studies showed the simultaneous expression of pro- and anti-inflammatory cytokines within the same active lesion (Whitney et al., 1999; Mycko et al., 2003). However, we found a quantitative shift in microglia/macrophage activation from the initial stages of active lesions into established lesions, which contained macrophages in the process of myelin digestion. Thus, active demyelination and neurodegeneration was clearly associated with a dominant pro-inflammatory phenotype of phagocytes, while the peak expression of anti-inflammatory M2 markers was seen in the macrophage-containing inactive lesion centre. M2 polarization of microglia/macrophages follows myelin phagocytosis (Boven et al., 2006; van Rossum et al., 2008). It has been suggested to stimulate remyelination (Butovsky et al., 2006; Miron et al., 2013) and early stages of remyelination within multiple sclerosis lesions are mainly seen in the core of active lesions (Raine et al., 1981; Prineas et al., 1993; Raine, 2017).
A simple differentiation between M1 and M2 phenotype is complicated by the fact that some of the respective molecules also have other functions, which are relevant in the context of a multiple sclerosis plaque. For example, iNOS expression in multiple sclerosis lesions has been found mainly in macrophages at the lesion edge, which are loaded with iron and iron was mainly taken up by M1-polarized human macrophages in vitro (Mehta et al., 2013). It is not only present in microglia/macrophages (Marik et al., 2007), but also in astrocytes, as has been shown previously (Bö et al., 1994). In a similar way, one of the classical M2 markers, CD163, is a haptoglobin/haemoglobin receptor, which is involved in iron uptake into macrophages (Andersen et al., 2017) and the main function of ferritins is iron storage and sequestration in the cytoplasm (Lane et al., 2015). Thus, the expression of certain microglia/macrophage molecules may also be in part regulated by the iron metabolism, which is disturbed within the multiple sclerosis brain and lesions (Hametner et al., 2013).
The phenotypic changes of microglia/macrophages we observed were similar in classical active lesions and in the active zone of slowly expanding (‘smouldering’) plaques, although in the latter the zone of active tissue injury was thin and did not allow an accurate separation of lesion stage-associated phenotype. In contrast to classical active lesions, macrophages were rare or absent in the demyelinated core of slowly expanding lesions and cells with an anti-inflammatory (M2) phenotype were extremely rare in these lesions, which also did not show signs of remyelination (Bramow et al., 2010).
An important question that we were able to address is the degree to which macrophages in multiple sclerosis lesions are derived from the original microglia cell pool or from recruited monocytes. We found at the active lesion edge that there is a transition from classical microglia cells expressing P2RY12, to phagocytic cells phenotypically resembling process-bearing activated microglia that contain early myelin degradation products to typical round to oval debris-loaded macrophages. Many of these cells express TMEM119, which is expressed on microglia, but not on recruited peripheral myeloid cells (Bennett et al., 2016). Using this marker, Satoh et al. (2016) described TMEM119-positive cells in the cortex of Alzheimer’s disease, but not in multiple sclerosis lesions. On this basis, the authors concluded that phagocytes in Alzheimer’s disease are derived from microglia, while in multiple sclerosis they come from recruited myeloid cells. In contrast, our study suggests that a major proportion of phagocytes present in early stages of multiple sclerosis lesions are indeed derived from the microglia pool, as suggested by Li et al. (1996). However, with the maturation of active lesions an increasing proportion of macrophages was TMEM119-negative and thus represents an additional pool of cells, which has entered the lesions from the blood. The proportion of recruited myeloid cells was higher in lesions following pattern II demyelination compared to pattern III lesions, as defined by Lucchinetti et al. (2000). Similar conclusions were reached through the analysis of the chemokine receptors CCR1 and CCR5 in multiple sclerosis lesions, which may allow differentiation between CCR5-positive resident microglia and CCR1/CCR5-double positive or MRP14-reactive recruited myeloid cells (Trebst et al., 2001). Also with this approach, a similar difference between pattern II and III lesions has been observed (Mahad et al., 2004).
In conclusion, our study provides evidence that during initial and early stages of plaque formation in multiple sclerosis a major fraction of the cells, which are associated with active demyelination and neurodegeneration, are derived from the original resident microglia pool, thus suggesting that therapeutic blockade of macrophage entry from the circulation into the lesions may be insufficient to halt the disease process. Active demyelination and neurodegeneration is associated with a pro-inflammatory activation of microglia and macrophages, while macrophages polarized into an anti-inflammatory or neuroprotective phenotype are mainly seen in the lesion core of classical active lesions, where attempts of remyelination are frequent. They are sparse or absent in slowly expanding lesions of progressive multiple sclerosis, which fail to remyelinate. Thus, development of new PET markers, which distinguish these different functional states of microglia, could become useful para clinical markers for disease activity not only in relapsing but also in progressive multiple sclerosis. In contrast to experimental models of inflammatory demyelination, lesion formation in multiple sclerosis occurs on a background of pro-inflammatory microglia activation, which is seen already in the normal white matter of age-matched controls, and this may contribute to neurodegeneration in the disease.
Supplementary Material
Acknowledgements
We thank Marianne Leiszer, Ulrike Köck and Angela Kury for expert technical assistance.
Funding
This study was funded by the Austrian Science Fund (F.W.F.), Project: P27744-B27 and Project I 2114 (Era Net Neuron Project Meltra-BBB), by the NIH-NINDS (R01NS088137) (O.B.), National Multiple Sclerosis Society (5092A1) (O.B.), Nancy Davis Foundation Award (O.B.) R01AG051812 (O.B.) and R01AG043975 (H.L.W.).
Supplementary material
Supplementary material is available at Brain online.
References
- Andersen CB, Stodkilde K, Saederup KL, Kuhlee A, Raunser S, Graversen JH. et al. Haptoglobin. Antioxid Redox Signal 2017; 26: 814–31. [DOI] [PubMed] [Google Scholar]
- Babinski J. Recherches sur l’anatomie pathologique de la sclerose en plaque et étude comparative des diverses variétés de la scleroses de la moelle. Arch Physiol 1885; 5: 186–207. [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 2004; 55: 458–68. [DOI] [PubMed] [Google Scholar]
- Bauer J, Lassmann H. Neuropathological techniques to investigate central nervous system sections in multiple sclerosis. Methods Mol Biol 2016; 1304: 211–29 [DOI] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB. et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci USA 2016; 113: E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bö L, Dawson TM, Wesselingh S, Möurk S, Choi S, Kong PA. et al. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol 1994; 36: 778–86. [DOI] [PubMed] [Google Scholar]
- Bogie JF, Stinissen P, Hendriks JJ. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol 2014; 128: 191–213. [DOI] [PubMed] [Google Scholar]
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG. et al. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 2006; 129: 517–26. [DOI] [PubMed] [Google Scholar]
- Bramow S, Frischer JM, Lassmann H, Koch-Henriksen N, Lucchinetti CF, Sørensen PS. et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain 2010; 133: 2983–98. [DOI] [PubMed] [Google Scholar]
- Brück W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmarch HA. et al. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol 1995; 38: 788–96. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Cialic R, Krasemann S, Murugaiyan G, Fanek Z. et al. Targeting miR‐155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann Neurol 2015; 77: 75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G. et al. Identification of a unique TGF-[beta]-dependent molecular and functional signature in microglia. Nat Neurosci 2014; 17: 131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S. et al. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 2006; 31: 149–60. [DOI] [PubMed] [Google Scholar]
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP. et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 2013; 4: 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol 2006; 65: 199–203. [DOI] [PubMed] [Google Scholar]
- Esiri M, Reading M. Macrophage populations associated with multiple sclerosis plaques. Neuropathol Appl Neurobiol 1987; 13: 451–65. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain 1997; 120: 393–9. [DOI] [PubMed] [Google Scholar]
- Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J. et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 2012; 135: 886–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MT, Wimmer I, Höftberger R, Gerlach S, Haider L, Zrzavy T. et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain 2013; 136:1799–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I. et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 2015; 78: 710–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 2012; 13: 1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330: 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G. et al. Oxidative damage in multiple sclerosis lesions. Brain 2011; 134: 1914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L, Zrzavy T, Hametner S, Höftberger R, Bagnato F, Grabner G. et al. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain 2016; 139: 807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hametner S, Wimmer I, Haider L, Pfeifenbring S, Brück W, Lassmann H. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013; 74: 848–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy LM, Perron G, Won SY, Rao VT, Guiot MC, Moore C. et al. Differential transcriptional response profiles in human myeloid cell populations. Clin Immunol 2016; pii: S1521–6616(16)30065‐1; doi: 10.1016/j.clim.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglia cells of the CNS are bone marrow-derived and present antigen in vivo. Science 1988; 239: 1035–292. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK. et al. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 2013; 16: 1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M. et al. Expression of major histocompatibility complex class l molecules on the different cell types in multiple sclerosis lesions. Brain Pathol 2004; 14: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky. Physiology of microglia. Physiol Rev 2011; 91: 461–553 [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG. et al. Microglia emerge from erythromyeloid precursors via Pu. 1-and Irf8-dependent pathways. Nat Neurosci 2013; 16: 273–80. [DOI] [PubMed] [Google Scholar]
- Lane D, Merlot A, Huang MH, Bae DH, Jansson P, Sahni S. et al. Cellular iron uptake, trafficking and metabolism: key molecules and mechanisms and their roles in disease. Biochim Biophys Acta 2015; 1853: 1130–44. [DOI] [PubMed] [Google Scholar]
- Lassmann H. The architectures of active multiple sclerosis lesions. Neuropathol Appl Neurobiol 2011; 37: 698–710. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol 2007; 17: 210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia 1993; 7: 19–24. [DOI] [PubMed] [Google Scholar]
- Li H, Cuzner M, Newcombe J. Microglia‐derived macrophages in early multiple sclerosis plaques. Neuropathol Appl Neurobiol 1996; 22: 207–15. [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassman H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000; 47: 707–17. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Trebst C, Kivisäkk P, Staugaitis SM, Tucky B, Wei T. et al. Expression of chemokine receptors CCR1 and CCR5 reflects differential activation of mononuclear phagocytes in pattern II and pattern III multiple sclerosis lesions. J Neuropathol Exp Neurol 2004; 63: 262–73. [DOI] [PubMed] [Google Scholar]
- Marburg O. Die sogenannte akute multiple Sklerose. J Psychiatric Neurol 1906; 27: 1–100 [Google Scholar]
- Marik C, Felts PA, Bauer J, Lassmann H, Smith KJ. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain 2007; 130: 2800–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V, Pei W, Yang G, Li S, Swamy E, Boster A. et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS One 2013; 8: e57573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell-Robinson MA, Touil H, Healy LM, Owen DR, Durafourt BA, Bar-Or A. et al. Roles of microglia in brain development, tissue maintenance and repair. Brain 2015; 138: 1138–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Huang H, Radke J, Stenzel W, Priller J. P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia 2016; 65: 375–87. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 2007; 10: 1544–53. [DOI] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL. et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 2013; 16: 1211–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Ase AR, Kinsara A, Rao VT, Michell-Robinson M, Leong SY. et al. P2Y12 expression and function in alternatively activated human microglia. Neurol Neuroimmunol Neuroinflamm 2015; 2: e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mycko MP, Papoian R, Boschert U, Raine CS, Selmaj KW. cDNA microarray analysis in multiple sclerosis lesions: detection of genes associated with disease activity. Brain 2003; 126: 1048–57. [DOI] [PubMed] [Google Scholar]
- Peferoen LA, Vogel DY, Ummenthum K, Breur M, Heijnen PD, Gerritsen WH. et al. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J Neuropathol Exp Neurol 2015; 74: 48–63. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol 2010; 6: 193–201. [DOI] [PubMed] [Google Scholar]
- Piddlesden S, Lassmann H, Zimprich F, Morgan B, Linington C. The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol 1993; 143: 555–64. [PMC free article] [PubMed] [Google Scholar]
- Prineas J, Barnard R, Kwon E, Sharer L, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol 1993; 33: 137–51. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL. et al. Immunopathology of secondary‐progressive multiple sclerosis. Ann Neurol 2001; 50: 646–57. [DOI] [PubMed] [Google Scholar]
- Prinz M, Tay TL, Wolf Y, Jung S. Microglia: unique and common features with other tissue macrophages. Acta Neuropathol 2014; 128: 319–31. [DOI] [PubMed] [Google Scholar]
- Raine C, Scheinberg L, Waltz J. Multiple sclerosis. Oligodendrocyte survival and proliferation in an active established lesion. Lab Invest 1981; 45: 534–46. [PubMed] [Google Scholar]
- Raine CS. Multiple sclerosis: the resolving lesion revealed. J Neuroimmunol 2017; 304: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 2016; 19: 987–91. [DOI] [PubMed] [Google Scholar]
- Satoh Ji, Kino Y, Asahina N, Takitani M, Miyoshi J, Ishida T. et al. TMEM119 marks a subset of microglia in the human brain. Neuropathology 2016; 36: 39–49. [DOI] [PubMed] [Google Scholar]
- Schuh C, Wimmer I, Hametner S, Haider L, Van Dam AM, Liblau RS. et al. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol 2014; 128: 247–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam N, Vroom TM, Peters P, Pastoors E, Ploegh H. HLA-A-and HLA-B-specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin-fixed, paraffin-embedded tissue sections and in cryo-immuno-electron microscopy. Int Immunol 1990; 2: 113–25. [DOI] [PubMed] [Google Scholar]
- Trebst C, Sørensen TL, Kivisäkk P, Cathcart MK, Hesselgesser J, Horuk R. et al. CCR1+/CCR5+ mononuclear phagocytes accumulate in the central nervous system of patients with multiple sclerosis. Am J Pathol 2001; 159: 1701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum D, Hilbert S, Strassenburg S, Hanisch Uk, Brück W. Myelin‐phagocytosing macrophages in isolated sciatic and optic nerves reveal a unique reactive phenotype. Glia 2008; 56: 271–83. [DOI] [PubMed] [Google Scholar]
- Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P. et al. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation 2013; 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Lue LF. Immune phenotypes of microglia in human disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther 2015; 7: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney LW, Becker KG, Tresser NJ, Caballero-Ramos CI, Munson PJ, Prabhu VV. et al. Analysis of gene expression in multiple sclerosis lesions using cDNA microarrays. Ann Neurol 1999; 46: 425–8. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang ZY, Schittenhelm J, Wu Y, Meyermann R, Schluesener HJ. Parenchymal accumulation of CD163+ macrophages/microglia in multiple sclerosis brains. J Neuroimmunol 2011; 237: 73–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.