The cellular origins of a precancerous condition called Barrett's oesophagus have been unclear. Tracking and analysis of epithelial cells at the affected site could shed light on the problem. See Letter p.529
A precancerous condition called Barrett's oesophagus is of much interest, because it arises when some cells of the oesophagus take on a new identity — a phenomenon known as metaplasia. If we could understand where these altered cells come from, we might hold the key to preventing oesophageal cancer. On page 529, Jiang et al.1 provide fresh insight into the cell of origin for Barrett's oesophagus.
Epithelial cells, which line the body's surfaces and cavities, can be organized into a multi-layered tissue called squamous epithelium (as in the oesophagus and skin) or a single layer of columnar cells (as in the stomach and intestine). During embryonic development, conversion between epithelial-cell types is expected, as tissues adopt specialized functions. After birth, epithelial organization becomes more fixed. However, there are several pathological conditions in which a patch of adult squamous tissue becomes columnar epithelium or vice versa 2. Although the resulting metaplastic tissue is itself benign, it can be prone to precancerous changes.
One might imagine that identifying the cell type from which metaplasia arises would be easy. But in the case of Barrett's oesophagus, metaplasia occurs at the gastro-oesophageal junction (GEJ) — the boundary between oesophageal and gastric epithelial cells. The condition is characterized by replacement of the oesophageal epithelium with a mosaic of gastric- and intestinal-like columnar cells, along with mucus-producing intestinal-like goblet cells 3,4. The condition's origins have been a matter of rigorous debate.
Several theories have been proposed 3. First, oesophageal squamous cells might undergo direct conversion to metaplastic cells. Second, metaplastic cells might originate from submucosal glands that lie beneath the squamous epithelium. Third, gastric cells that have stem-cell-like properties might extend up into the oesophagus. Fourth, a small population of embryonic cells that persists into adulthood and gives rise to columnar cells might expand. Finally, the condition might develop from a multi-layered tissue called a transitional epithelium, found at the GEJ, that has both squamous and columnar features. The transitional epithelium has been seen in people with Barrett's oesophagus 5, but is not always clearly visible in healthy tissues, as would be expected if it were the site of origin for this condition.
Jiang et al. provide evidence to support the idea that the transitional epithelium is innate and gives rise to metaplasia. The authors showed that differential expression of three protein markers — the cytokeratins KRT5 and KRT7, and the transcriptional regulator p63 — distinguishes cell types in the mouse GEJ. They found that the healthy transitional epithelium consisted of two cell types: a basal (bottom) layer expressing all three markers; and a luminal layer lining the oesophagus that expressed only KRT7. The cells in this region were distinct from those of the neighbouring oesophageal epithelium, which expressed KRT5 but not KRT7, and the gastric epithelium, which expressed none of the proteins (Fig. 1). KRT7, which is expressed in cells of Barrett's oesophagus in humans 6, is thus specific to the transitional epithelium.
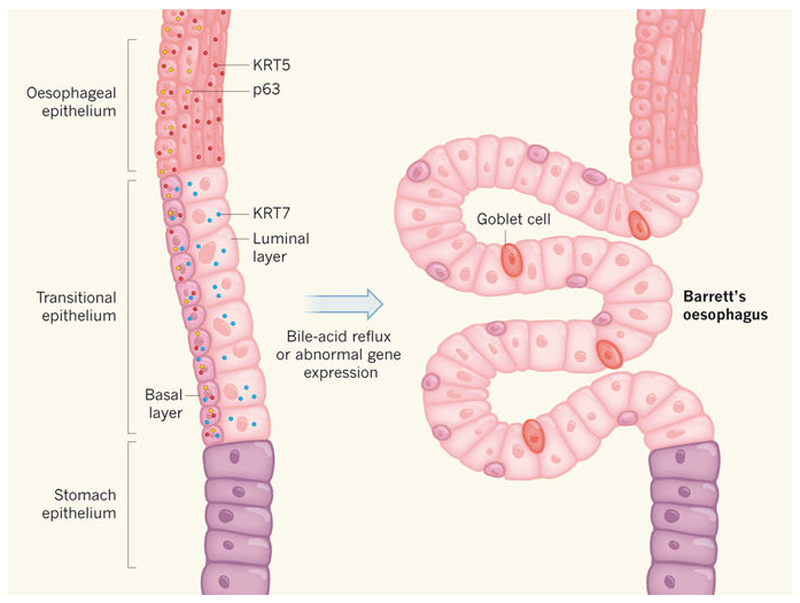
Figure 1. Dissection of epithelial-cell types in the oesophagus.
The gastro-oesophageal junction between the oesophagus and stomach of mammals is composed of various types of epithelial cell. Jiang et al.1 describe a transitional epithelial zone between the oesophagus and stomach, and show that the basal and luminal layers of this region, and the epithelia around them, are characterized by differential expression of three marker proteins (KRT5, KRT7 and p63; depicted only on the left-hand side, for simplicity). The authors provide evidence in mice and humans that bile-acid reflux or abnormal gene expression can cause the transitional epithelium to undergo abnormal expansion to form a precancerous tissue containing mucus-secreting goblet cells — a condition called Barrett's oesophagus. (Figure adapted from epithelial graphics in ref. 1.)
Next, Jiang and colleagues surgically redirected bile acid to the oesophagus of mice to mimic severe reflux, which is a major risk factor for Barrett's oesophagus 7. This led to expansion of the transitional epithelium, which contained epithelial cells expressing the protein CDX2 and goblet cells — both markers of Barrett's oesophagus 8. By contrast, the neighbouring squamous oesophagus, which was also exposed to bile-acid reflux, contained neither cell type.
So it seems that there is a link between the transitional epithelium and Barrett's oesophagus. The authors' next step was to exclude the possibility that cells from the neighbouring epithelia migrate to the GEJ, acquire KRT7 expression and transform into Barrett's oesophagus cells.
Jiang et al. used a genetic technique to generate mice in which KRT7-expressing cells of the transitional epithelium were indelibly labelled with a fluorescent protein, so that they and all their descendants fluoresced. The authors labelled the cells at birth. After 14 days, all the cells in the transitional epithelium — but none in the surrounding tissues — were marked. Therefore, formation and maintenance of this tissue is independent of the oesophagus and stomach. The authors also demonstrated that forced expression of CDX2 in both the transitional epithelium and oesophagus led to metaplasia arising from the transitional epithelium only, further suggesting that this tissue is an origin for Barrett's oesophagus.
How relevant are these findings to human disease? Jiang et al. examined the human GEJ and found that the transitional epithelium expressed the same marker proteins as in mice. To better study the human tissue, the authors turned to organoids — three-dimensional in vitro 'mini-organs', which maintain the biological features of their tissue of origin. The researchers grew organoids from KRT7-expressing cells isolated from a normal human GEJ, and induced expression of CDX2 in the cells. These organoids gave rise to metaplastic cells similar to those seen in Barrett's oesophagus. By contrast, organoids formed by cells that did not express KRT7 — presumably derived from oesophageal epithelium — did not give rise to metaplastic cells, even after CDX2 overexpression.
Jiang and co-workers' findings are important on two levels. First, they imply that the transitional epithelium arises during embryonic development, and is distinct from the oesophagus and stomach (at least in mice). Second, the transitional epithelium seems to be more susceptible than the oesophageal and gastric epithelia to metaplasia following environmental damage or genetic manipulation.
The work also raises further questions. For instance, it is not clear what triggers abnormal CDX2 expression in the transitional epithelium. Another human organoid study 9 suggested that mutations gradually accumulate in adult stem cells, and it is possible that the transitional epithelium contains stem cells that are mutation-prone. Indeed, the cells of Barrett's oesophagus are highly mutated 10. The mutational landscape of the transitional epithelium remains unknown, and this should be investigated in the future.
In addition, the authors did not exclude the possibility that their KRT7-expressing organoids originated from cells of the submucosal glands. It has been reported 11 that patients in whom the transitional epithelium has been removed can still acquire Barrett's oesophagus or a metaplasia resembling the condition.
Nonetheless, Jiang and colleagues' comprehensive characterization of the transitional epithelium sheds some light on the possible origin of Barrett's oesophagus. The stage is set to investigate whether the transitional epithelium is the sole origin of the condition, and what role this tissue has in the progression to oesophageal cancer.
References
- 1.Jiang M, et al. Nature. 2017;550:529–533. doi: 10.1038/nature24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giroux V, Rustgi AK. Nature Rev Cancer. 2017;17:594–604. doi: 10.1038/nrc.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald SA, Lavery D, Wright NA, Jansen M. Nature Rev Gastroenterol Hepatol. 2015;12:50–60. doi: 10.1038/nrgastro.2014.181. [DOI] [PubMed] [Google Scholar]
- 4.Spechler SJ, Souza RF. N Engl J Med. 2014;371:836–845. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 5.Glickman JN, Chen YY, Wang HH, Antonioli DA, Odze RD. Am J Surg Pathol. 2001;25:569–578. doi: 10.1097/00000478-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Cabibi D, et al. Med Sci Monit. 2009;15:CR203–CR210. [PubMed] [Google Scholar]
- 7.Coleman HG, Xie S-H, Lagergren J. Gastroenterology. 2017 doi: 10.1053/j.gastro.2017.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Phillips RW, Frierson HF, Moskaluk CA. Am J Surg Pathol. 2003;27:1442–1447. doi: 10.1097/00000478-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Blokzijl F, et al. Nature. 2016;538:260–264. doi: 10.1038/nature19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross-Innes CS, et al. Nature Genet. 2015;47:1038–1046. doi: 10.1038/ng.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton SR, Yardley JH. Gastroenterology. 1977;72:669–675. [PubMed] [Google Scholar]