Abstract
Background
Studies have demonstrated relationships between polyunsaturated fatty acids (PUFAs) and adiposity. It is unclear whether PUFAs in pregnancy have an effect on maternal weight retention after childbirth, which can contribute to long-term obesity.
Objective
We examined the association of maternal plasma PUFAs in pregnancy with 18 months postpartum weight retention (PPWR) in a multi-ethnic Asian cohort.
Design
We studied pregnant women (n=653) recruited between June 2009 and September 2010 from a prospective cohort. At 26-28 weeks’ gestation, plasma phosphatidylcholine PUFA concentrations were measured and determined as percentages of total fatty acids. PPWR was calculated based on the difference between measured weight at the first antenatal clinic visit and 18 months postpartum.
Results
The medium retained weight of women was 0.90 kg (interquartile range -1.40, 3.25) at 18 months postpartum. Of 653 women, 544 (83.3%) women had PPWR<5 kg and 109 (16.7%) had PPWR≥5 kg. In adjusted linear regression models, higher plasma eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and total omega-3 PUFAs were associated with lower PPWR [EPA: β = -0.62 kg per % increase of total fatty acids (95% CI -1.18, -0.05); DHA: β = -0.24 kg per % increase (95% CI -0.45, -0.02); total omega-3 PUFAs: β = -0.20 kg per % increase (95% CI -0.36, -0.03)], while higher plasma omega-6/omega-3 PUFA ratio was associated with higher PPWR [β = 0.21 kg per unit increase (95% CI 0.05, 0.36)].
Conclusions
Higher plasma percentages of omega-3 PUFAs and a lower ratio of omega-6 to omega-3 PUFAs in the late-second trimester of pregnancy are associated with less weight retention at 18 months postpartum. This may offer an alternative strategy to assist postpartum weight reduction by increasing EPA and DHA status, together with decreased omega-6 to omega-3 PUFA ratio through diet or fish oil supplementation during pregnancy.
Keywords: adiposity, obesity, polyunsaturated fatty acids, postpartum weight, pregnancy
Introduction
Obesity prevalence continues to increase and obesity is a growing burden in women of childbearing age (1). Many women attribute substantial weight gain and fat deposition to childbearing (2). Pregnancy is a life stage which can potentially affect future weight gain trajectory (1, 2). An increase in body weight following pregnancy or postpartum weight retention (PPWR) has been reported as a risk factor predisposing women to obesity and related long-term adverse health outcomes (1).
PPWR is referred to the average weight change from preconception until a time point after delivery (1, 3). It includes the weight gain during gestation (preconception through gestation), early postpartum weight loss (delivery to 6 weeks postpartum) and later postpartum weight changes (after 6 weeks until weight prior to next pregnancy) (1, 3). Retaining weight of at least 5 kg above preconception weight at one to two years postpartum is considered to be substantial PPWR (1, 3). PPWR appears to be more physiologically harmful than weight gain during other life periods as the retained body fat is preferentially deposited in central rather than in peripheral sites, thus increasing risk for development of metabolic and cardiovascular disease (1).
Recently, there is growing interest on the role of omega-3 (n-3) and omega-6 (n-6) polyunsaturated fatty acids (PUFAs) in adiposity development. Both short-chain [i.e. α-linolenic acid (ALA)] and long-chain n-3 PUFAs [i.e. eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] have been shown to reduce adiposity in animal feeding studies (4, 5), but evidence from human studies has been less consistent. While some observational studies have reported that n-3 PUFA levels are inversely associated with body weight or fat mass (6, 8), other studies conducted among Canadian Inuit and Cree Indian populations have shown n-3 PUFA levels to be associated with increased abdominal obesity (9, 10). Dietary supplementation studies have also provided conflicting findings, with some studies reporting weight/fat loss after n-3 PUFA supplementation (11–13), and others reporting no effect on body weight/fat (14–17). While n-6 PUFAs [i.e. linoleic acid (LA) and arachidonic acid (AA)] have been shown to stimulate adipogenesis in animal studies (18, 19), there is no clear link with obesity in human epidemiological studies. There has been a large increase in the n-6/n-3 PUFA ratio of the diet from an estimated 1:1 earlier in human evolution to 16:1 or even higher today (20). A raised n-6/n-3 PUFA ratio has been associated with increased risk of obesity in humans (20).
A few studies have investigated maternal PUFAs in relation to infant body weight at birth but none examined association with body weight of mothers (21). In this study, we examined the associations of maternal plasma PUFA concentrations during pregnancy with PPWR. We hypothesized that higher maternal plasma concentrations of n-3 PUFAs and lower n-6/n-3 PUFA ratio in the late-second trimester of pregnancy would be associated with decreased 18 months PPWR.
Methods
Study design and participants
Data were drawn from the Growing Up in Singapore Towards healthy Outcomes (GUSTO) prospective cohort study (www.clinicaltrials.gov, NCT01174875) (22). This study was conducted according to the guidelines laid down in the Helsinki Declaration. Ethical approval was obtained from the Domain Specific Review Board of Singapore National Healthcare Group (reference D/09/021) and the Centralised Institutional Review Board of SingHealth (reference 2009/280/D).
Pregnant women attending antenatal care (<14 weeks’ gestation) from June 2009 to September 2010 in KK Women’s and Children’s Hospital or National University Hospital were recruited. These women were aged at least 18 years and had homogeneous parental ethnic groups (Chinese, Malay or Indian). Women who became pregnant again before 18 months postpartum were excluded from this analysis. Informed written consent was obtained from all women.
Data collection
Detailed interviews and measurements were conducted in the clinics at recruitment and at 26-28 weeks’ gestation. Data on socioeconomic status, educational attainment, obstetric history, smoking status, physical activity and fish oil supplementation were collected. Smoking exposure was defined as current smoking or exposed to second hand smoke at home and/or at work on a daily basis. Physical activity during pregnancy was assessed using a structured interviewer-administered questionnaire which was designed based on three types of activities: light-moderate, moderate and vigorous intensity activities. Examples for each type of activity were provided to help women to recall their activities or exercise in the past 6 months. Total score of physical activity was computed from the summation of the duration (in minutes) and frequency (days) of these activities, which was expressed in metabolic equivalent task (MET-minutes/week) (23, 24).
Data on mode of infant feeding were collected through interviewer-administered questionnaires at 3 months and 6 months postpartum. At each interview, women were asked to classify the types of infant feeding, i.e. 1) exclusive/predominant (only breast milk and water is given), 2) partial (mixture of breast milk and formula milk is given) or 3) no breastfeeding (only formula milk is given). In this analysis, we defined ‘breastfeeding’ as those women who fed their infants via method 1 for the entire first 6 months postpartum; ‘formula feeding’ as those women who fed their infants via method 3 for the entire first 6 months postpartum; ‘mixed breastfeeding’ as those women who fed their infants via method 2 or those who did not meet the criteria for ‘breastfeeding’ and ‘formula feeding’ in the first 6 months postpartum (25).
Dietary assessments
A 24-hour dietary recall was administered face-to-face by trained clinical staff at 26-28 weeks’ gestation using the 5-stage, multiple-pass interviewing technique (26). Standardized household measuring utensils and food pictures of various portion sizes were used to assist women in quantifying their food and beverage intakes. Total daily energy intake was assessed using a nutrient analysis software (Dietplan, Forestfield Software) with a food composition database of locally available foods (27). For food items not found in the database, nutrient information was obtained from either food labels or the United States Department of Agriculture (USDA) national nutrient database (28).
Anthropometric measurements
Maternal height was measured to the nearest 0.1 cm using a Seca 213 Portable Stadiometer (SECA, Hamburg, Germany) at 26-28 weeks’ gestation. Self-reported pre-pregnancy weight and measured weight at the first antenatal visit (≤14 weeks of gestation) were collected. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). Since maternal BMI at the first antenatal visit was strongly correlated with pre-pregnancy BMI (r=0.96, p<0.001) and without subject to recall bias, it was used for analyses in this study. Serial measurements of maternal weight throughout pregnancy were collected from the medical records. Linear mixed-effects model with the Best Linear Unbiased Predictor was used to estimate linear trajectory of gestational weight gain (GWG) per week between 15 to 35 weeks’ gestation for each individual (29). Total GWG was not computed as not all women had weight data near to their delivery (within four weeks of delivery). Maternal weights at ≤14 weeks’ gestation and 18 months postpartum were measured to the nearest 0.1 kg using an electronic weighing scale (SECA, Hamburg, Germany). PPWR was calculated as the difference between measured weight at ≤14 weeks’ gestation and measured weight at 18 months postpartum.
Plasma glucose and plasma phosphatidylcholine (PC) fatty acids analyses
At 26-28 weeks’ gestation, maternal fasting blood samples were collected for plasma glucose and PUFAs analyses. At the same visit, women underwent a 75-g Oral Glucose Tolerance Test for the diagnosis of gestational diabetes mellitus (GDM). Plasma glucose concentrations at 0 and 120 minutes following the oral glucose load were measured by colorimetry [Advia 2400 Chemistry system (Siemens Medical Solutions Diagnostics) and Beckman LX20 Pro analyser (Beckman Coulter)]. GDM was diagnosed according to the 1999 World Health Organization criteria: ≥7.0 mmol/l for fasting glucose and/or ≥7.8 mmol/l for 2-hour post-glucose (30).
Analysis of plasma PC fatty acids has been described elsewhere (31). Briefly, lipid extraction was carried out with chloroform/methanol (Fisher Scientific) and PC was separated by solid-phase extraction. After purification and extraction, PC fatty acid methyl esters were separated by gas chromatography (BPX-70 column mounted on a Hewlett-Packard HP6890) and detected by flame ionization. Plasma PC concentrations of fatty acids were expressed as percentages of total fatty acids. For all fatty acids identified in plasma PC, inter- and intra-assay variation coefficients were lower than 6% and 3%, respectively. In this study, we examined the percentages of ALA, EPA, DHA, LA, AA, total n-3 PUFAs, total n-6 PUFAs and n-6/n-3 PUFA ratio.
Statistical analysis
Categorical data are presented as frequencies and percentages, while continuous data are presented as means and standard deviations. Comparisons between maternal characteristics and PPWR were performed using Pearson’s Chi-square test for categorical variables and independent t-test for continuous variables. Multiple regression analysis was performed to examine the association between individual maternal plasma PC PUFA and PPWR in continuous form. Binary logistic regression analysis was performed to examine the association between individual maternal plasma PC PUFA and PPWR in categorical form. Normal and substantial PPWR were defined as <5 kg and ≥5 kg respectively.
In the main adjusted model, we controlled for maternal age, education, ethnicity, parity, GDM, physical activity, total energy intake, smoking exposure during pregnancy and early pregnancy BMI, which were selected a priori based on literature review (1, 2, 3). We additionally controlled for GWG per week and mode of infant feeding in the main adjusted model. These two factors were not included in our main adjusted model as they may be in the causal pathway between maternal PUFAs (measured at mid-gestation) and PPWR, which could result in over-adjustment, but additional analyses were conducted to examine for any potential mediating effect. To examine the contributing role of fish oil supplementation on maternal PUFA status in relation to PPWR, we further adjusted for maternal fish oil supplementation.
PPWR was computed based on the change score (post minus baseline score), which has the advantage of being more intuitive to interpret than an absolute value. The use of a change score as a dependent variable in regression analysis without adjusting for baseline score as a covariate is equivalent to assuming that the coefficient of regressing post-score (dependent variable) upon baseline score is 1 (32). This assumption of coefficient = 1 is unrealistic because higher baseline score tends to associate with a lower change score. Analysis of change score with adjustment for baseline score as a covariate removes this unrealistic assumption (32). Hence, baseline BMI which serves a function similar to baseline weight was adjusted.
Missing values for maternal education (n=7), gestational diabetes (n=35), gestational weight gain per week (n=64), physical activity (n=10), smoking exposure (n=2), total daily energy intake (n=7), fish oil supplement intake (n=57) and mode of feeding (n=38) were imputed 100 times using multiple imputation analyses by chained equation (33). The results of the 100 analyses were pooled using the Rubin's rule (34). A sensitivity analysis was performed by only including women with complete dataset for all covariates (n=508). All point estimates were presented with 95% confidence intervals (CI). All statistical analyses were performed using IBM SPSS statistics, Version 19 (USA) or StataCorp Stata Statistical Software, Release 13 (USA).
Results
Participant characteristics
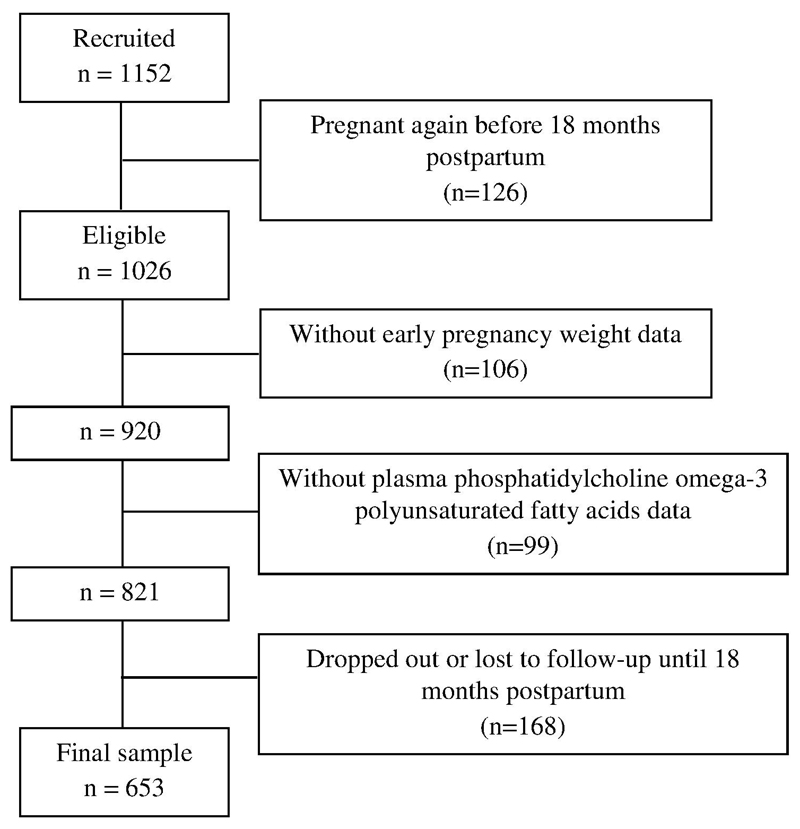
Of 1152 enrolled women with singleton pregnancies, 126 (10.9%) women became pregnant again before 18 months postpartum, leaving 1026 (89.1%) women who were eligible for this study. Of those, 920 women had early pregnancy weight data and 821 had an adequate volume of plasma for analysis of PC-PUFAs. At 18 months postpartum, 168 women were lost to follow-up or missed their 18 month visit. A final sample of 653 (63.6%) women was included in the present analysis (Figure 1). In comparison to excluded women (n=373, 36.4%), those included were found to: be older (P<0.001), be multiparous (P=0.001), be less likely exposed to cigarette smoke during pregnancy (P=0.005) and have ‘breastfeeding’ or ‘mixed feeding’ practice (P=0.011). No statistically significant differences in maternal characteristics were observed for education, ethnicity, GDM, early pregnancy BMI, GWG per week, physical activity, total daily energy intake and fish oil supplement intake between included and excluded women.
Figure 1.
Flowchart of women included for analysis in the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study, Singapore.
Table 1 shows the characteristics of women categorized by normal and substantial PPWR. The median PPWR for all women (n=653) was 0.90 kg (interquartile range -1.40, 3.25); 544 (83.3%) women had normal PPWR (median 0.30 kg, interquartile range -1.20, 1.95) and 109 (16.7%) had substantial PPWR (median 7.00 kg, interquartile range 5.63, 8.80). Women with substantial PPWR were younger (P<0.001), were more likely to belong to the Malay or Indian ethnic group (P=0.002), were primiparous (P<0.001), were less likely to have GDM (P<0.001), had higher early pregnancy BMI (P=0.003), had higher GWG per week (P<0.001) and had a lower tendency to take fish oil supplement during pregnancy (P=0.012). There were no differences between the groups with regard to education levels, physical activity levels, smoking exposure, total daily energy intake and mode of feeding. In comparison to women with normal PPWR, women with substantial PPWR had lower plasma PC percentages of EPA (P<0.001), DHA (P=0.020), total n-3 PUFAs (P=0.001) and AA (P=0.024), but higher plasma PC n-6/n-3 PUFA ratio (P=0.012). Plasma PC percentages of ALA, LA, and total n-6 PUFAs were not different between the two groups of women.
Table 1.
Descriptive characteristics of women1
| Variable | Total (n=653) |
PPWR <5 kg (n=544) |
PPWR ≥5kg (n=109) |
P |
|---|---|---|---|---|
| PPWR (kg), median (IQR) | 0.90 (-1.40 – 3.25) | 0.30 (-1.20 – 1.95) | 7.00 (5.63 – 8.80) | <0.001 |
| Maternal age (years) | 31.18 ± 5.19 | 31.50 ± 5.16 | 29.56 ± 5.12 | <0.001 |
| Education, n (%) | 0.961 | |||
| None/ Primary/ Secondary | 436 (66.8) | 363 (66.7) | 73 (67.0) | |
| University and above | 217 (33.2) | 181 (33.3) | 36 (33.0) | |
| Ethnicity, n (%) | 0.002 | |||
| Chinese | 359 (55.0) | 316 (58.1) | 43 (39.4) | |
| Malay | 169 (25.9) | 130 (23.9) | 39 (35.8) | |
| Indian | 125 (19.1) | 98 (18.0) | 27 (24.8) | |
| Parity, n (%) | <0.001 | |||
| 0 | 236 (36.1) | 169 (31.1) | 67 (61.5) | |
| ≥1 | 417 (63.9) | 375 (68.9) | 42 (38.5) | |
| Gestational diabetes, n (%) | <0.001 | |||
| No | 533 (81.6) | 431 (79.2) | 102 (93.6) | |
| Yes | 120 (18.4) | 113 (20.8) | 7 (6.4) | |
| Early pregnancy body mass index (kg/m2) | 23.62 ± 4.45 | 23.39 ± 4.36 | 24.75 ± 4.73 | 0.003 |
| Gestational weight gain per week (kg/week) | 0.47 ± 0.12 | 0.46 ± 0.12 | 0.53 ± 0.13 | <0.001 |
| Physical activity (MET-min/week), n (%) | 0.240 | |||
| Not highly active (<3000) | 531 (81.3) | 438 (80.5) | 93 (85.3) | |
| Highly active (≥3000) | 122 (18.7) | 106 (19.5) | 16 (14.7) | |
| Smoking exposure, n (%) | 0.075 | |||
| No | 368 (56.4) | 315 (57.9) | 53 (48.6) | |
| Yes | 285 (43.6) | 229 (42.1) | 56 (51.4) | |
| Total daily energy intake (kcal) | 1889 ± 595 | 1885 ± 590 | 1907 ± 625 | 0.723 |
| Fish oil supplement intake, n (%) | 0.012 | |||
| No | 372 (57.0) | 298 (54.8) | 74 (67.9) | |
| Yes | 281 (43.0) | 246 (45.2) | 35 (32.1) | |
| Mode of infant feeding, n (%) | 0.136 | |||
| Breastfeeding | 88 (13.5) | 79 (14.5) | 9 (8.3) | |
| Mixed feeding | 406 (62.2) | 338 (62.2) | 68 (62.3) | |
| Formula feeding | 159 (24.3) | 127 (23.3) | 32 (29.4) | |
| ALA (%) | 0.21 ± 0.14 | 0.21 ± 0.14 | 0.21 ± 0.13 | 0.625 |
| EPA (%) | 0.70 ± 0.57 | 0.73 ± 0.60 | 0.53 ± 0.35 | <0.001 |
| DHA (%) | 4.74 ± 1.45 | 4.80 ± 1.47 | 4.45 ± 1.31 | 0.020 |
| Total Omega-3 (%) | 6.41 ± 1.89 | 6.51 ± 1.93 | 5.91 ± 1.64 | 0.001 |
| LA (%) | 21.74 ± 3.37 | 21.68 ± 3.41 | 22.04 ± 3.12 | 0.317 |
| AA (%) | 7.92 ± 1.73 | 7.98 ± 1.77 | 7.57 ± 1.50 | 0.024 |
| Total Omega-6 (%) | 34.20 ± 3.38 | 34.19 ± 3.43 | 34.22 ± 3.15 | 0.923 |
| Omega-6:Omega-3 ratio | 5.86 ± 2.01 | 5.77 ± 1.99 | 6.30 ± 2.07 | 0.012 |
Means ± standard deviations are shown, unless otherwise stated. Chi-square tests for categorical variables and independent-samples t tests for continuous variables were used to compare the 2 groups. AA, arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IQR, interquartile range; LA, linoleic acid; MET, Metabolic equivalent task; PPWR, postpartum weight retention.
Plasma PC PUFA concentrations and PPWR
Table 2 shows the linear regression models of individual maternal plasma PC concentration of PUFAs with 18 months PPWR. After adjustment for confounders (Model 2), plasma PC EPA, DHA and total n-3 PUFAs during pregnancy were inversely associated with PPWR [EPA: β= -0.62 kg (95% CI -1.18, -0.05); DHA: β= -0.24 kg (95% CI -0.45, -0.02); total n-3 PUFAs: β= -0.20 kg (95% CI -0.36, -0.03)], while plasma PC ratio of n-6/n-3 PUFAs was positively associated with PPWR [β= 0.21 kg (95% CI 0.05, 0.36)]. No associations were seen for ALA, or individual and total n-6 PUFAs. When adjustments for GWG per week and mode of feeding were conducted in the additional analyses (Model 3), the degree of associations remained similar. When further adjustment was made for fish oil supplementation (Model 4), there was generally an attenuation of the associations between maternal plasma PC PUFAs and 18 months PPWR. Effect sizes of PPWR were reduced by 20 to 25% for plasma PC EPA, DHA, total n-3 PUFAs and ratio of n-6/n-3 PUFAs, indicating fish oil supplementation contributed to approximately one-quarter of plasma PC n-3 PUFAs.
Table 2.
Maternal plasma phosphatidylcholine PUFAs in pregnancy and PPWR at 18 months (n=653)1
| Omega-3 PUFAs (%) | PPWR (kg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||||||
| β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | β | 95% CI | P | |
| ALA | 0.32 | -1.95, 2.60 | 0.781 | 0.19 | -2.00, 2.39 | 0.863 | 0.03 | -2.16, 2.21 | 0.981 | 0.12 | -2.06, 2.29 | 0.915 |
| EPA | -0.99 | -1.55, -0.43 | 0.001 | -0.62 | -1.18, -0.05 | 0.032 | -0.64 | -1.19, -0.09 | 0.024 | -0.49 | -1.05, 0.08 | 0.091 |
| DHA | -0.26 | -0.48, -0.04 | 0.021 | -0.24 | -0.45, -0.02 | 0.030 | -0.24 | -0.46, -0.03 | 0.026 | -0.19 | -0.40, 0.03 | 0.090 |
| Total Omega-3 | -0.25 | -0.42, -0.08 | 0.004 | -0.20 | -0.36, -0.03 | 0.022 | -0.21 | -0.37, -0.04 | 0.013 | -0.15 | -0.32, 0.02 | 0.079 |
| LA | 0.05 | -0.04, 0.15 | 0.265 | 0.02 | -0.07, 0.11 | 0.698 | 0.01 | -0.09, 0.10 | 0.958 | 0.02 | -0.07, 0.12 | 0.617 |
| AA | -0.14 | -0.32, 0.05 | 0.145 | -0.03 | -0.22, 0.16 | 0.763 | -0.01 | -0.19, 0.19 | 0.990 | -0.04 | -0.23, 0.15 | 0.710 |
| Total Omega-6 | 0.06 | -0.03, 0.16 | 0.198 | 0.05 | -0.04, 0.14 | 0.289 | 0.04 | -0.06, 0.13 | 0.465 | 0.05 | -0.05, 0.14 | 0.345 |
| Omega-6:Omega-3 ratio | 0.24 | 0.08, 0.40 | 0.003 | 0.21 | 0.05, 0.36 | 0.011 | 0.20 | 0.05, 0.36 | 0.011 | 0.16 | 0.01, 0.32 | 0.042 |
Data were analyzed using multiple linear regressions. Values are presented in regression coefficients (β) and 95% confidence intervals (CIs). Model 1 was unadjusted; Model 2 was adjusted for maternal age, education, ethnicity, parity, gestational diabetes, early pregnancy body mass index, physical activity, smoking exposure and total energy intake; Model 3 was adjusted as for model 2 and additionally adjusted for gestational weight gain per week and mode of infant feeding; Model 4 was adjusted as for model 2 and additionally adjusted for fish oil supplementation. AA, arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; CI, confidence interval; PPWR, postpartum weight retention; PUFAs, polyunsaturated fatty acids.
Table 3 shows the logistic regression models of individual maternal plasma PC PUFA concentration with risk of retaining substantial postpartum weight. As indicated in Model 2, women with higher plasma PC EPA, DHA and total n-3 PUFAs during pregnancy had lower likelihood of retaining at least 5kg weight at 18 months postpartum after confounders adjustment [EPA: odds ratio (OR)= 0.48 (95% CI 0.26, 0.91); DHA: OR= 0.84 (95% CI 0.71, 0.99); total n-3 PUFAs: OR= 0.84 (95% CI 0.75, 0.98)]. The associations of plasma PC AA and n-6/n-3 ratio with risk of retaining substantial postpartum weight were attenuated after adjustment for confounders. Plasma PC concentrations of ALA, LA and total n-6 PUFAs were not significantly associated with PPWR in the adjusted models.
Table 3.
Maternal plasma phosphatidylcholine PUFAs in pregnancy and risk of PPWR ≥5kg at 18 months (n=653)1
| Omega-3 PUFAs (%) | PPWR (≥5 kg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| ALA | 1.42 | 0.35, 5.82 | 0.624 | 1.58 | 0.34, 7.34 | 0.563 | 1.58 | 0.32, 7.71 | 0.575 | 1.43 | 0.30, 6.73 | 0.651 |
| EPA | 0.37 | 0.20, 0.67 | 0.001 | 0.48 | 0.26, 0.91 | 0.025 | 0.46 | 0.24, 0.89 | 0.020 | 0.53 | 0.28, 0.99 | 0.045 |
| DHA | 0.84 | 0.72, 0.97 | 0.020 | 0.84 | 0.71, 0.99 | 0.039 | 0.84 | 0.71, 0.99 | 0.043 | 0.86 | 0.73, 1.02 | 0.084 |
| Total Omega-3 | 0.83 | 0.74, 0.94 | 0.003 | 0.85 | 0.75, 0.98 | 0.020 | 0.85 | 0.74, 0.97 | 0.017 | 0.87 | 0.76, 0.99 | 0.048 |
| LA | 1.03 | 0.97, 1.10 | 0.317 | 1.01 | 0.94, 1.08 | 0.867 | 1.01 | 0.95, 1.09 | 0.703 | 1.01 | 0.95, 1.08 | 0.733 |
| AA | 0.86 | 0.75, 0.98 | 0.023 | 0.87 | 0.74, 1.02 | 0.089 | 0.88 | 0.74, 1.03 | 0.112 | 0.87 | 0.74, 1.02 | 0.076 |
| Total Omega-6 | 1.00 | 0.94, 1.07 | 0.923 | 0.98 | 0.92, 1.05 | 0.564 | 0.98 | 0.92, 1.05 | 0.627 | 0.98 | 0.91, 1.05 | 0.528 |
| Omega-6:Omega-3 ratio | 1.13 | 1.03, 1.24 | 0.013 | 1.10 | 0.98, 1.22 | 0.096 | 1.10 | 0.99, 1.23 | 0.087 | 1.08 | 0.96, 1.20 | 0.192 |
Data were analyzed using binary logistic regressions. Values are presented in odds ratios (OR) and 95% confidence intervals (CI). Model 1 was unadjusted; Model 2 was adjusted for maternal age, education, ethnicity, parity, gestational diabetes, early pregnancy body mass index, physical activity, smoking exposure and total energy intake; Model 3 was adjusted as for model 2 and additionally adjusted for gestational weight gain per week and mode of infant feeding; Model 4 was adjusted as for model 2 and additionally adjusted for fish oil supplementation. AA, arachidonic acid; ALA, α-linolenic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; CI, confidence interval; PPWR, postpartum weight retention; PUFAs, polyunsaturated fatty acids.
In sensitivity analyses based on women with complete dataset (n=508), results remained similar for the outcomes using PPWR as continuous (Supplemental Table 1) or categorical variable (Supplemental Table 2) in relation to plasma PC PUFA concentrations.
Discussion
In this multi-ethnic cohort, one in six women (16.7%) were found to have substantial weight retention (≥5 kg) at 18 months postpartum, with significantly higher rates found in Malay and Indian women compared to Chinese women. We showed that higher maternal plasma PC percentages of n-3 PUFAs, and a lower plasma PC n-6/n-3 PUFA ratio at 26-28 weeks’ gestation were significantly associated with lower 18 months PPWR, after adjusting for demographic and health covariates. This association was largely driven by EPA and DHA, rather than ALA. n-6 PUFAs such as LA, AA and total n-6 PUFAs were not related to PPWR. GWG per week and mode of infant feeding in the first 6 months postpartum did not seem to mediate the associations. Overall, our data suggests that increased maternal plasma long-chain n-3 PUFAs in pregnancy may play a role to reduce weight retention during the postpartum period.
Our findings are consistent with existing epidemiological studies (6, 7) and nutritional intervention trials using EPA and DHA supplementation (11–13) in non-pregnant populations, showing increased plasma EPA and DHA concentrations were associated with decreased body weight. A recent study involving 291 middle-aged women reported that erythrocyte concentrations of DHA and n-3 PUFA index (red blood cell EPA + DHA) were inversely associated with BMI, waist circumference and body fat (7). Another cross-sectional study revealed that higher plasma concentration of total n-3 PUFAs was associated with a healthier BMI in 124 men and women, aged 18-70 years (6). An experimental study in healthy participants has demonstrated a greater fat loss effect following supplementation with EPA and DHA for six weeks, compared with safflower oil (n-6 PUFA) supplementation (13). Additional effects of EPA-rich and DHA-rich diets or supplementation (e.g. fish or fish oil) on weight loss have also been observed in energy-restricted overweight or obese individuals (11–12). These findings suggest that long-chain n-3 PUFAs have a role in weight regulation. In contrast, some intervention studies indicate no relationship between n-3 PUFA supplementation and obesity (15, 16). This however, may be attributable to study power, confounding factors, n-3 PUFA dose, proportions of EPA and DHA used and inter-individual variations in the responses to fish oil supplements.
Multiple mechanisms have been proposed to explain the effects of long-chain n-3 PUFAs on obesity. In animal models, EPA and DHA have been shown to counteract obesity through suppression of hepatic lipogenesis (35), stimulation of fat oxidation (36) and enhancement of energy expenditure (37) which in turn could suppress fat synthesis and deposition. EPA and DHA may also reduce adiposity by improving gut health through reduction of oxidative stress and inflammation (38). An in vitro study found that EPA has greater anti-inflammatory effect than DHA in human adipose tissue (39), which is in accordance with our findings, showing plasma PC EPA had the most pronounced association with PPWR.
Despite several plausible biological mechanisms suggesting that ALA too may have anti-obesity effects (4), our data showed no statistically significant association with weight retention in postpartum women. This null finding is supported by two clinical studies, showing increased plasma ALA through chia seed supplementation had no influence on body weight in overweight adults (14, 17). A recent investigation revealed that ALA induced lipid redistribution away from the abdominal cavity, but did not decrease total body fat (40).
Epidemiologic studies on n-6 PUFAs and human obesity remain limited. In this study, no associations were observed between maternal plasma PC n-6 PUFAs and PPWR. In contrast, recent findings from the Women’s Health Study showed that erythrocyte concentrations of n-6 PUFAs were positively associated with weight gain over a period of 10 years in initially normal weight healthy women (8).
An increased n-6/n-3 ratio in our current diet, primarily due to a shift towards high use of vegetable oils, has been implicated in causing greater adipose tissue accumulation and as a potential contributor to the rise in obesity prevalence (20). A longitudinal study in 534 normal weight women showed that high n-6/n-3 PUFA ratio in red blood cell membrane phospholipids was associated with increased risk of weight gain (8). Though a positive association between plasma PC n-6/n-3 PUFA ratio and PPWR was observed in our study, this is most likely due to the high concentrations of n-3 PUFAs as no associations were noted between plasma PC n-6 PUFAs and PPWR.
We recognized and considered the following limitations. The measured weight at the first antenatal clinic visit was used as the baseline to compute PPWR which could underestimate postpartum weight retention. However, the first trimester weight has been shown to be a sufficiently accurate measurement to represent pre-pregnancy weight, where weight change is minimal at this stage (41). Weight at the end of pregnancy was not available which restricted our ability to confirm whether the association observed was mediated through excessive GWG. However, our current findings should remain valid since the adjustment for GWG per week did not attenuate the association between maternal plasma PC PUFAs and PPWR. Maternal body composition was not measured, so changes in fat mass and lean mass could not be examined. Plasma PC PUFAs were measured at a single point in time during pregnancy without assessment of any change over time. No measurements of body weight and no diet assessments were made in the 18 months follow-up period. Some differences in characteristics (i.e. age, parity, smoking exposure and mode of feeding) were noted between included and excluded women, which could introduce a potential selection bias that affected generalizability of the results to a wider population. However, we controlled for these variables in the statistical analysis. Additionally, our study recruited Asian participants which may limit the generalizability of our findings to populations of other ethnic groups.
The present findings should be interpreted cautiously. Recognising plasma concentrations of fatty acids are useful biomarkers to reflect habitual dietary intake (42), it is tempting to speculate that increased intakes of n-3 PUFAs (e.g. from fish or fish oil) during pregnancy may help to control weight retention after giving birth. However, we cannot exclude the possibility that lower plasma or dietary n-3 PUFAs and higher PPWR may both be secondary to other independent factors, such as poor quality diet or lack of health awareness in women. Nevertheless, our analysis included covariates like education that should reflect these factors. The measurements of n-3 and n-6 PUFAs were made in plasma PC, which is not as good a long term marker of PUFA status as erythrocyte membrane phospholipids. This may result in exposure misclassification and lead to underestimation of the true associations. Although various potential confounding factors were considered and adjusted in our analyses, there might be unadjusted or residual confounding factors that remain.
In summary, this study, to our knowledge, is the first demonstration that higher maternal plasma PC percentages of EPA, DHA and total n-3 PUFAs, and a lower n-6/n-3 PUFA ratio in the late-second trimester of pregnancy are associated with lesser weight retention at 18 months postpartum. At present, recommendations to reduce PPWR are mainly based on dietary energy restriction and increased physical activity (43, 44) which are less likely to be complied with by many women. Our results may offer an alternative strategy to assist postpartum weight reduction by increasing EPA and DHA status, together with decreased n-6/n-3 PUFA ratio through diet or fish oil supplementation during pregnancy. However, more nutritional intervention trials are required to confirm the effects of maternal dietary and plasma PUFAs in pregnancy as well as in the postpartum period on later weight regulation and metabolic outcomes.
Supplementary Material
2Supplemental Tables 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://ajcn.nutrition.org.
Acknowledgements
We would like to thank the GUSTO study group, which includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, George Seow Heong Yeo, Helen Chen, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Joanna D. Holbrook, Joshua J. Gooley, Kenneth Kwek, Krishnamoorthy Niduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Marielle V. Fortier, Mark Hanson, Mary Rauff, Mei Chien Chua, Michael Meaney, Mya Thway Tint, Neerja Karnani, Oon Hoe Teoh, P. C. Wong, Pratibha Agarwal, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Chin-Ying Stephen Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yiong Huak Chan and Yung Seng Lee.
The authors’ responsibilities were as follows - FY, Y-SC, KHT, LPCS: designed and led the GUSTO cohort study; SLL: designed the present study; SLL, MJHN, PCC, FMR, PN: performed data management and analysis; YBC: advised on the statistical analysis; SLL, MJHN, YBC, KMG, PCC, NL, MFFC and JKYC: interpreted the findings; SLL and MJHN: wrote the paper; SLL and JKYC had primary responsibility for the final content and all authors participated in the critical review, revision and approval of the final manuscript.
KMG, PCC and YSC report receiving reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG and YSC are part of an academic consortium that has received research funding from Abbott Nutrition, Nestle and Danone. Other authors declared no conflict of interest.
Footnotes
Supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. YBC is supported by the National Research Foundation, Singapore, under its Clinician Scientist Award (Award No. NMRC/CSA/0039/2012) administered by the Singapore Ministry of Health’s National Medical Research Council. KMG and PCC are supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre. KMG is supported by the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement n°289346. JKYC received salary support from the Ministry of Health’s National Medical Research Council, Singapore (NMRC/CSA/043/2012).
Abbreviations used: AA, arachidonic acid; ALA, α-linolenic acid; BMI, body mass index; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; CI, confidence interval; GDM, gestational diabetes mellitus; GUSTO, Growing Up in Singapore Towards healthy Outcomes; GWG, gestational weight gain; MET, metabolic equivalent task; PC, plasma phosphatidylcholine; PPWR, postpartum weight retention; PUFAs, polyunsaturated fatty acids.
This trial was registered at www.clinicaltrials.gov as NCT01174875.
References
- 1.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009;36:317–32. doi: 10.1016/j.ogc.2009.04.001. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011;94:1225–31. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 3.Oken E, Taveras EM, Popoola FA, Rich-Edwards JW, Gillman MW. Television, walking, and diet: associations with postpartum weight retention. Am J Prev Med. 2007;32:305–11. doi: 10.1016/j.amepre.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliva ME, Ferreira MR, Chicco A, Lombardo YB. Dietary Salba (Salvia hispanica L) seed rich in alpha-linolenic acid improves adipose tissue dysfunction and the altered skeletal muscle glucose and lipid metabolism in dyslipidemic insulin-resistant rats. Prostaglandins Leukot Essent Fatty Acids. 2013;89:279–89. doi: 10.1016/j.plefa.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Buckley JD, Howe PR. Long-chain omega-3 polyunsaturated fatty acids may be beneficial for reducing obesity-a review. Nutrients. 2010;2:1212–30. doi: 10.3390/nu2121212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micallef M, Munro I, Phang M, Garg M. Plasma n-3 Polyunsaturated Fatty Acids are negatively associated with obesity. Br J Nutr. 2009;102:1370–4. doi: 10.1017/S0007114509382173. [DOI] [PubMed] [Google Scholar]
- 7.Howe PR, Buckley JD, Murphy KJ, Pettman T, Milte C, Coates AM. Relationship between erythrocyte omega-3 content and obesity is gender dependent. Nutrients. 2014;6:1850–60. doi: 10.3390/nu6051850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Manson JE, Rautiainen S, Gaziano JM, Buring JE, Tsai MY, Sesso HD. A prospective study of erythrocyte polyunsaturated fatty acid, weight gain, and risk of becoming overweight or obese in middle-aged and older women. Eur J Nutr. 2016;55:687–697. doi: 10.1007/s00394-015-0889-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewailly E, Blanchet C, Lemieux S, Sauve L, Gingras S, Ayotte P, Holub BJ. n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr. 2001;74:464–73. doi: 10.1093/ajcn/74.4.464. [DOI] [PubMed] [Google Scholar]
- 10.Dewailly E, Blanchet C, Gingras S, Lemieux S, Holub BJ. Cardiovascular disease risk factors and n-3 fatty acid status in the adult population of James Bay Cree. Am J Clin Nutr. 2002;76:85–92. doi: 10.1093/ajcn/76.1.85. [DOI] [PubMed] [Google Scholar]
- 11.Thorsdottir I, Tomasson H, Gunnarsdottir I, Gisladottir E, Kiely M, Parra MD, Bandarra NM, Schaafsma G, Martinéz JA. Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int J Obes (Lond) 2007;31:1560–6. doi: 10.1038/sj.ijo.0803643. [DOI] [PubMed] [Google Scholar]
- 12.Munro IA, Garg ML. Prior supplementation with long chain omega-3 polyunsaturated fatty acids promotes weight loss in obese adults: a double-blinded randomised controlled trial. Food Funct. 2013;4:650–8. doi: 10.1039/c3fo60038f. [DOI] [PubMed] [Google Scholar]
- 13.Noreen EE, Sass MJ, Crowe ML, Pabon VA, Brandauer J, Averill LK. Effects of supplemental fish oil on resting metabolic rate, body composition, and salivary cortisol in healthy adults. J Int Soc Sports Nutr. 2010;7:31. doi: 10.1186/1550-2783-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieman DC, Cayea EJ, Austin MD, Henson DA, McAnulty SR, Jin F. Chia seed does not promote weight loss or alter disease risk factors in overweight adults. Nutr Res. 2009;29:414–8. doi: 10.1016/j.nutres.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Munro IA, Garg ML. Dietary supplementation with n-3 PUFA does not promote weight loss when combined with a very-low-energy diet. Br J Nutr. 2012;108:1466–74. doi: 10.1017/S0007114511006817. [DOI] [PubMed] [Google Scholar]
- 16.Munro IA, Garg ML. Dietary supplementation with long chain omega-3 polyunsaturated fatty acids and weight loss in obese adults. Obes Res Clin Pract. 2013;7:e173–81. doi: 10.1016/j.orcp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Nieman DC, Gillitt N, Jin F, Henson DA, Kennerly K, Shanely RA, Ore B, Su M, Schwartz S. Chia seed supplementation and disease risk factors in overweight women: a metabolomics investigation. J Altern Complement Med. 2012;18:700–8. doi: 10.1089/acm.2011.0443. [DOI] [PubMed] [Google Scholar]
- 18.Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T, Narumiya S, Guesnet P, Amri EZ, Negrel R, Ailhaud G. Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res. 2003;44:271–9. doi: 10.1194/jlr.M200346-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Alvheim AR, Malde MK, Osei-Hyiaman D, Lin YH, Pawlosky RJ, Madsen L, Kristiansen K, Frøyland L, Hibbeln JR. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 2012;20:1984–94. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simopoulos AP, DiNicolantonio JJ. The importance of a balanced omega-6 to omega-3 ratio in the prevention and management of obesity. Open Heart. 2016;3:e000385. doi: 10.1136/openhrt-2015-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Victoria E, Yago MD. Omega 3 polyunsaturated fatty acids and body weight. Br J Nutr. 2012;107(Suppl 2):S107–16. doi: 10.1017/S000711451200150X. [DOI] [PubMed] [Google Scholar]
- 22.Soh SE, Tint MT, Gluckman PD, Godfrey KM, Rifkin-Graboi A, Chan YH, Stünkel W, Holbrook JD, Kwek K, Chong YS, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43:1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 23.Padmapriya N, Shen L, Soh SE, Shen Z, Kwek K, Godfrey KM, Gluckman PD, Chong YS, Saw SM, Müller-Riemenschneider F. Physical Activity and Sedentary Behavior Patterns Before and During Pregnancy in a Multi-ethnic Sample of Asian Women in Singapore. Matern Child Health J. 2015;19:2523–35. doi: 10.1007/s10995-015-1773-3. [DOI] [PubMed] [Google Scholar]
- 24.IPAQ. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) – Short and Long Forms, revised on November 2005. [assessed 30 March 2016]; Version current 5 November 2010. Internet: https://sites.google.com/site/theipaq/scoring-protocol.
- 25.Cheng TS, Loy SL, Cheung YB, Chan JK, Pang WW, Godfrey KM, Gluckman PD, Kwek K, Saw SM, Chong YS, et al. Sexually dimorphic response to feeding mode in the growth of infants. Am J Clin Nutr. 2016;103:398–405. doi: 10.3945/ajcn.115.115493. [DOI] [PubMed] [Google Scholar]
- 26.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–8. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 27.Health Promotion Board Singapore. Singapore: Healthy living and disease prevention information. [accessed 25 April 2015];2012 Internet: http://www.hpb.gov.sg/HOPPortal/
- 28.NDL/FNIC Food Composition Database. USDA National nutrient database for standard reference. [accessed 30 April 2015]; Version current 2011. Internet: http://ndb.nal.usda.gov/
- 29.Cheung YB. Statistical Analysis of Human Growth and Development. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 30.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Chong MF, Ong YL, Calder PC, Colega M, Wong JX, Tan CS, Lim AL, Fisk HL, Cai S, Pang WW, et al. Long-chain polyunsaturated fatty acid status during pregnancy and maternal mental health in pregnancy and the postpartum period: results from the GUSTO study. J Clin Psychiatry. 2015;76:e848–56. doi: 10.4088/JCP.14m09191. [DOI] [PubMed] [Google Scholar]
- 32.Senn S. Baseline and covariates information. 2nd. ed. New York, US: John Wiley & Sons; 2008. Statistical Issues in Drug Development; pp. 100–101. [Google Scholar]
- 33.Royston P. Multiple imputation of missing values. Stata Journal. 2004;4:227–41. [Google Scholar]
- 34.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, US: John Wiley & Sons; 2004. [Google Scholar]
- 35.Sato A, Kawano H, Notsu T, Ohta M, Nakakuki M, Mizuguchi K, Itoh M, Suganami T, Ogawa Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes. 2010;59:2495–504. doi: 10.2337/db09-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–9S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 37.Bjursell M, Xu X, Admyre T, Bottcher G, Lundin S, Nilsson R, Stone VM, Morgan NG, Lam YY, Storlien LH, et al. The beneficial effects of n-3 polyunsaturated fatty acids on diet induced obesity and impaired glucose control do not require Gpr120. PLoS One. 2014;9:e114942. doi: 10.1371/journal.pone.0114942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem. 2014;25:270–80. doi: 10.1016/j.jnutbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Murumalla RK, Gunasekaran MK, Padhan JK, Bencharif K, Gence L, Festy F, Cesari M, Roche R, Hoareau L. Fatty acids do not pay the toll: effect of SFA and PUFA on human adipose tissue and mature adipocytes inflammation. Lipids Health Dis. 2012;11:175–183. doi: 10.1186/1476-511X-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poudyal H, Panchal SK, Ward LC, Brown L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J Nutr Biochem. 2013;24:1041–52. doi: 10.1016/j.jnutbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Krukowski RA, West DS, DiCarlo M, Shankar K, Cleves MA, Saylors ME, Andres A. Are early first trimester weights valid proxies for preconception weight? BMC pregnancy childbirth. 2016;16:357. doi: 10.1186/s12884-016-1159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuriki K, Nagaya T, Tokudome Y, Imaeda N, Fujiwara N, Sato J, Goto C, Ikeda M, Maki S, Tajima K, et al. Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: a cross-sectional study. J Nutr. 2003;133:3643–50. doi: 10.1093/jn/133.11.3643. [DOI] [PubMed] [Google Scholar]
- 43.van der Pligt P, Willcox J, Hesketh KD, Ball K, Wilkinson S, Crawford D, Campbell K. Systematic review of lifestyle interventions to limit postpartum weight retention: implications for future opportunities to prevent maternal overweight and obesity following childbirth. Obes Rev. 2013;14:792–805. doi: 10.1111/obr.12053. [DOI] [PubMed] [Google Scholar]
- 44.Harrison CL, Lombard CB, Teede HJ. Limiting postpartum weight retention through early antenatal intervention: the HeLP-her randomised controlled trial. Int J Behav Nutr Phys Act. 2014;11:134. doi: 10.1186/s12966-014-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.