Abstract
Background
The aim of this study was to investigate the impact of decreased skeletal muscle (SM) volume on survival outcomes in patients undergoing surgical resection for pancreatic ductal adenocarcinoma (PDAC).
Methods
Between March 2000 and February 2015, 323 patients who underwent upfront surgical resection for PDAC were identified from the Mayo Clinic SPORE in Pancreatic Cancer. Body composition data, including SM area, subcutaneous adipose tissue area, and visceral adipose tissue area were calculated using an abdominal computed tomography (CT) image at the third lumbar spinal level. The body composition data were normalized by patients’ height (e.g., SM index, cm2/m2) and analyzed as continuous variables. Clinicopathological findings and body composition data at initial diagnosis were evaluated for association with overall survival and recurrence-free survival.
Results
Because the median SM index was significantly different between males vs. females (49.9 cm2/m2 [range, 32.0–70.3] vs. 39.4 cm2/m2 [range, 29.2–66.2], P < 0.001), it was standardized for each sex and used for further analyses. Parameters independently associated with a shorter overall survival were a larger tumor size (P = 0.007), a greater tumor extent (P = 0.037), a higher carbohydrate antigen 19–9 level (P < 0.001), and a smaller sex-standardized SM index (P = 0.011). Parameters independently associated with a shorter recurrence-free survival were female sex (P = 0.029), a larger tumor size (P < 0.001), a higher carbohydrate antigen 19–9 level (P = 0.001), and a smaller sex-standardized SM index (P = 0.007).
Conclusions
A smaller sex-standardized SM index is a predictive factor for shorter overall and recurrence-free survival in PDAC patients undergoing surgery.
Keywords: Skeletal muscle, Sarcopenia, Pancreatic ductal adenocarcinoma, Pancreatectomy, Survival
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a very aggressive malignancy with a reported 5-year survival rate of 6% in the overall patient cohort.1 Surgical resection is the only therapeutic option for long-term survival; however, majority of the patients develop recurrence and the 5-year survival rate is 20% after complete resection.2,3 In patients with PDAC, as disease progresses, cachexia is prevalent in 80% of the patients and correlates with worse survival.4–7 A key component of cachexia in PDAC patients is hypercatabolism due to direct tumor metabolism, systemic inflammation, or other tumor-mediated effects.8,9
In 2011, an international consensus statement defined cancer cachexia as a progressive condition with anorexia, catabolic drive, muscle weakness, and/or functional/psychosocial impairment.10 Sarcopenia, which is characterized as involuntary loss of skeletal muscle (SM), is regarded as an objective and measurable feature of cachexia, because SM is the major consumer of energy and contributor to basal metabolic rate in the body.11–13 Sarcopenia can be objectively evaluated using a single slice of abdominal CT, and has increasingly been reported to be associated with poor survival or postoperative complications among various types of cancer patients.14–23
A recent systematic review of the impact of sarcopenia on surgical patients for gastrointestinal and hepatopancreatobiliary malignancies showed that preoperative sarcopenia was independently associated with a shorter overall survival in seven of ten studies.24 However, means of the measurement of SM area (i.e., CT cross-sectional area of the total skeletal muscle or only the psoas muscle at the third lumbar spinal (L3) level and its threshold of the CT attenuation value) or its cutoff values to define sarcopenia widely varied among those studies. Because sarcopenia is a progressive disease condition,10 SM volume should be evaluated quantitatively. Sex difference of the body habitus should also be properly acknowledged. The aim of this study was to investigate the predictive value of decreased SM volume for poorer survival outcomes in patients who underwent upfront surgical resection for PDAC.
Materials and Methods
Patients and Clinical Data Collection
The study was approved by the Mayo Clinic’s Institutional Review Board. Clinical data of the patients who were enrolled in the Mayo Clinic SPORE database were used. Between March 2000 and February 2015, 374 patients who had been examined with abdominal contrast-enhanced computed tomography (CT) at initial diagnosis and later underwent surgical resection for PDAC were identified. Among them, 51 patients who received neoadjuvant therapy were excluded, to analyze “predictive” factors for survival that could be obtained only at diagnosis. The remaining 323 patients were studied.
Curative-intent surgical resection for PDAC was performed using a pancreaticoduodenectomy (PD), distal pancreatectomy (DP), or total pancreatectomy (TP), depending on the location or distribution of the tumor. Details of the procedures were described in previous papers.25,26 For all patients, PDAC was pathologically proven on the surgical specimen. The study sample was composed of 176 males and 147 females, with a median age of 65 years (range, 38–88).
Selected clinicopathological findings at diagnosis of PDAC were collected both prospectively and retrospectively. Basic patient demographics included age, sex, and race. Condition at initial diagnosis included body mass index, Charlson comorbidity index, Eastern Cooperative Oncology Group (ECOG) score, and weight loss > 10%. CT findings at initial diagnosis included tumor location, tumor size, and tumor extent. Tumor extent was categorized into potentially resectable, borderline resectable, or locally advanced, according to the National Comprehensive Cancer Network Guidelines version 1.2016.27 All CT images were re-reviewed by an experienced radiologist (N.T.). Laboratory data at initial diagnosis included serum levels of albumin and carbohydrate antigen 19-9 (CA19-9). Logarithmic converted CA 19-9 values were used for analyses.
Protocol of Body Composition Measurements
Body composition data were measured from contrast-enhanced abdominal CT obtained at the time of diagnosis using a software program developed by the study team.28 The program automatically places three boundary lines between external air and subcutaneous fat (boundary 1), between subcutaneous fat and abdominal wall/paraspinal muscles (boundary 2), and between abdominal wall/paraspinal muscles and visceral fat (boundary 3) on a single slice at L3 level of the CTand calculates areas between the boundaries (Fig. 1). A single investigator (M.S.), blinded to the clinical data, carefully inspected the boundaries, and manually corrected the boundaries using the mouse-computer interface as necessary. When the correction of all boundaries was complete, the program calculated the skeletal muscle (SM) area, subcutaneous adipose tissue (SAT) area, and visceral adipose tissue (VAT) area (cm2 for all). SM area was calculated as an area containing pixels between boundaries 2 and 3, and having a CT attenuation value of − 30 to 150 HU, but excluding the spine and spinal canal. SAT area was calculated as the area containing pixels between boundaries 1 and 2, and having a CT attenuation value of − 190 to − 30 HU. VAT area was calculated as the area containing pixels within boundary 3, and having a CT attenuation value of − 190 to − 30 HU, but excluding bowel content. The program automatically created masks for bone and colonic content; these masks were used to exclude bone and colonic content from being included as muscle or fat. Those three areas were divided by the square of height for each patient (cm2/m2): SM index, SAT index, and VAT index were calculated. These body composition data were analyzed to clarify their difference between sexes and to search for association with survival outcomes.
Fig. 1.
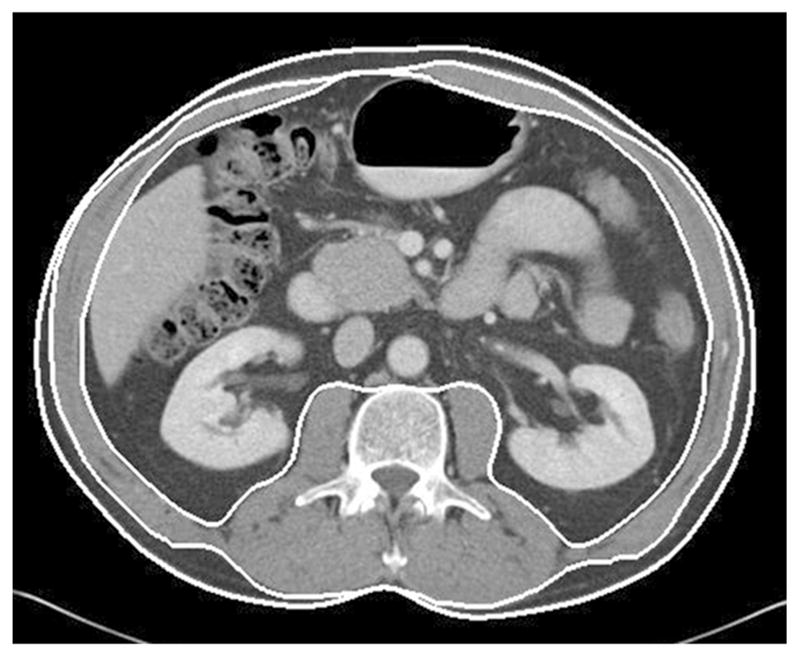
Axial CT image of the abdomen at the third lumbar level. Boundary lines between subcutaneous fat, skeletal muscle, and visceral fat compartments are shown (lines are thickened for ease of visualization)
Statistical Analysis
Clinicopathological findings and body composition data that could be obtained only at initial diagnosis of PDAC were used for analyses. First, all those parameters were compared between sexes. Categorical variables were analyzed using Pearson’s chi-square test. Continuous variables were analyzed using Mann-Whitney U test or Student’s t test. Continuous variables are shown in median with [range] or mean ± standard deviation. When the body composition data were significantly different between sexes, those data were standardized for each sex: males: [X − mean value for all males]/standard deviation for all males; females: [Y − mean value for all females]/standard deviation for all females). Sex-standardized SM index, SAT index, and VAT index were used for further analyses to represent SM volume, SAT volume, and VAT volume for each patient. Correlations between the parameters of clinicopathological findings and body composition data were evaluated using Spearman’s correlation coefficient r.
Then, those clinicopathological findings and body composition data were evaluated for association with overall survival and recurrence-free survival by Cox-regression analysis. Body composition data were analyzed as continuous variables. Hazard ratio (HR) was obtained. Parameters that were found to be significant in univariable analysis were included in multivariable analysis. Overall survival was defined as the time from initial diagnosis to death or the date censored at last follow-up. Recurrence-free survival was calculated as the time from surgery to tumor relapse or death or the date censored at last follow-up. The observation period was until December 2015, and the median duration of the estimated follow-up was 25.3 months (95% confidence interval [CI], 22.1–28.4]. Overall survival and recurrence-free survival were compared between patient groups using dichotomization of the SM index, by Kaplan-Meier survival curve and log-rank test. All P values were based on two-sided statistical tests, and the significance level was set at 0.05. All statistical analyses were performed using SPSS Statistics software (version 19.0; SPSS, Chicago, IL, USA).
Results
Comparison of Clinicopathological Findings and Body Composition Data Between Sexes
There were 176 males and 147 females who underwent up-front surgical resection for PDAC. Basic clinicopathological findings and body composition data at the time of diagnosis were compared between sexes (Table 1). The median body mass index was greater in males (27.4 kg/m2 [18.1–43.3] vs. 26.1 kg/m2 [14.8–48.4], P = 0.028). In terms of the body composition data, the median SAT index was greater in females (49.8 cm2/m2 [1.2–153.8] vs. 72.9 cm2/m2 [3.3–216.5], P < 0.001); the median VAT index was greater in males (70.1 cm2/m2 [1.5–173.8] vs. 34.9 cm2/m2 [1.2–138.9], P < 0.001), as was the median SM index (49.9 cm2/m2 [32.0–70.3] vs. 39.4 cm2/m2 [29.2–66.2], P < 0.001). These variables showing such differences were therefore standardized by sex using a mean value and standard deviation for further analysis. Among those parameters of clinicopathological findings and body composition data, a strong correlation was observed only between sex-standardized body mass index and sex-standardized SAT index and between sex-standardized body mass index and sex-standardized VAT index (Spearman’s correlation coefficient r, 0.828 and 0.745, respectively).
Table 1.
Clinicopathological findings and body composition data between males and females (n = 323)
| Males (n = 176) | Females (n = 147) | P | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, median [range] | 65 | [40–88] | 66 | [38–86] | 0.601 |
| Race (non-Caucasian) | 3 | (2%) | 2 | (1%) | 0.803 |
| Condition at diagnosis | |||||
| Body mass index (kg/m2) | |||||
| Median [range] | 27.4 | [18.1–43.3] | 26.1 | [14.8–48.4] | 0.028 |
| Mean ± standard deviation | 27.9 | ± 4.2 | 27.1 | ± 6.1 | 0.202 |
| Charlson comorbidity index ≥ 2 | 23 | (13%) | 20 | (14%) | 0.887 |
| ECOG score ≥ 1 | 13 | (7%) | 20 | (14%) | 0.066 |
| Weight loss > 10% | 36 | (20%) | 32 | (22%) | 0.773 |
| CT findings at diagnosis | |||||
| Tumor location at head/uncinate (vs. body/tail) | 140 | (80%) | 124 | (84%) | 0.265 |
| Tumor size (mm) | 25.0 | [9.0–150.0] | 24.0 | [12.0–73.0] | 0.069 |
| Tumor extent | 0.689 | ||||
| PR | 144 | (82%) | 125 | (85%) | |
| BR | 30 | (17%) | 20 | (14%) | |
| LA | 2 | (1%) | 2 | (1%) | |
| Laboratory data at diagnosis | |||||
| Albumin (g/dl) | 4.1 | [2.9–5.0] | 4.0 | [2.9–4.9] | 0.324 |
| CA19-9 (logarithmic converted) | 4.9 | [0.7–8.4] | 4.9 | [0.7–9.6] | 0.869 |
| Body composition data at diagnosis | |||||
| SAT index (cm2/m2) | |||||
| Median [range] | 49.8 | [1.2–153.8] | 72.9 | [3.3–216.5] | < 0.001 |
| Mean ± standard deviation | 53.2 | ± 27.4 | 79.4 | ± 45.1 | < 0.001 |
| VAT index (cm2/m2) | |||||
| Median [range] | 70.1 | [1.5–173.8] | 34.9 | [1.2–138.9] | < 0.001 |
| Mean ± standard deviation | 71.0 | ± 33.7 | 41.9 | ± 30.2 | < 0.001 |
| SM index (cm2/m2) | |||||
| Median [range] | 49.9 | [32.0–70.3] | 39.4 | [29.2–66.2] | < 0.001 |
| Mean ± standard deviation | 50.6 | ± 7.5 | 40.4 | ± 6.7 | < 0.001 |
BR borderline resectable, CA 19-9 carbohydrate antigen 19-9, ECOG Eastern Cooperative Oncology Group, LA locally advanced, PR potentially resectable, SAT subcutaneous adipose tissue, SM skeletal muscle, VAT visceral adipose tissue
Risk Analysis for a Shorter Overall Survival
Clinicopathological findings and body composition data that could be obtained at diagnosis were evaluated for the relationship with a shorter overall survival (Table 2). On univariable analysis, parameters that were significantly associated with a shorter overall survival were a larger tumor size (HR, 1.015; P = 0.001), a greater tumor extent (HR, 1.451; P = 0.016), a higher CA19-9 level (HR, 1.263; P < 0.001), and a smaller sex-standardized SM index (HR, 1.143; P = 0.046). On multivariable analysis, a larger tumor size (HR, 1.014; 95% CI, 1.004–1.024; P = 0.007), a greater tumor extent (HR, 1.378; 95% CI, 1.019–1.865; P = 0.037), a higher CA19-9 level (HR, 1.258; 95% CI, 1.152–1.374; P < 0.001), and a smaller sex-standardized SM index (HR, 1.188; 95% CI, 1.041–1.355; P = 0.011) were independently associated with a shorter overall survival.
Table 2.
Risk analysis for poorer overall survival in patients who underwent surgical resection for pancreatic ductal adenocarcinoma (n = 323)
| HR | 95% CI | P | ||
|---|---|---|---|---|
| Univariable analysis | ||||
| Demographics | ||||
| Age | 1.008 | 0.996 | 1.019 | 0.190 |
| Sex (female) | 1.183 | 0.920 | 1.522 | 0.191 |
| Race (non-Caucasian) | 0.937 | 0.348 | 2.519 | 0.897 |
| Condition at diagnosis | ||||
| Sex-standardized body mass index | 0.997 | 0.877 | 1.133 | 0.960 |
| Charlson comorbidity index ≥ 2 | 0.842 | 0.571 | 1.241 | 0.385 |
| ECOG score ≥ 1 | 1.051 | 0.694 | 1.590 | 0.815 |
| Weight loss > 10% | 1.298 | 0.959 | 1.756 | 0.091 |
| CT findings at diagnosis | ||||
| Tumor location (head/uncinate vs. body/tail) | 0.865 | 0.621 | 1.207 | 0.394 |
| Tumor size | 1.015 | 1.006 | 1.023 | 0.001 |
| Tumor extent (LA > BR > PR) | 1.451 | 1.072 | 1.964 | 0.016 |
| Laboratory data at diagnosis | ||||
| Albumin | 0.914 | 0.610 | 1.370 | 0.664 |
| CA19-9 | 1.263 | 1.156 | 1.381 | 0.001 |
| Body composition data | ||||
| Sex-standardized SAT index | 1.028 | 0.903 | 1.170 | 0.680 |
| Sex-standardized VAT index | 1.032 | 0.914 | 1.165 | 0.608 |
| Sex-standardized SM index | 0.875 | 0.768 | 0.998 | 0.046 |
| Multivariable analysis | ||||
| Tumor size | 1.014 | 1.004 | 1.024 | 0.007 |
| Tumor extent (LA > BR > PR) | 1.378 | 1.019 | 1.865 | 0.037 |
| CA19-9 | 1.258 | 1.152 | 1.374 | 0.001 |
| Sex-standardized SM index | 0.842 | 0.738 | 0.961 | 0.011 |
95% CI 95% confidence interval, BR borderline resectable, CA 19-9 carbohydrate antigen 19-9, ECOG Eastern Cooperative Oncology Group, LA locally advanced, PR potentially resectable, SAT subcutaneous adipose tissue, SM skeletal muscle, VAT visceral adipose tissue
Risk Analysis for a Shorter Recurrence-Free Survival
Next, predictive analysis for a shorter recurrence-free survival was performed (Table 3). On univariable analysis, parameters that were significantly associated with a shorter recurrence-free survival were female sex (HR, 1.306; P = 0.031), a larger tumor size (HR, 1.015; P < 0.001), a higher CA19-9 level (HR, 1.175; P < 0.001), and a smaller sex-standardized SM index (HR, 1.168; P = 0.015). On multivariable analysis, female sex (HR, 1.316; 95% CI, 1.028–1.685; P = 0.029), a larger tumor size (HR, 1.017; 95% CI, 1.008–1.026; P < 0.001), a higher CA19-9 level (HR, 1.153; 95% CI, 1.061–1.254; P = 0.001), and a smaller sex-standardized SM index (HR, 1.186; 95% CI, 1.048–1.342; P = 0.007) were independently associated with a shorter recurrence-free survival.
Table 3.
Risk analysis for poorer recurrence-free survival in patients who underwent surgical resection for pancreatic ductal adenocarcinoma (n = 323)
| HR | 95% CI | P | ||
|---|---|---|---|---|
| Univariable analysis | ||||
| Demographics | ||||
| Age | 1.006 | 0.995 | 1.018 | 0.266 |
| Sex (female) | 1.306 | 1.024 | 1.665 | 0.031 |
| Race (non-Caucasian) | 0.535 | 0.171 | 1.672 | 0.282 |
| Condition at diagnosis | ||||
| Sex-standardized body mass index | 0.958 | 0.844 | 1.088 | 0.510 |
| Charlson comorbidity index ≥ 2 | 0.947 | 0.654 | 1.371 | 0.772 |
| ECOG score ≥ 1 | 1.091 | 0.737 | 1.615 | 0.663 |
| Weight loss > 10% | 1.066 | 0.792 | 1.435 | 0.674 |
| CT findings at diagnosis | ||||
| Tumor location (head/uncinate vs. body/tail) | 0.960 | 0.700 | 1.317 | 0.802 |
| Tumor size | 1.015 | 1.007 | 1.024 | 0.001 |
| Tumor extent (LA > BR > PR) | 1.324 | 0.990 | 1.773 | 0.059 |
| Laboratory data at diagnosis | ||||
| Albumin | 0.866 | 0.592 | 1.266 | 0.457 |
| CA19-9 | 1.175 | 1.079 | 1.279 | 0.001 |
| Body composition data | ||||
| Sex-standardized SAT index | 0.997 | 0.879 | 1.132 | 0.969 |
| Sex-standardized VAT index | 0.985 | 0.875 | 1.108 | 0.795 |
| Sex-standardized SM index | 0.856 | 0.756 | 0.970 | 0.015 |
| Multivariable analysis | ||||
| Sex (female) | 1.316 | 1.028 | 1.685 | 0.029 |
| Tumor size | 1.017 | 1.008 | 1.026 | 0.001 |
| CA19-9 | 1.153 | 1.061 | 1.254 | 0.001 |
| Sex-standardized SM index | 0.843 | 0.745 | 0.954 | 0.007 |
95% CI 95% confidence interval, BR borderline resectable, CA 19-9 carbohydrate antigen 19-9, ECOG Eastern Cooperative Oncology Group, LA locally advanced, PR potentially resectable, SAT subcutaneous adipose tissue, SM skeletal muscle, VAT visceral adipose tissue
Survival of the Patients Using Dichotomized Variables of SM Index
First, 323 patients of the study cohort were divided into two groups: the lowest quartile of the sex-standardized SM index (n = 80) and the other (n = 243). Patients in the lowest quartile of the sex-standardized SM index tended to show a shorter median overall survival than those in the other (23 vs. 26 months, P = 0.075) (Fig. 2a). They did not show a significant association with a shorter median recurrence-free survival (14 vs. 15 months, P = 0.172) (Fig. 2b).
Fig. 2.
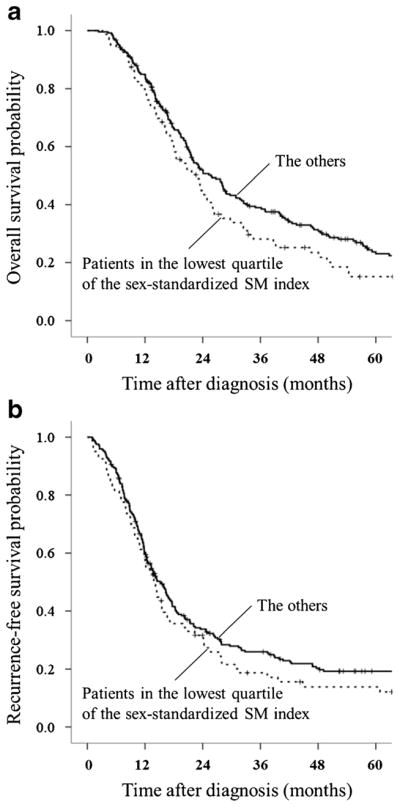
a Comparison of overall survival between patients in the lowest quartile of the sex-standardized SM index (n = 80) vs. the others (n = 243): median; 23 months [95% CI, 17–28] vs. 26 months [95% CI, 21–30], P = 0.075. b Comparison of recurrence-free survival between patients in the lowest quartile of the sex-standardized SM index (n = 80) vs. the others (n = 243): median; 14 months [95% CI, 11–16] vs. 15 months [95% CI, 13–17], P = 0.172
Second, 323 patients were divided into two groups using a cutoff of the SM index according to the international consensus for sarcopenia (males < 55.4 cm2/m2; females < 38.9 cm2/m2). Two hundred patients (62%) met this criterion of the lower SM index. They did not show significant associations with a shorter median overall survival (23 vs. 25 months, P = 0.412) (Fig. 3a) or a shorter median recurrence-free survival than the others (14 vs. 14 months, P = 0.390) (Fig. 3b).
Fig. 3.
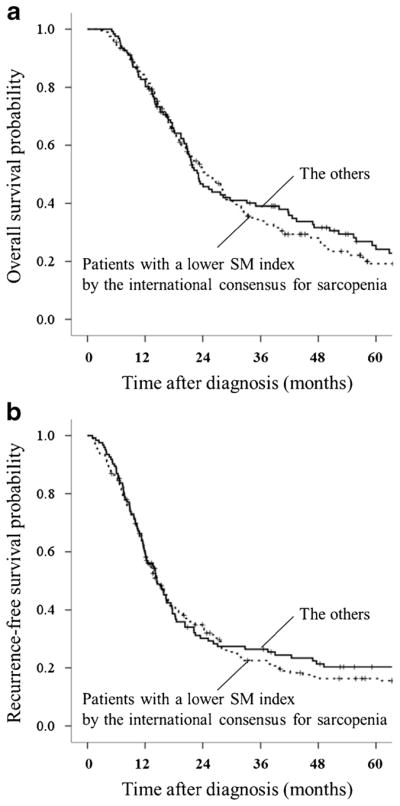
a Comparison of overall survival between patients with a lower SM index by the international consensus for sarcopenia (n = 200) vs. the others (n = 123): median; 23 months [95% CI, 20–27] vs. 25 months [95% CI, 21–29], P = 0.412. b Comparison of recurrence-free survival between patients with a lower SM index by the international consensus for sarcopenia (n = 200) vs. the others (n = 123): median; 14 months [95% CI, 12–17] vs. 14 months [95% CI, 12–17], P = 0.390
Discussion
In 2011, an international consensus defined cancer cachexia as a multifactorial syndrome characterized by ongoing loss of SM volume that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment.10 According to the consensus, diagnostic criteria of cancer cachexia include the following: weight loss greater than 5% over the past 6 months, weight loss greater than 2% in individuals with body mass index less than 20 kg/m2, or SM index at L3 level on a CT image consistent with sarcopenia (males < 55.4 cm2/m2; females < 38.9 cm2/m2)10,29 and any degree of weight loss greater than 2%. Sarcopenia can be determined by a SM index quantitatively and objectively using abdominal CT.
The impact of sarcopenia on survival outcomes after surgery for cancer patients has increasingly been reported.14–23 However, definition of sarcopenia and means of the measurement of SM area varied among studies. Peng et al. defined sarcopenia as a group of the patients in the lowest sex-specific quartile of the total psoas muscle index in the population, and showed that sarcopenia was associated with 3-year mortality after surgery for PDAC.21 In their multivariable analysis, a hazard ratio for the effect of sarcopenia on mortality was 1.63. They used CTs within 30 days before or after surgery, and included postoperative complications and pathological factors for survival analysis. They did not include preoperative CA 19-9 level, although it is widely recognized as a parameter being strongly associated with survival of PDAC patients. Okumura et al. determined a sex-specific cutoff value for the psoas muscle mass index in relation with death, using a receiver operating characteristic curve, and showed that a low psoas muscle mass index was independently associated with a shorter overall and recurrence-free survival after surgery for PDAC.22 In their multivariable analysis including demographic, nutritional, and pathological data, a hazard ratio for the effect of the low psoas muscle mass index on overall survival was 2.00. To compare the effect of sarcopenia between the studies, we conducted multivariable analysis by including a dichotomized parameter of the SM index (patients in the lowest sex-specific quartile of the SM index vs. the others). The hazard ratio for poorer overall survival in patients in the lowest sex-specific quartile of SM index was 1.359 (P = 0.035) (supplemental table). The effect of sarcopenia on overall survival may be smaller than previously reported in two studies by Peng et al. and Okumura et al. This difference might be due to different patient population, variety in the methodology of measurement of SM volume, or difference in the parameters included in multivariable analysis between the studies.
Because SM volume decreases over time as tumor progression and cachexia in clinical course of the patients with cancer,10 it should ideally be evaluated quantitatively as a continuous variable. When we divided our 323 patients into two groups using the lowest quartile of the SM index or using a cutoff of the SM index as sarcopenia according to the international consensus for cancer cachexia, they did not show statistically significant association with survival outcomes (Figs. 2 and 3). There were total of 200 patients (132 males, 75% of the total males and 68 females, 46% of the total females) satisfying the criterion of the international consensus for sarcopenia, indicating a relatively high rate of sarcopenia patients in the cohort and maldistribution between sexes. Because high incidence of cancer cachexia has been suggested in PDAC patients,4–7 setting a cutoff of the SM index might not be appropriate to evaluate survival outcomes. Based on these results, we emphasize significance to evaluate the SM index as a continuous variable. In addition, because body habitus may differ between sexes and a sex difference may not be relevant to the process of sarcopenia, a difference between sexes should be adequately considered to analyze SM index.29,30 Moreover, although a threshold of CT attenuation value of − 30 to 150 HU for the SM area has been widely used as described by the traditional studies,31,32 some recent studies used a different threshold of − 30 to 110 HU.19,21 Furthermore, in interpreting the SM area on a single CT slice, myosteatosis, which is interpreted as fatty infiltration in skeletal muscle, might be exclusively considered to evaluate the function of SM deliberately.22,33,34
Cachexia in patients with PDAC is considered as a complex multifactorial syndrome. A multimodal approach involving nutritional supplementation and pharmacological management has been studied to treat patients with cachexia in a series of randomized controlled trials and clinical studies. Fearon et al. showed that n-3 fatty acid enriched energy and protein dense supplement provided patients with net gain of weight, lean tissue, and improved quality of life.35 Bauer and Capra showed that nutritional supplement by eicosapentaenoic acid together with chemotherapy improved nutritional status, Karnofsky performance status, and quality of life in patients with PDAC or non-small-cell lung cancer.36 In a randomized multicenter trial, Kraft et al. showed that oral L-carnitine intake improved nutritional status and quality of life.37 Mantovani et al. showed that a combination of medroxyprogesterone or megestrol acetate, eicosapentaenoic acid, L-carnitine, and thalidomide increased the lean body mass; decreased the resting energy expenditure, fatigue, and serum level of interleukin-6; and improved the performance status.38 van Dijk et al. studied the protein balance between cachectic PDAC patients and non-cachectic PDAC patients, and showed that cachectic patients had a high basal protein turnover.39 They suggested a key role of stimulating protein synthesis to develop more effective nutritional intervention. To our knowledge, there have not been sufficient data to show whether reversing sarcopenia by nutritional support improves survival outcome in cancer patients. However, since nutritional support improves not only nutritional status but also performance status and quality of life in patients with PDAC, it may also improve survival outcomes. Moreover, because neoadju-vant therapy and planned surgical resection have increasingly been used for patients with PDAC,40,41 treatment to restore skeletal muscle volume in combination with anti-cancer therapy might also be effective to obtain better survival outcomes. Further study is necessary to reach such conclusion.
Limitations of the present study include the retrospective study design, lack of disease-specific outcomes, enrollment of only surgical cases, and unavailable data to analyze impact of adjuvant therapy after surgery. Our measure of defining the SM index should be validated in other studies.
Conclusion
The quantification of the height-adjusted and sex-standardized amount of the skeletal muscle area measured at the L3 level on CT was shown to have associations with overall survival and recurrence-free survival in patients undergoing upfront surgical resection for PDAC.
Supplementary Material
Acknowledgments
The authors thank William R. Bamlet, M.S., and Brendan Broderick for their contributions. This study was supported in part by a NIH grant P50CA102701, Mayo Clinic SPORE in Pancreatic Cancer.
Footnotes
Author Contribution Sugimoto designed the study, and wrote the initial draft of the manuscript. Takahashi contributed to the interpretation of the data and the critical revision of the manuscript for important intellectual content. All the other authors (Farnell, Nagorney, Kendrick, Truty, Smoot, Chari, Moynagh, Petersen, and Carter) contributed to the data collection and interpretation and critically reviewed the manuscript. All the authors have read and approved the final version of the manuscript, and have agreed to be accountable for all aspects of the study, ensuring that any questions related to the accuracy or integrity of any part of the work are answerable.
Compliance with Ethical Standards
The study was approved by the Mayo Clinic’s Institutional Review Board.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11605-018-3695-z) contains supplementary material, which is available to authorized users.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 4.Fearon KC, Voss AC, Hustead DS Cancer Cachexia Study Group. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 5.Wigmore SJ, Plester CE, Richardson RA, Fearon KC. Changes in nutritional status associated with unresectable pancreatic cancer. Br J Cancer. 1997;75:106–109. doi: 10.1038/bjc.1997.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Büchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg. 2008;12:1193–1201. doi: 10.1007/s11605-008-0505-z. [DOI] [PubMed] [Google Scholar]
- 7.Pausch T, Hartwig W, Hinz U, Swolana T, Bundy BD, Hackert T, Grenacher L, Büchler MW, Werner J. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery. 2012;152:S81–88. doi: 10.1016/j.surg.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep. 2009;21:1091–1095. doi: 10.3892/or_00000328. [DOI] [PubMed] [Google Scholar]
- 9.Miura T, Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Takahashi H, Furuse J, Inagaki M, Higashi S, Kato H, Terao K, Ochiai A. Characterization of patients with advanced pancreatic cancer and high serum interleukin-6 levels. Pancreas. 2015;44:756–763. doi: 10.1097/MPA.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 10.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 11.Buettner S, Wagner D, Kim Y, Margonis GA, Makary MA, Wilson A, Sasaki K, Amini N, Gani F, Pawlik TM. Inclusion of Sarcopenia Outperforms the Modified Frailty Index in Predicting 1-Year Mortality among 1,326 Patients Undergoing Gastrointestinal Surgery for a Malignant Indication. J Am Coll Surg. 2016;222:397–407. doi: 10.1016/j.jamcollsurg.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 13.Tzankoff SP, Norris AH. Longitudinal changes in basal metabolism in man. J Appl Physiol Respir Environ Exerc Physiol. 1978;45:536–539. doi: 10.1152/jappl.1978.45.4.536. [DOI] [PubMed] [Google Scholar]
- 14.Reisinger KW, Bosmans JW, Uittenbogaart M, Alsoumali A, Poeze M, Sosef MN, Derikx JP. Loss of Skeletal Muscle Mass During Neoadjuvant Chemoradiotherapy Predicts Postoperative Mortality in Esophageal Cancer Surgery. Ann Surg Oncol. 2015;22:4445–4452. doi: 10.1245/s10434-015-4558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, Salloum C, Luciani A, Azoulay D. Sarcopenia Impacts on Short-and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann Surg. 2015;261:1173–1183. doi: 10.1097/SLA.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 16.Hamaguchi Y, Kaido T, Okumura S, Ito T, Fujimoto Y, Ogawa K, Mori A, Hammad A, Hatano E, Uemoto S. Preoperative intramuscular adipose tissue content is a novel prognostic predictor after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2015;22:475–485. doi: 10.1002/jhbp.236. [DOI] [PubMed] [Google Scholar]
- 17.Peng PD, van Vledder MG, Tsai S, de Jong MC, Makary M, Ng J, Edil BH, Wolfgang CL, Schulick RD, Choti MA, Kamel I, Pawlik TM. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 2011;13:439–446. doi: 10.1111/j.1477-2574.2011.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki Y, Okamoto T, Fujishita T, Katsura M, Akamine T, Takamori S, Morodomi Y, Tagawa T, Shoji F, Maehara Y. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016;101:92–97. doi: 10.1016/j.lungcan.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, Poeze M. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345–352. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 20.Psutka SP, Boorjian SA, Moynagh MR, Schmit GD, Frank I, Carrasco A, Stewart SB, Tarrell R, Thapa P, Tollefson MK. Mortality after radical cystectomy: impact of obesity versus adiposity after adjusting for skeletal muscle wasting. J Urol. 2015;193:1507–1513. doi: 10.1016/j.juro.2014.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng P, Hyder O, Firoozmand A, Kneuertz P, Schulick RD, Huang D, Makary M, Hirose K, Edil B, Choti MA, Herman J, Cameron JL, Wolfgang CL, Pawlik TM. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:1478–1486. doi: 10.1007/s11605-012-1923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okumura S, Kaido T, Hamaguchi Y, Fujimoto Y, Masui T, Mizumoto M, Hammad A, Mori A, Takaori K, Uemoto S. Impact of preoperative quality as well as quantity of skeletal muscle on survival after resection of pancreatic cancer. Surgery. 2015;157:1088–1098. doi: 10.1016/j.surg.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Onesti JK, Wright GP, Kenning SE, Tierney MT, Davis AT, Doherty MG, Chung MH. Sarcopenia and survival in patients undergoing pancreatic resection. Pancreatology. 2016;16:284–289. doi: 10.1016/j.pan.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448–1458. doi: 10.1002/bjs.9893. [DOI] [PubMed] [Google Scholar]
- 25.Kirihara Y, Takahashi N, Hashimoto Y, Sclabas GM, Khan S, Moriya T, Sakagami J, Huebner M, Sarr MG, Farnell MB. Prediction of pancreatic anastomotic failure after pancreatoduodenectomy: the use of preoperative, quantitative computed tomography to measure remnant pancreatic volume and body composition. Ann Surg. 2013;257:512–519. doi: 10.1097/SLA.0b013e31827827d0. [DOI] [PubMed] [Google Scholar]
- 26.Christein JD, Kendrick ML, Iqbal CW, Nagorney DM, Farnell MB. Distal pancreatectomy for resectable adenocarcinoma of the body and tail of the pancreas. J Gastrointest Surg. 2005;9:922–927. doi: 10.1016/j.gassur.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma. Version 1. (2016) 2016 Available from: https://www.tri-kobe.org/nccn/guideline/pancreas/english/pancreatic.pdf.
- 28.Takahashi N, Sugimoto M, Psutka SP, Chen B, Moynagh M, Carter RE. Validation study of a new semi-automated software program for CT body composition analysis. Abdom Radiol. 2017;42:2369–2375. doi: 10.1007/s00261-017-1123-6. [DOI] [PubMed] [Google Scholar]
- 29.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 30.Miller VM, Rice M, Schiebinger L, Jenkins MR, Werbinski J, Núñez A, Wood S, Viggiano TR, Shuster LT. Embedding concepts of sex and gender health differences into medical curricula. J Womens Health (Larchmt) 2013;22:194–202. doi: 10.1089/jwh.2012.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 32.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 33.Joglekar S, Asghar A, Mott SL, Johnson BE, Button AM, Clark E, Mezhir JJ. Sarcopenia is an independent predictor of complications following pancreatectomy for adenocarcinoma. J Surg Oncol. 2015;111:771–775. doi: 10.1002/jso.23862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rollins KE, Tewari N, Ackner A, Awwad A, Madhusudan S, Macdonald IA, Fearon KC, Lobo DN. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 2016;35:1103–1109. doi: 10.1016/j.clnu.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Fearon KC, Von Meyenfeldt MF, Moses AG, Van Geenen R, Roy A, Gouma DJ, Giacosa A, Van Gossum A, Bauer J, Barber MD, Aaronson NK, Voss AC, Tisdale MJ. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut. 2003;52:1479–1486. doi: 10.1136/gut.52.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauer JD, Capra S. Nutrition intervention improves outcomes in patients with cancer cachexia receiving chemotherapy—a pilot study. Support Care Cancer. 2005;13:270–274. doi: 10.1007/s00520-004-0746-7. [DOI] [PubMed] [Google Scholar]
- 37.Kraft M, Kraft K, Gärtner S, Mayerle J, Simon P, Weber E, Schütte K, Stieler J, Koula-Jenik H, Holzhauer P, Gröber U, Engel G, Müller C, Feng YS, Aghdassi A, Nitsche C, Malfertheiner P, Patrzyk M, Kohlmann T, Lerch MM. L-Carnitine-supplementation in advanced pancreatic cancer (CARPAN)—a randomized multicentre trial. Nutr J. 2012;11:52. doi: 10.1186/1475-2891-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantovani G, Macciò A, Madeddu C, Serpe R, Massa E, Dessì M, Panzone F, Contu P. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist. 2010;15:200–211. doi: 10.1634/theoncologist.2009-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dijk DP, van de Poll MC, Moses AG, Preston T, Olde Damink SW, Rensen SS, Deutz NE, Soeters PB, Ross JA, Fearon KCh, Dejong CH. Effects of oral meal feeding on whole body protein breakdown and protein synthesis in cachectic pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2015;6:212–221. doi: 10.1002/jcsm.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winner M, Goff SL, Chabot JA. Neoadjuvant therapy for non-metastatic pancreatic ductal adenocarcinoma. Semin Oncol. 2015;42:86–97. doi: 10.1053/j.seminoncol.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Evans DB, Ritch PS, Erickson BA. Neoadjuvant therapy for localized pancreatic cancer: support is growing? Ann Surg. 2015;261:18–20. doi: 10.1097/SLA.0000000000000996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


