Abstract
Traumatic brain injury (TBI) is a worldwide medical problem, and currently, there are few therapeutic interventions that can protect the brain and improve functional outcomes in patients. Over the last several decades, experimental studies have investigated the pathophysiology of TBI and tested various pharmacological treatment interventions targeting specific mechanisms of secondary damage. Although many preclinical treatment studies have been encouraging, there remains a lack of successful translation to the clinic and no therapeutic treatments have shown benefit in phase 3 multicenter trials. Therapeutic hypothermia and targeted temperature management protocols over the last several decades have demonstrated successful reduction of secondary injury mechanisms and, in some selective cases, improved outcomes in specific TBI patient populations. However, the benefits of therapeutic hypothermia have not been demonstrated in multicenter randomized trials to significantly improve neurological outcomes. Although the exact reasons underlying the inability to translate therapeutic hypothermia into a larger clinical population are unknown, this failure may reflect the suboptimal use of this potentially powerful therapeutic in potentially treatable severe trauma patients. It is known that multiple factors including patient recruitment, clinical treatment variables, and cooling methodologies are all important in yielding beneficial effects. High-quality multicenter randomized controlled trials that incorporate these factors are required to maximize the benefits of this experimental therapy. This article therefore summarizes several factors that are important in enhancing the beneficial effects of therapeutic hypothermia in TBI. The current failures of hypothermic TBI clinical trials in terms of clinical protocol design, patient section, and other considerations are discussed and future directions are emphasized.
Keywords: Clinical trials, fever, pathophysiology, targeted temperature management, therapeutic hypothermia, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a serious worldwide health problem that includes mild, moderate, and severe injuries.[1,2] Within the United States, there are over 1.7 million new patients each year who sustain some type of TBI with a vast majority of those patients having mild TBI (mTBI) or concussive insults.[3,4] Depending on the location of the primary impact and injury severity, patients can be left with a spectrum of functional problems including sensorimotor, cognitive, and a range of postconcussive symptoms.[5] The pathophysiology of TBI is complex and previous research has clarified a number of secondary injury mechanisms important in the generation of structural and functional deficits in patients.[6,7] These injury mechanisms include excitotoxicity, apoptosis, free radical generation, as well as inflammatory processes that contribute to neural dysfunction, cell death, axonal and vascular damage, and circuit dysfunction.[7,8] Based on this complex pathophysiology, a spectrum of pharmacological interventions have been developed and tested using a variety of preclinical models with different degrees of success.[9,10] To date, however, no therapeutic interventions have successfully improved behavioral outcomes in multicenter phase 3 clinical trials for TBI.[11,12,13,14,15] For example, in recent clinical testing, the neuroprotective agents progesterone and erythropoietin both failed to improve outcomes in well-designed multicenter clinical trials.[11,13,14,15]
Profound focal levels of hypothermia have been known for many years to be effective in reducing brain edema and improving functional outcomes in models of TBI.[16,17,18] However, more recent preclinical studies also demonstrated that relatively mild reductions in systemic brain temperature were also neuroprotective in models of global and focal cerebral ischemia.[19,20,21,22,23] TBI studies have been initiated to test the benefits of mild systemic hypothermia on histopathological and behavioral outcomes.[24,25,26,27,28,29,30,31,32,33,34] Clifton et al. first utilized moderate hypothermia (30°C) in a rat moderate lateral fluid percussion injury (FPI) and showed that the induction of hypothermia before or soon after the primary insult improved motor function using beam-walking and beam-balance outcome measures.[29] Subsequently, Dietrich et al. reported that early posttraumatic cooling significantly reduced histopathological damage after moderate FPI.[35] In that study, a reduction of brain temperature to 30°C starting 5 min after TBI and extended for 3 h significantly reduced overall contusion volume as well as the frequency of dead neurons within the adjacent cerebral cortex [Figure 1].[35] Encouraging findings with posttraumatic hypothermia were also reported from other laboratories using different animal models with variable levels and durations of cooling.[30,36,37] Together, these preclinical studies showed that the early induction of mild-to-moderate hypothermia in models of both focal as well as diffuse TBI was beneficial in terms of a variety of histopathological outcomes and functional outcomes including cognitive assessment.[31,34,38,39,40,41,42,43,44,45]
Figure 1.
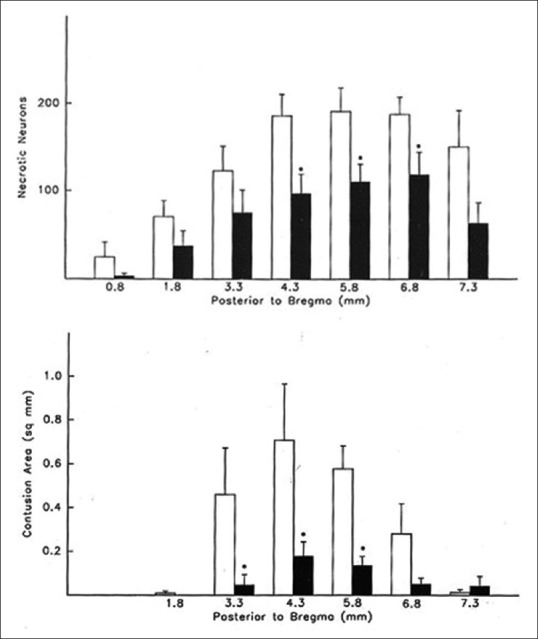
Bar graph of mean + standard error of the mean number of cortical necrotic neurons per microscopic field (1.65 mm2) at seven coronal levels. Data taken from normothermic (clear bars) and posttraumatic hypothermic (black bars) rats. (*, significantly reduced compared to normothermia. Bar graph of mean + standard error of the mean contusion area from normothermic (clear bars) and posttraumatic hypothermia (black bars) rats at 6 coronal levels (*, significantly reduced compared to normothermia). Reprinted from Acta Neuropathologica, Posst-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat, Vol 87, 1994, pages 250-258, Dietrich WD, Alonso O, Busto R, Globus, MY and Ginsberg MD with permission of Springer
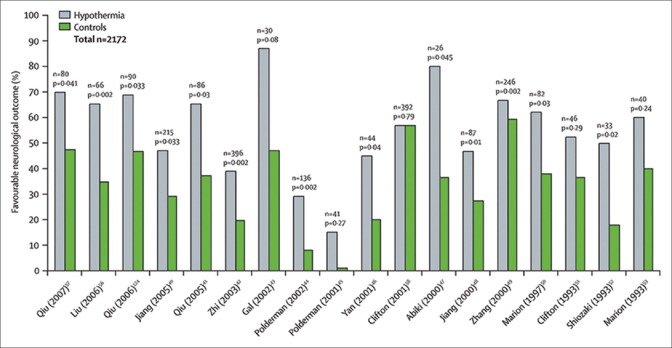
Based on these encouraging findings from multiple research groups, a number of single institutional clinical studies using a relatively small number of subjects were initiated in severe TBI patients to test the beneficial effects of moderate systemic hypothermia [Figure 2].[46,47,48,49,50,51] Importantly, several of these clinical investigations reported that the induction of early hypothermia reduced abnormal elevations in intracranial pressure (ICP) as well as improved neurological function at chronic survival periods.[52] However, results from additional randomized controlled clinical trials resulted in conflicting findings.[53,54,55]
Figure 2.
Clinical trials assessing the effects of hypothermia on neurological outcome in patients with traumatic brain injury and intracranial hypertension. Reprinted from The Lancet, Vol 371, Polderman KH, Induced hypothermia and fever control for prevention and treatment of neurological injuries, pages 1955-1969, 2008, with permission from Elsevier
The first multicenter trial, National Acute Brain Injury Study: Hypothermia (NABIS: H) that involved a number of recruitment sites throughout the United States, failed to show beneficial effects in terms of improving functional outcomes.[56] Many questions emerged from this multicenter trial suggested that this negative finding might be due to a delay in initiating the cooling protocol as well as patient management protocols that may have varied between recruitment sites.[57] Although negative, overall hypothermia treatment appeared to work best in younger patients who were cooled early after the traumatic insult.[56] A second multicenter trial was initiated based on these observations where several changes in the treatment protocol were initiated.[58] Unfortunately, therapeutic hypothermia was again shown to be ineffective in this second multicenter trial, resulting in the study being stopped. Interestingly, post hoc analysis of the data sets from the two previous NABIS: H trials indicated that hypothermia might work best in the treatment group where patients had undergone early cooling combined with decompression surgery.[58] In contrast to this observation, there appeared to be a lack of therapeutic efficacy in patients who had diffuse axonal injury and were cooled.
Although the current literature including systematic reviews and meta-analyses does not support the routine use of hypothermia for the management of severe TBI in pediatric and adult patients, more recent studies suggest that specific patient populations may benefit from this experimental treatment.[59,60,61,62,63,64,65,66,67] Over the last several years, there have been reports suggesting various explanations for the lack of efficacy of therapeutic hypothermia with severe TBI.[68] Furthermore, considerable work has continued using preclinical TBI models to more clearly define the most critical factors that may be important when designing clinical trials for the use of therapeutic hypothermia. In this regard, variables including the therapeutic window, duration and level of cooling, as well as the rewarming protocol have been emphasized.[46,49,50,57,68,69,70,71] Each of these factors is now appreciated to be highly relevant for maximizing the beneficial effects of hypothermic treatment, and these concepts need to be considered and integrated into the design of future clinical trials.
Level of Hypothermia
Studies initiated in the 1940s and 1950s for cardiac by-pass surgery utilized very profound levels of hypothermia to protect the heart and brain.[16,17,21,68,72,73,74] As previously mentioned, encouraging preclinical studies for transient global cerebral ischemia first reported that more mild-to-moderate levels of systemic hypothermia were protective in reducing ischemic cell death as well as improving behavioral outcome measures.[19,20,22,23,75] Indeed, it was discovered that in some circumstances, only a 1- or 2-degree difference in intra-ischemic brain temperature significantly altered the severity of hippocampal CA1 neuronal cell death.[20] Based on these findings, moderate levels of systemic hypothermia were tested in several models of TBI.[21,26,29,30,35,76,77] Early studies showed that hypothermic levels ranging from 30°C to 34°C were effective in improving a variety of clinically relevant outcomes.[8,30,32] However, in terms of the potential use of systemic hypothermia in TBI patients, it was determined that reducing core temperature to levels below 33° could potentially increase the frequency of risk factors, including changes in clotting factors, increased incidence of pneumonia and cardiac arrhythmias as well as reducing heart rate or blood pressure.[78] Thus, in clinical studies including cardiac arrest, TBI, and spinal cord injury (SCI), levels of systemic hypothermia ranging from 33°C to 36°C have been commonly utilized.[49,56,78] An important question remains whether lower levels of hypothermia might be more protective in the experimental or clinical setting under certain situations. This question is being currently addressed by utilizing more selective or focal-cooling strategies with new cooling devices.[79,80,81] Interestingly, a recent clinical study of severe TBI patients reported that metabolic-targeted hypothermia treatment reduced metabolic rate to 50%–60% in contrast to testing a predetermined temperature target which would significantly reduce mortality.[82]
Recent findings, specifically from the cardiac arrest field, have suggested that possibly more mild levels of hypothermia may be just as effective as moderate-cooling strategies.[83] For example, in the recent 33 versus 36 cardiac arrest study, Nielsen et al. reported that a postcardiac arrest patient population that was cooled to either 33°C or 36°C both showed similar behavioral outcomes.[83] This study emphasized that moderate levels of hypothermia previously reported to significantly improve outcomes after cardiac arrest may not be necessary to promote protection.[84,85,86] Several issues have subsequently been raised in the literature regarding this well-conducted multicenter cardiac arrest trial, including a significant delay in the initiation of cooling, the lack of consistently reaching cooling levels in the 33°C group, and a relatively rapid rewarming phase.[87,88] Thus, there is a continued need to consider optimal cooling levels and whether specific patient populations may benefit from specific levels of cooling depending on their condition and injury severity. Because TBI is also a very heterogeneous patient population, it will be important in future studies to develop diagnostic strategies including imaging and surrogate protein biomarker approaches to better select appropriate patients and to monitor temperature-sensitive injury cascades.[89,90] Such an approach would allow physicians to vary therapeutic treatments based on an individual's specific status. Furthermore, because many patients have focal lesions that can be identified with high-resolution computed tomography or magnetic resonance imaging, it might be important in future investigations to consider more focal-cooling strategies for these patients.[80,91] This strategy would permit more profound levels of cooling to be utilized, thereby potentially producing more neuroprotective efficacy with reduced risk factors associated with cooling.
Therapeutic Window
One of the most important factors on whether a therapy can be successfully translated to the clinic is whether the delayed initiation of the therapy remains significantly protective in a preclinical study.[92] In the previous brain injury studies, investigators have administered treatments relatively early after the insult to evaluate the effects of new treatments on specific pathomechanisms as well as structural or behavioral outcomes.[29,30,32,35,93,94] In contrast, many of these studies have not included a systematic examination of whether a delayed treatment protocol remains effective several hours after injury. This is a critical factor when thinking about clinical translation since many TBI patients may not be brought into the emergency room or assessed by a clinician until hours after the primary insult. If a treatment can only work when administered before or at early postinjury periods, it may be difficult to translate that treatment protocol to the clinical arena.
In the area of therapeutic hypothermia, Markgraf et al. first evaluated the therapeutic window for therapeutic hypothermia after experimental TBI.[33] That study reported that significant beneficial effects of moderate hypothermia were seen when treatment was initiated as late as 90 min.[33] However, if cooling was initiated after that period of time, there was a lack of benefit in terms of behavioral outcomes. In contrast, other subsequent studies have reported that systemic hypothermia remains effective even when it is delayed up to 3–4 h after injury.[92] Importantly, the therapeutic window of hypothermia appears to be based on several factors including level of hypothermia, injury severity, specific injury model utilized, and whether the trauma is focal or diffuse. Nevertheless, in the clinic, cooling strategies are generally initiated as soon as possible to target early occurring secondary injury mechanisms.
One challenge for early cooling is the lack of safe and established strategies for rapid cooling. In some studies, the infusion of cold saline has been used to reduce core temperature in a rapid fashion.[95] Importantly, recent technological advances in the development of effective intravascular, surface, and other cooling approaches have reduced the delay in reaching hypothermia temperature targets.[96,97,98,99]
Duration of Cooling
Many early preclinical studies tested the beneficial effects of posttraumatic hypothermia using relatively restricted periods of cooling.[29,30,32,35,100,101,102] In early studies of TBI, for example, many published studies reported positive effects with relatively restricted duration.[29,30,32,35,76] Interestingly, studies from the transient and focal cerebral ischemia field have reported that restricted periods of cooling may only transiently protect against ischemic brain injury.[103,104,105] In a recent TBI study, Lu et al. demonstrated the beneficial effects of an extended period of selective brain cooling in a model of penetrating ballistic-brain injury. These and other observations have led to investigations to determine the optimal periods of cooling required to produce permanent benefits including clinically meaningful neurological improvements.[106] In previous hypothermia studies, TBI patients have also been cooled using a variety of durations ranging from 24 h up to several days after trauma.[46,57,69,70,107,108,109]
When considering the importance of cooling duration on traumatic outcome, one should also consider what secondary injury mechanisms are being targeted by the cooling protocol.[24,36,38,43,44,75,110,111,112,113] Previous studies have reported that hypothermia can influence a number of injury mechanisms that are active at variable periods during the posttraumatic period.[114] In this regard, injury mechanisms such as free radical formation and excitotoxicity are active fairly early after injury. In contrast, other important secondary mechanisms such as apoptotic cell death and inflammatory processes may be more delayed but remain active for days after injury. In terms of acute pathophysiological mechanisms, hypothermia initiated early after the insult and continued during the time when secondary injury mechanisms are active in the patient should therefore be considered.[21,27,31,75,115] Thus, early cooling continued for up to several days after injury may be necessary to successfully target these injury cascades.
A second mechanism that is considered to be an important therapeutic target for TBI is the elevations in ICP.[6,48,49,70,71,107,108,116,117,118] Many patients after moderate or severe TBI or other types of brain injury experience focal or diffuse brain swelling that can lead to increases in ICP that can be life threatening.[6,7,107] The temporal pattern of ICP elevations can also be highly variable from patient to patient.[48] Thus, it is important when developing a clinical hypothermia protocol to consider cooling or targeted temperature management being on board before or rapidly initiated when ICP elevations occur.[116,119,120] Because early secondary injury mechanisms and delayed increases in ICP can each significantly affect patient outcomes, an optimal approach for the use of therapeutic hypothermia may be initiating cooling strategies as early as possible and extending them through the period of elevated ICP.[56,58,68,116,118,121]
In this regard, several clinical studies have used early cooling protocols that were only sustained for a 24- or 48-h period and therefore may not have extended to the period of increased ICP.[58,70,78] Some clinical studies that have utilized early and more prolonged cooling strategies have reported improvements in neurological outcomes.[122,123] In the study by Jiang et al., for example, extending the cooling period up to 5 days was reported to provide better neurological outcomes compared to a 2-day cooling protocol.[122] Further, in a recent multicenter clinical trial where hypothermic treatment was delayed and only restricted to the period of ICP elevation, no long-term benefits on neurological outcomes were reported although ICP elevations appeared to be successfully managed with the targeted cooling treatment.[108,113]
Rewarming Phase
Following a period of extended hypothermia, another important factor to maximize the benefits of cooling is using a relatively slow and controlled rewarming protocol.[8,124,125] Rapid-rewarming strategies, especially following a prolonged period of cooling, have been reported not to be optimal in terms of improving long-term outcome.[126] A study by Suehiro et al. reported that a slow-progressive cooling approach compared to rapid cooling was more effective in protecting against traumatically induced axonal damage commonly reported in experimental models of TBI.[127] In another study that assessed a complicated model of TBI that included FPI combined with secondary hypoxia, slow but not rapid rewarming again produced the best effect in terms of behavioral outcomes.[34] Impairments in cerebral vascular reactivity have been reported in TBI patients after rewarming from therapeutic hypothermia, leading to increased neuronal vulnerability.[125] Although underlying mechanisms are not known, inadvertent cerebral hyperthermia has been suggested in some situations.[128]
Based on these preclinical findings, recent clinical studies have developed established protocols for conducting therapeutic hypothermia in patients combined with a slow and controlled rewarming phase.[121,129] In one clinical study that used therapeutic hypothermia to target severe SCI, for example, Levi et al. after 2 days of systematic therapeutic hypothermia (33°) used a protocol that included the slow normalization of core temperature over a 24-h period.[129] In that study, induction of hypothermia combined with such a rewarming phase led to improved neurological function in cervical SCI patients at 1 year after injury compared to historical data. In a recent TBI trial for hypothermia, prolonged mild (33°C) combined with slow rewarming was also used with encouraging results.[121]
In operating room settings, slow rewarming after a surgical procedure may not be consistent with normal-operating procedures that commonly necessitate high throughput. Rapid rewarming, especially after a prolonged period of hypothermia, may stress vulnerable tissues leading to the aggravation of secondary injury mechanisms, including brain edema and other detrimental consequences.[8,130,131] Further, it is important to emphasize that a rapid-rewarming protocol can lead to temperature overshoots and periods of posttreatment hyperthermia which also may be detrimental to long-term outcomes, especially when associated with an extended hypothermia treatment protocol.[88,128]
Gender
A shortcoming of many preclinical studies is that only one gender is used to investigate traumatic pathomechanisms or new therapeutic interventions.[132,133] In the area of TBI, for example, the majority of preclinical studies have been restricted to only male animals for a number of stated or unstated reasons. However, in the clinical arena, it is clear that both sexes have TBIs and any sex-specific differences in the pathophysiology or potential treatment effects should be first evaluated before clinical translation.[3,134,135,136]
Previous investigations have reported gender differences that may be important when attempting to translate new therapies to the clinic.[137,138,139] Early studies by Roof et al. showed that sex hormones including estrogen and progesterone were neuroprotective after TBI and therefore important when considering gender-specific differences in traumatic vulnerability.[139] In those and other studies, the removal of the ovaries before producing a TBI enhanced the vulnerability of the female brain, leading to increased contusion volumes and behavioral deficits compared to intact animals.[132,133] Bramlett et al. directly compared the effects of moderate FPI in both male and female rats and reported that female rats displayed smaller contusion volumes compared to males.[132] In addition, removing the ovaries several days before TBI increased tissue vulnerability, resulting in similar contusion volumes between both male and ovariectomized female rats. Together, these studies emphasize the importance of sex hormones in the pathophysiology of TBI and the potential benefits of hormonal treatments on neurological disorders such as stroke. These types of preclinical studies have also led to clinical trials where progesterone and other hormonal treatments have been tested after severe TBI.[140,141]
In terms of injury mechanisms, recent preclinical studies have emphasized gender differences in specific pathophysiological events following TBI.[137,138,142] In a recent study, for example, Villapol et al. reported significant differences in the neuroinflammatory response to TBI in male versus female rats.[142] In those studies, male rats were reported to show a more aggressive inflammatory response compared to female rats after TBI. The importance of gender in the effects of temperature modifications after brain injury has also been emphasized.[138,143,144,145] For example, in a model of neonatal hypoxia-ischemia, Smith et al. reported that the beneficial effect of therapeutic hypothermia differed between male and female.[145] In a TBI study, posttraumatic hypothermia was also reported to protect males but not intact females after moderate FPI.[138] However, ovariectomized females showed increased contusion volumes that were comparable to males and were protected by posttraumatic hypothermia. In contrast to hypothermia, other studies have shown that ovariectomized female rats are more affected by posttraumatic hyperthermia than intact female rats. In a study by Suzuki et al., posttraumatic hyperthermia in ovariectomized female rats resulted in a dramatic increase in diffuse axonal injury compared to intact females.[137] These preclinical findings are important as we continue to develop novel therapeutic agents and clinical protocols for the future TBI trials, where neuroprotective or reparative strategies are tested. It will be important to recruit adequate numbers of male and female patients in clinical trials to clarify the importance of sex in the therapeutic efficacy of experimental treatments.
Heterogeneity of Traumatic Brain Injury Patient Population
One of the major challenges in conducting clinical trials for severe TBI as well as other neurological disorders is the heterogeneity of the patient population.[57,146,147] Different types of TBI commonly occur in the general population, which include focal, diffuse, as well as a combination of both types of injuries. In addition, many severe TBI patients can experience multiorgan injury that may also complicate treatment approaches. The acute severity and duration of clinical consequences after a brain injury can also be highly variable in patients undergoing mTBI or concussive insults. This potential heterogeneity therefore has to be taken into account when developing clinical trial protocols to test a new therapeutic treatment in TBI patients. For example, the temporal or regional profile of inflammatory cascades would be expected to significantly vary between patients with different degrees of pathological damage while exhibiting similar early neurological score assessments. Precision or specialized medical approaches are now being used to potentially reduce patient heterogeneity and improve treatment outcomes.
An important area of the current clinical research is the development of minimally invasive surrogate biomarkers including imaging and protein biomarkers which may help identify subsets of patients who may most benefit from a particular treatment.[148,149,150,151] In this regard, failure of previous stroke and TBI clinical trials have been suggested to be the result of enrolling highly heterogeneous patient populations. To respond to this apparent shortcoming in clinical trial design, studies are now utilizing more restricted inclusion and exclusion criteria to help recruit a more homogenous patient population. It is anticipated that such an approach may lead to a more successful translation of preclinical findings to the clinic.
Posttraumatic Hyperthermia
Periods of hyperthermia occur in a large number of stroke and trauma patients hours to days after the primary insult.[12,47,152,153,154,155,156,157,158] Importantly, preclinical and clinical studies have concluded that periods of posttraumatic hyperthermia may worsen outcome by aggravating secondary injury mechanisms, leading to increases in contusion volume, diffuse axonal injury, and ICP.[159,160] In an early preclinical study, Dietrich and Bramlett reported that an induced period of posttraumatic hyperthermia 24 h after TBI worsened histopathological damage and aggravated behavioral deficits compared to normothermic animals.[161] Similar to therapeutic hypothermia, periods of posttraumatic hyperthermia target multiple secondary injury mechanisms that are thought to contribute to the long-term consequences of TBI.[20,153] For example, published studies have reported that hyperthermia following brain injury aggravates patterns of excitotoxicity, free radical generation, apoptotic cell death, as well as a variety of inflammatory cascades. In terms of inflammation, Chatzipanteli et al. reported that an induced period of posttraumatic hyperthermia increased polymorphonuclear leukocyte extravasation into the vulnerable brain tissue associated with increases in tissue levels of pro-inflammatory cytokines.[27] These and other studies have led to the use of targeted temperature management protocols to inhibit periods of hyperthermia that are commonly observed in many TBI patients. In a recent clinical TBI study, Hifumi et al. reported that fever control management was preferable to mild hypothermia in reducing TBI-related mortality.[162] Indeed, targeted temperature management protocols are used in many clinical situations to reduce the incidence of hyperthermia in Intensive Care Unit patients following brain and SCI and to maintain normothermic conditions.
Most recently, the importance of brain hyperthermia in models of mTBI or concussion has also been reported.[163,164,165] Sakurai et al. first demonstrated that increasing brain temperature to 39°C at the time of impact and continued for 4 h significantly increased histopathological damage compared to normothermic mTBI animals.[164] In a subsequent study, Titus et al. using a similar experimental protocol reported that hyperthermic mTBI led to the emergence of long-term cognitive problems which are not present in animals that underwent normothermic mTBI [Figure 3].[163] These studies are important because many individuals prone to concussion such as athletes or military personnel frequently undergo strenuous activities such as sports-related events or stressful activities that can lead to increased core and brain temperature. Indeed, a variety of clinical studies have been reported that individuals exercising specifically in warm climates can demonstrate significant elevations in jugular blood temperature above 39°C.[166,167,168] These studies emphasize that brain temperature at the time of a relatively mild impact may vary from individual to individual and potentially participate in the severity of functional consequences including postconcussive syndromes. Recent studies have reported that elevated mild hyperthermic mTBI significantly aggravates neuroinflammatory and microvascular responses compared to normothermia.[165] In addition to more severe TBI injuries, mTBI or concussive insults may also require targeted temperature management strategies to minimize the detrimental effects of these more common types of milder insults to the brain.
Figure 3.
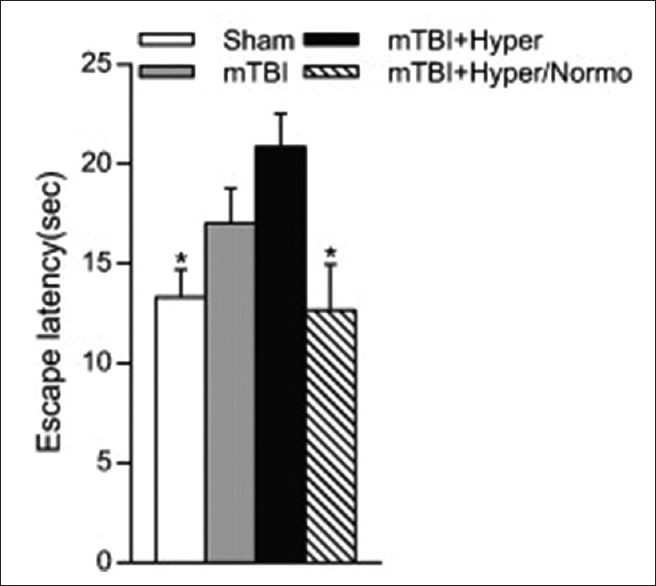
Effects of temperature manipulations on water maze performance. Analysis of escape latency on day 4 of testing 2 weeks postinjury. Hyperthermic mild traumatic brain injury animals had significantly longer escape latencies as compared to sham animals or hyperthermic/normothermic mild traumatic brain injury animals. *P < 0.05, one-way ANOVA and Tukey's post hoc analysis. Reprinted from Experimental Neurology, Vol 263, Emergence of cognitive deficits afer mild traumatic brain injury due to hyperthermia, pages 254-262, 2015, with permission from Elsevier
Recent Clinical Trial Failure
Previous and more recent multicenter trials using pharmacological compounds or therapeutic hypothermia for severe TBI have failed to show efficacy in large numbers of patients.[11,14,15,56,57,69] Earlier clinical studies by Clifton and colleagues indicated that the early initiation of hypothermia as well as the patient population could be important factors in determining the benefits of hypothermia. Indeed, the heterogeneity of the TBI patient population has emphasized the importance of patient selection and recruitment in terms of demonstrating benefits from hypothermic therapy.[68,70]
Recently, a large multicenter trial that used cooling to target increases in ICP also failed to show functional benefits of therapeutic hypothermia.[108] In the Eurotherm 3235 trial, the investigators sought to utilize hypothermia only when evidence of increased ICP occurred in the patients.[107,108] In this specific protocol, targeting early secondary pathophysiological mechanisms was therefore not included. Unfortunately, the clinical trial although showing a benefit in terms of attenuating ICP elevations did not demonstrate any long-term improvements in neurological outcomes.[113] Mechanisms underlying the ultimate consequences of TBI are complicated and involve multiple injury cascades including early and later pathophysiological events.[6,147] It will therefore be important in the future to develop and test clinical protocols that include both early- and prolonged-cooling strategies that extend past the period when ICP elevations remain present.[40,121]
Ongoing studies are also incorporating more extended periods of cooling that again may provide better outcomes in specific patient populations.[40,121] A recent study in Japan will be utilizing an extended-cooling strategy that includes both early and prolonged cooling. This trial will therefore target a variety of injury processes at extended posttraumatic periods and may therefore have the best chance of providing long-term outcomes.[40]
A recent development in terms of clinical trials using therapeutic hypothermia for TBI is the HOPES trial.[45,169] This trial is currently recruiting severe TBI patients from several institutions within the United States as well as Japan and China. Inclusion criteria include patients where early decompression surgery is required. The protocol involves early cooling with decompression surgery in severe TBI patients. The overall hypothesis is that cooling before the decompression surgery attenuates some of the detrimental effects of reperfusion injury that can occur when blood re-enters the ischemic area. Reperfusion injury has been studied extensively in the heart and involves a variety of injury mechanisms including free radical generation, glutamate neurotoxicity, and other injury mechanisms.[45] Whether this particular targeted therapy in this specific TBI patient population will provide beneficial effects remains to be demonstrated.[170]
Pharmacologically Induced Hypothermia
In addition to physical strategies for local or focal hypothermia, new investigations are clarifying the potential for pharmacologically induced hypothermia to also benefit patients with cerebral ischemia or TBI.[171,172,173,174,175] Various research groups have identified drugs or compounds that target mechanisms underlying temperature homeostasis which may allow for an efficient pharmacological approach for inducing hypothermia. For example, compounds that target adenosine A1 receptors, opioid receptors, transient receptor potential (TRP) channels, and dopamine receptors have been reported to produce hypothermia.[176,177,178] In neonatal rats, Gu et al. reported that the neurotensin receptor agonist HPI 201-induced hypothermia reduced neuronal damage and blood–brain barrier in a pediatric model of TBI [Figure 4].[172] Alterations in the hypothermia regulatory set point or peripheral temperature sensitive channels are among the mechanisms underlying pharmacological hypothermia.[179,180,181] An exciting future direction therefore could be the use of therapeutic hypothermia-inducing drugs in combination with passive-cooling strategies. This new approach could enhance the benefits of hypothermia in terms of accelerating the hypothermic phase and maximize neuroprotective benefits.
Figure 4.
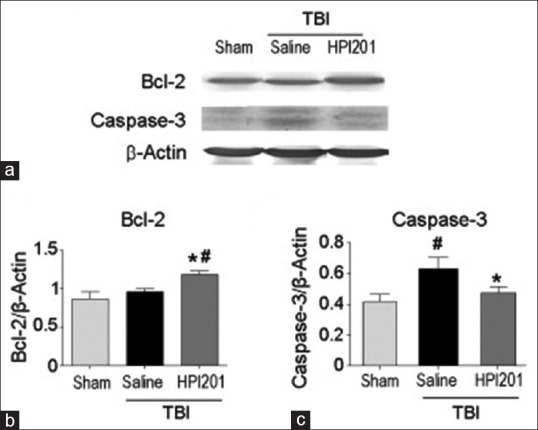
HPI 201-induced hypothermia attenuates apoptosis. Activation of the apoptotic gene caspase-3 was detected in the traumatic brain injury (a and b). At 24 h posttraumatic brain injury, the caspase-3 levels declined to the sham control levels in the HPI 201 group. There was also an observed significant increase of the antiapoptotic gene Bcl-2 in HPI 201-treated animals (c). #P < 0.05 versus sham; *P < 0.05 versus saline. Mean ± standard error of the mean n = 6–8 per group. Reprinted from Experimental Neurology, Vol 267, Gu X, Wei ZZ, Espinera A, Lee JH, Ji X, Dix TA, and Yu SP, Pharmacologically induced hypothermia attenuates traumatic brain injury in neonatal rats, Pages 135-142, 2015, with permission from Elsevier
Summary
Although therapeutic hypothermia remains one of the most potent neuroprotective strategies investigated to date, it is clear from the current literature that there remain many challenges for successfully utilizing therapeutic hypothermia in severe TBI patients.[65] Only through the continued translation of supportive preclinical data to the clinic will important advancements be made in this exciting field. Controlled, hypothesis-driven approaches are required to treat TBI patients with specialized targeted temperature management protocols that have a chance of improving outcomes. The potential use of therapeutic hypothermia in combination with FDA-approved therapeutic drugs also represents an exciting direction for continued research. The combination of therapeutic hypothermic and targeted temperature management approaches with pharmacotherapy to protect or repair the injured nervous system may lead to true improvements in long-term outcomes in this important clinical condition.
Financial support and sponsorship
The research was supported by NIH grants R01 NS 042133 and NS 089443.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank Erika Suazo for editorial support.
References
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–15. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 4.Faul MX, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA. 2010 [Google Scholar]
- 5.Finkelstein EA, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. New York: Oxford University Press; 2006. [Google Scholar]
- 6.Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Robertson CS, Valadka AB, Hannay HJ, Contant CF, Gopinath SP, Cormio M, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27:2086–95. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: Where do we go from here? Br J Pharmacol. 2011;164:1207–29. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radosevich JJ, Patanwala AE, Erstad BL. Emerging pharmacological agents to improve survival from traumatic brain injury. Brain Inj. 2013;27:1492–9. doi: 10.3109/02699052.2013.823658. [DOI] [PubMed] [Google Scholar]
- 11.Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371:2457–66. doi: 10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 13.Chase A. Traumatic brain injury. No benefit of progesterone therapy in patients with TBI. Nat Rev Neurol. 2015;11:65. doi: 10.1038/nrneurol.2014.258. [DOI] [PubMed] [Google Scholar]
- 14.Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, et al. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. 2014;371:2467–76. doi: 10.1056/NEJMoa1411090. [DOI] [PubMed] [Google Scholar]
- 15.Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: A randomized clinical trial. JAMA. 2014;312:36–47. doi: 10.1001/jama.2014.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fay T. Observations on generalized refrigeration in cases of severe cerebral trauma. Res Publ Assoc Res Nerv Ment Dis. 1945;4:611–9. [Google Scholar]
- 17.Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol. 1954;179:85–8. doi: 10.1152/ajplegacy.1954.179.1.85. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed AI, Bullock MR, Dietrich WD. Hypothermia in traumatic brain injury. Neurosurg Clin N Am. 2016;27:489–97. doi: 10.1016/j.nec.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich WD. The importance of brain temperature in cerebral injury. J Neurotrauma. 1992;9(Suppl 2):S475–85. [PubMed] [Google Scholar]
- 20.Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD, et al. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab. 1987;7:729–38. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- 21.Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma. 2009;26:301–12. doi: 10.1089/neu.2008.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh FA, Sims RE, Harris VA. Mild hypothermia prevents ischemic injury in gerbil hippocampus. J Cereb Blood Flow Metab. 1990;10:557–63. doi: 10.1038/jcbfm.1990.98. [DOI] [PubMed] [Google Scholar]
- 23.Onesti ST, Baker CJ, Sun PP, Solomon RA. Transient hypothermia reduces focal ischemic brain injury in the rat. Neurosurgery. 1991;29:369–73. doi: 10.1097/00006123-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Smith SL, Hall ED. Mild pre- and posttraumatic hypothermia attenuates blood-brain barrier damage following controlled cortical impact injury in the rat. J Neurotrauma. 1996;13:1–9. doi: 10.1089/neu.1996.13.1. [DOI] [PubMed] [Google Scholar]
- 25.Ma M, Matthews BT, Lampe JW, Meaney DF, Shofer FS, Neumar RW, et al. Immediate short-duration hypothermia provides long-term protection in an in vivo model of traumatic axonal injury. Exp Neurol. 2009;215:119–27. doi: 10.1016/j.expneurol.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bramlett HM, Green EJ, Dietrich WD, Busto R, Globus MY, Ginsberg MD, et al. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J Neurotrauma. 1995;12:289–98. doi: 10.1089/neu.1995.12.289. [DOI] [PubMed] [Google Scholar]
- 27.Chatzipanteli K, Alonso OF, Kraydieh S, Dietrich WD. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: Biochemical and immunocytochemical studies. J Cereb Blood Flow Metab. 2000;20:531–42. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Chatzipanteli K, Wada K, Busto R, Dietrich WD. Effects of moderate hypothermia on constitutive and inducible nitric oxide synthase activities after traumatic brain injury in the rat. J Neurochem. 1999;72:2047–52. doi: 10.1046/j.1471-4159.1999.0722047.x. [DOI] [PubMed] [Google Scholar]
- 29.Clifton GL, Jiang JY, Lyeth BG, Jenkins LW, Hamm RJ, Hayes RL, et al. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. 1991;11:114–21. doi: 10.1038/jcbfm.1991.13. [DOI] [PubMed] [Google Scholar]
- 30.Dixon CE, Markgraf CG, Angileri F, Pike BR, Wolfson B, Newcomb JK, et al. Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat. J Neurotrauma. 1998;15:95–103. doi: 10.1089/neu.1998.15.95. [DOI] [PubMed] [Google Scholar]
- 31.Lotocki G, de Rivero Vaccari JP, Perez ER, Sanchez-Molano J, Furones-Alonso O, Bramlett HM, et al. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: Effects of post-traumatic hypothermia. J Neurotrauma. 2009;26:1123–34. doi: 10.1089/neu.2008.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyeth BG, Jiang JY, Liu S. Behavioral protection by moderate hypothermia initiated after experimental traumatic brain injury. J Neurotrauma. 1993;10:57–64. doi: 10.1089/neu.1993.10.57. [DOI] [PubMed] [Google Scholar]
- 33.Markgraf CG, Clifton GL, Moody MR. Treatment window for hypothermia in brain injury. J Neurosurg. 2001;95:979–83. doi: 10.3171/jns.2001.95.6.0979. [DOI] [PubMed] [Google Scholar]
- 34.Matsushita Y, Bramlett HM, Alonso O, Dietrich WD. Posttraumatic hypothermia is neuroprotective in a model of traumatic brain injury complicated by a secondary hypoxic insult. Crit Care Med. 2001;29:2060–6. doi: 10.1097/00003246-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich WD, Alonso O, Busto R, Globus MY, Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994;87:250–8. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- 36.Jiang JY, Lyeth BG, Kapasi MZ, Jenkins LW, Povlishock JT. Moderate hypothermia reduces blood-brain barrier disruption following traumatic brain injury in the rat. Acta Neuropathol. 1992;84:495–500. doi: 10.1007/BF00304468. [DOI] [PubMed] [Google Scholar]
- 37.Yokobori S, Gajavelli S, Mondello S, Mo-Seaney J, Bramlett HM, Dietrich WD, et al. Neuroprotective effect of preoperatively induced mild hypothermia as determined by biomarkers and histopathological estimation in a rat subdural hematoma decompression model. J Neurosurg. 2013;118:370–80. doi: 10.3171/2012.10.JNS12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin Y, Lin Y, Feng JF, Jia F, Gao G, Jiang JY, et al. Attenuation of cell death in injured cortex after post-traumatic brain injury moderate hypothermia: Possible involvement of autophagy pathway. World Neurosurg. 2015;84:420–30. doi: 10.1016/j.wneu.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 39.Kinoshita K, Chatzipanteli IK, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD, et al. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: Importance of injury severity and brain temperature. Neurosurgery. 2002;51:195–203. doi: 10.1097/00006123-200207000-00027. [DOI] [PubMed] [Google Scholar]
- 40.Lei J, Gao G, Mao Q, Feng J, Wang L, You W, et al. Rationale, methodology, and implementation of a nationwide multicenter randomized controlled trial of long-term mild hypothermia for severe traumatic brain injury (the LTH-1 trial) Contemp Clin Trials. 2015;40:9–14. doi: 10.1016/j.cct.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Li YH, Zhang CL, Zhang XY, Zhou HX, Meng LL. Effects of mild induced hypothermia on hippocampal connexin 43 and glutamate transporter 1 expression following traumatic brain injury in rats. Mol Med Rep. 2015;11:1991–6. doi: 10.3892/mmr.2014.2928. [DOI] [PubMed] [Google Scholar]
- 42.Lotocki G, de Rivero Vaccari JP, Alonso O, Molano JS, Nixon R, Safavi P, et al. Oligodendrocyte vulnerability following traumatic brain injury in rats. Neurosci Lett. 2011;499:143–8. doi: 10.1016/j.neulet.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Truettner JS, Suzuki T, Dietrich WD. The effect of therapeutic hypothermia on the expression of inflammatory response genes following moderate traumatic brain injury in the rat. Brain Res Mol Brain Res. 2005;138:124–34. doi: 10.1016/j.molbrainres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD, et al. Tumor necrosis factor alpha expression and protein levels after fluid percussion injury in rats: The effect of injury severity and brain temperature. Neurosurgery. 2004;55:416–24. doi: 10.1227/01.neu.0000130036.52521.2c. [DOI] [PubMed] [Google Scholar]
- 45.Yokobori S, Frantzen J, Bullock R, Gajavelli S, Burks S, Bramlett H, et al. The use of hypothermia therapy in traumatic ischemic/reperfusional brain injury: Review of the literatures. Ther Hypothermia Temp Manag. 2011;1:185–92. doi: 10.1089/ther.2011.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marion D, Bullock MR. Current and future role of therapeutic hypothermia. J Neurotrauma. 2009;26:455–67. doi: 10.1089/neu.2008.0582. [DOI] [PubMed] [Google Scholar]
- 47.Natale JE, Joseph JG, Helfaer MA, Shaffner DH. Early hyperthermia after traumatic brain injury in children: Risk factors, influence on length of stay, and effect on short-term neurologic status. Crit Care Med. 2000;28:2608–15. doi: 10.1097/00003246-200007000-00071. [DOI] [PubMed] [Google Scholar]
- 48.Polderman KH, Tjong Tjin Joe R, Peerdeman SM, Vandertop WP, Girbes AR. Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med. 2002;28:1563–73. doi: 10.1007/s00134-002-1511-3. [DOI] [PubMed] [Google Scholar]
- 49.Polderman KH. Application of therapeutic hypothermia in the ICU: Opportunities and pitfalls of a promising treatment modality. Part 1: Indications and evidence. Intensive Care Med. 2004;30:556–75. doi: 10.1007/s00134-003-2152-x. [DOI] [PubMed] [Google Scholar]
- 50.Polderman KH, Andrews PJ. Hypothermia in patients with brain injury: The way forward? Lancet Neurol. 2011;10:404–5. doi: 10.1016/S1474-4422(11)70084-9. [DOI] [PubMed] [Google Scholar]
- 51.Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–69. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- 52.Polderman KH, Mayer SA, Menon D. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;359:1178. doi: 10.1056/NEJMc081418. [DOI] [PubMed] [Google Scholar]
- 53.Kramer C, Freeman WD, Larson JS, Hoffman-Snyder C, Wellik KE, Demaerschalk BM, et al. Therapeutic hypothermia for severe traumatic brain injury: A critically appraised topic. Neurologist. 2012;18:173–7. doi: 10.1097/NRL.0b013e318253f8ef. [DOI] [PubMed] [Google Scholar]
- 54.Jiang JY. Clinical study of mild hypothermia treatment for severe traumatic brain injury. J Neurotrauma. 2009;26:399–406. doi: 10.1089/neu.2008.0525. [DOI] [PubMed] [Google Scholar]
- 55.Maas A, Stocchetti N. Hypothermia and the complexity of trials in patients with traumatic brain injury. Lancet Neurol. 2011;10:111–3. doi: 10.1016/S1474-4422(10)70312-4. [DOI] [PubMed] [Google Scholar]
- 56.Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Jr, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–63. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- 57.Clifton GL, Choi SC, Miller ER, Levin HS, Smith KR, Jr, Muizelaar JP, et al. Intercenter variance in clinical trials of head trauma – Experience of the National Acute Brain Injury Study: Hypothermia. J Neurosurg. 2001;95:751–5. doi: 10.3171/jns.2001.95.5.0751. [DOI] [PubMed] [Google Scholar]
- 58.Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S, et al. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): A randomised trial. Lancet Neurol. 2011;10:131–9. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madden LK, Hill M, May TL, Human T, Guanci MM, Jacobi J, et al. The implementation of targeted temperature management: An evidence-based guideline from the neurocritical care society. Neurocrit Care. 2017;27:468–87. doi: 10.1007/s12028-017-0469-5. [DOI] [PubMed] [Google Scholar]
- 60.Wallisch JS, Kochanek PM. A “Metamorphosis” in our approach to treatment is not likely to result from a meta-analysis of the use of therapeutic hypothermia in severe traumatic brain injury. Crit Care Med. 2017;45:744–5. doi: 10.1097/CCM.0000000000002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang BF, Wang J, Liu ZW, Zhao YL, Li DD, Huang TQ, et al. Meta-analysis of the efficacy and safety of therapeutic hypothermia in children with acute traumatic brain injury. World Neurosurg. 2015;83:567–73. doi: 10.1016/j.wneu.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Newmyer R, Mendelson J, Pang D, Fink EL. Targeted temperature management in pediatric central nervous system disease. Curr Treat Options Pediatr. 2015;1:38–47. doi: 10.1007/s40746-014-0008-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis SR, Evans DJ, Butler AR, Schofield-Robinson OJ, Alderson P. Hypothermia for traumatic brain injury. Cochrane Database Syst Rev. 2017;9:CD001048. doi: 10.1002/14651858.CD001048.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crompton EM, Lubomirova I, Cotlarciuc I, Han TS, Sharma SD, Sharma P, et al. Meta-analysis of therapeutic hypothermia for traumatic brain injury in adult and pediatric patients. Crit Care Med. 2017;45:575–83. doi: 10.1097/CCM.0000000000002205. [DOI] [PubMed] [Google Scholar]
- 65.Shaefi S, Mittel AM, Hyam JA, Boone MD, Chen CC, Kasper EM, et al. Hypothermia for severe traumatic brain injury in adults: Recent lessons from randomized controlled trials. Surg Neurol Int. 2016;7:103. doi: 10.4103/2152-7806.194816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirst TC, Watzlawick R, Rhodes JK, Macleod MR, Andrews PJ. Study protocol - A systematic review and meta-analysis of hypothermia in experimental traumatic brain injury: Why have promising animal studies not been replicated in pragmatic clinical trials? Evid Based Preclin Med. 2016;3:e00020. doi: 10.1002/ebm2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tasker RC, Vonberg FW, Ulano ED, Akhondi-Asl A. Updating evidence for using hypothermia in pediatric severe traumatic brain injury: Conventional and bayesian meta-analytic perspectives. Pediatr Crit Care Med. 2017;18:355–62. doi: 10.1097/PCC.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 68.Marion DW, Regasa LE. Revisiting therapeutic hypothermia for severe traumatic brain injury. Again Crit Care. 2014;18:160. doi: 10.1186/cc13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adelson PD, Wisniewski SR, Beca J, Brown SD, Bell M, Muizelaar JP, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool kids): A phase 3, randomised controlled trial. Lancet Neurol. 2013;12:546–53. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 70.Crossley S, Reid J, McLatchie R, Hayton J, Clark C, MacDougall M, et al. A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care. 2014;18:R75. doi: 10.1186/cc13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madden LK, DeVon HA. A systematic review of the effects of body temperature on outcome after adult traumatic brain injury. J Neurosci Nurs. 2015;47:190–203. doi: 10.1097/JNN.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alzaga AG, Salazar GA, Varon J. Resuscitation great. Breaking the thermal barrier: Dr. Temple Fay. Resuscitation. 2006;69:359–64. doi: 10.1016/j.resuscitation.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 73.Leonov Y, Sterz F, Safar P, Radovsky A, Oku K, Tisherman S, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10:57–70. doi: 10.1038/jcbfm.1990.8. [DOI] [PubMed] [Google Scholar]
- 74.Safar P. Resuscitation from clinical death: Pathophysiologic limits and therapeutic potentials. Crit Care Med. 1988;16:923–41. doi: 10.1097/00003246-198810000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: Effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–11. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- 76.Bramlett HM, Dietrich WD, Green EJ, Busto R. Chronic histopathological consequences of fluid-percussion brain injury in rats: Effects of post-traumatic hypothermia. Acta Neuropathol. 1997;93:190–9. doi: 10.1007/s004010050602. [DOI] [PubMed] [Google Scholar]
- 77.Bramlett HM, Dietrich WD. The effects of posttraumatic hypothermia on diffuse axonal injury following parasaggital fluid percussion brain injury in rats. Ther Hypothermia Temp Manag. 2012;2:14–23. doi: 10.1089/ther.2012.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med. 1997;336:540–6. doi: 10.1056/NEJM199702203360803. [DOI] [PubMed] [Google Scholar]
- 79.Inoue T, Fujii M, Kida H, Yamakawa T, Maruta Y, Tokiwa T, et al. Epidural focal brain cooling abolishes neocortical seizures in cats and non-human primates. Neurosci Res. 2017;122:35–44. doi: 10.1016/j.neures.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 80.Szczygielski J, Müller A, Mautes AE, Sippl C, Glameanu C, Schwerdtfeger K, et al. Selective brain hypothermia mitigates brain damage and improves neurological outcome after post-traumatic decompressive craniectomy in mice. J Neurotrauma. 2017;34:1623–35. doi: 10.1089/neu.2016.4615. [DOI] [PubMed] [Google Scholar]
- 81.Smyth MD, Han RH, Yarbrough CK, Patterson EE, Yang XF, Miller JW, et al. Temperatures achieved in human and canine neocortex during intraoperative passive or active focal cooling. Ther Hypothermia Temp Manag. 2015;5:95–103. doi: 10.1089/ther.2014.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng JZ, Wang WY, Zeng J, Zhou ZY, Peng J, Yang H, et al. Optimization of brain metabolism using metabolic-targeted therapeutic hypothermia can reduce mortality from traumatic brain injury. J Trauma Acute Care Surg. 2017;83:296–304. doi: 10.1097/TA.0000000000001522. [DOI] [PubMed] [Google Scholar]
- 83.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 84.Bray JE, Stub D, Bloom JE, Segan L, Mitra B, Smith K, et al. Changing target temperature from 33°C to 36°C in the ICU management of out-of-hospital cardiac arrest: A before and after study. Resuscitation. 2017;113:39–43. doi: 10.1016/j.resuscitation.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 85.Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, et al. Induction of prehospital therapeutic hypothermia after resuscitation from nonventricular fibrillation cardiac arrest*. Crit Care Med. 2012;40:747–53. doi: 10.1097/CCM.0b013e3182377038. [DOI] [PubMed] [Google Scholar]
- 86.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 87.Chandrasekaran PN, Dezfulian C, Polderman KH. What is the right temperature to cool post-cardiac arrest patients? Crit Care. 2015;19:406. doi: 10.1186/s13054-015-1134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Polderman KH, Varon J. Interpreting the results of the targeted temperature management trial in cardiac arrest. Ther Hypothermia Temp Manag. 2015;5:73–6. doi: 10.1089/ther.2014.0031. [DOI] [PubMed] [Google Scholar]
- 89.Figaji AA, Graham Fieggen A, Mankahla N, Enslin N, Rohlwink UK. Targeted treatment in severe traumatic brain injury in the age of precision medicine. Childs Nerv Syst. 2017;33:1651–61. doi: 10.1007/s00381-017-3562-3. [DOI] [PubMed] [Google Scholar]
- 90.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Westermaier T, Nickl R, Koehler S, Fricke P, Stetter C, Rueckriegel SM, et al. Selective brain cooling after traumatic brain injury: Effects of three different cooling methods-case report. J Neurol Surg A Cent Eur Neurosurg. 2017;78:397–402. doi: 10.1055/s-0036-1596057. [DOI] [PubMed] [Google Scholar]
- 92.Zhao WY, Chen SB, Wang JJ, Xu C, Zhao ML, Dong HJ, et al. Establishment of an ideal time window model in hypothermic-targeted temperature management after traumatic brain injury in rats. Brain Res. 2017;1669:141–9. doi: 10.1016/j.brainres.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 93.Feng JF, Zhang KM, Jiang JY, Gao GY, Fu X, Liang YM, et al. Effect of therapeutic mild hypothermia on the genomics of the hippocampus after moderate traumatic brain injury in rats. Neurosurgery. 2010;67:730–42. doi: 10.1227/01.NEU.0000378023.81727.6E. [DOI] [PubMed] [Google Scholar]
- 94.Jia F, Mao Q, Liang YM, Jiang JY. Effect of post-traumatic mild hypothermia on hippocampal cell death after traumatic brain injury in rats. J Neurotrauma. 2009;26:243–52. doi: 10.1089/neu.2008.0670. [DOI] [PubMed] [Google Scholar]
- 95.Bernard SA, Smith K, Finn J, Hein C, Grantham H, Bray JE, et al. Induction of therapeutic hypothermia during out-of-hospital cardiac arrest using a rapid infusion of cold saline: The RINSE trial (Rapid infusion of cold normal saline) Circulation. 2016;134:797–805. doi: 10.1161/CIRCULATIONAHA.116.021989. [DOI] [PubMed] [Google Scholar]
- 96.Glover GW, Thomas RM, Vamvakas G, Al-Subaie N, Cranshaw J, Walden A, et al. Intravascular versus surface cooling for targeted temperature management after out-of-hospital cardiac arrest - an analysis of the TTM trial data. Crit Care. 2016;20:381. doi: 10.1186/s13054-016-1552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goury A, Poirson F, Chaput U, Voicu S, Garçon P, Beeken T, et al. Targeted temperature management using the “Esophageal Cooling Device” after cardiac arrest (the COOL study): A feasibility and safety study. Resuscitation. 2017;121:54–61. doi: 10.1016/j.resuscitation.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 98.Polderman KH, Noc M, Beishuizen A, Biermann H, Girbes AR, Tully GW, et al. Ultrarapid induction of hypothermia using continuous automated peritoneal lavage with ice-cold fluids: Final results of the cooling for cardiac arrest or acute ST-elevation myocardial infarction trial. Crit Care Med. 2015;43:2191–201. doi: 10.1097/CCM.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 99.Takeda Y, Kawashima T, Kiyota K, Oda S, Morimoto N, Kobata H, et al. Feasibility study of immediate pharyngeal cooling initiation in cardiac arrest patients after arrival at the emergency room. Resuscitation. 2014;85:1647–53. doi: 10.1016/j.resuscitation.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 100.Atkins CM, Oliva AA, Jr, Alonso OF, Chen S, Bramlett HM, Hu BR, et al. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. Eur J Neurosci. 2007;26:810–9. doi: 10.1111/j.1460-9568.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- 101.Atkins CM, Truettner JS, Lotocki G, Sanchez-Molano J, Kang Y, Alonso OF, et al. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci. 2010;32:1912–20. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bramlett HM, Dietrich WD. Long-term consequences of traumatic brain injury: Current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma. 2015;32:1834–48. doi: 10.1089/neu.2014.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–9. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- 104.Corbett D, Hamilton M, Colbourne F. Persistent neuroprotection with prolonged postischemic hypothermia in adult rats subjected to transient middle cerebral artery occlusion. Exp Neurol. 2000;163:200–6. doi: 10.1006/exnr.2000.7369. [DOI] [PubMed] [Google Scholar]
- 105.Colbourne F, Li H, Buchan AM. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. J Cereb Blood Flow Metab. 1999;19:742–9. doi: 10.1097/00004647-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 106.Lu XC, Shear DA, Deng-Bryant Y, Leung LY, Wei G, Chen Z, et al. Comprehensive evaluation of neuroprotection achieved by extended selective brain cooling therapy in a rat model of penetrating ballistic-like brain injury. Ther Hypothermia Temp Manag. 2016;6:30–9. doi: 10.1089/ther.2015.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andrews PJ, Sinclair HL, Battison CG, Polderman KH, Citerio G, Mascia L, et al. European society of intensive care medicine study of therapeutic hypothermia (32-35°C) for intracranial pressure reduction after traumatic brain injury (the eurotherm3235Trial) Trials. 2011;12:8. doi: 10.1186/1745-6215-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, et al. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015;373:2403–12. doi: 10.1056/NEJMoa1507581. [DOI] [PubMed] [Google Scholar]
- 109.Hutchison JS, Ward RE, Lacroix J, Hébert PC, Barnes MA, Bohn DJ, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447–56. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 110.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–78. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 111.Yenari MA, Han HS. Influence of therapeutic hypothermia on regeneration after cerebral ischemia. Front Neurol Neurosci. 2013;32:122–8. doi: 10.1159/000346428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 113.Flynn LM, Rhodes J, Andrews PJ. Therapeutic hypothermia reduces intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury: Preliminary data from the eurotherm3235 trial. Ther Hypothermia Temp Manag. 2015;5:143–51. doi: 10.1089/ther.2015.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Truettner JS, Bramlett HM, Dietrich WD. Posttraumatic therapeutic hypothermia alters microglial and macrophage polarization toward a beneficial phenotype. J Cereb Blood Flow Metab. 2017;37:2952–62. doi: 10.1177/0271678X16680003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bregy A, Nixon R, Lotocki G, Alonso OF, Atkins CM, Tsoulfas P, et al. Posttraumatic hypothermia increases doublecortin expressing neurons in the dentate gyrus after traumatic brain injury in the rat. Exp Neurol. 2012;233:821–8. doi: 10.1016/j.expneurol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shiozaki T, Sugimoto H, Taneda M, Yoshida H, Iwai A, Yoshioka T, et al. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg. 1993;79:363–8. doi: 10.3171/jns.1993.79.3.0363. [DOI] [PubMed] [Google Scholar]
- 117.Therapeutic Hypothermia for Severe Traumatic Brain Injury in Japan. [Last accessed on 2012 May 11]. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00134472 .
- 118.Nichol A, Gantner D, Presneill J, Murray L, Trapani T, Bernard S, et al. Protocol for a multicentre randomised controlled trial of early and sustained prophylactic hypothermia in the management of traumatic brain injury. Crit Care Resusc. 2015;17:92–100. [PubMed] [Google Scholar]
- 119.Sadaka F, Veremakis C. Therapeutic hypothermia for the management of intracranial hypertension in severe traumatic brain injury: A systematic review. Brain Inj. 2012;26:899–908. doi: 10.3109/02699052.2012.661120. [DOI] [PubMed] [Google Scholar]
- 120.Schreckinger M, Marion DW. Contemporary management of traumatic intracranial hypertension: Is there a role for therapeutic hypothermia? Neurocrit Care. 2009;11:427–36. doi: 10.1007/s12028-009-9256-2. [DOI] [PubMed] [Google Scholar]
- 121.Maekawa T, Yamashita S, Nagao S, Hayashi N, Ohashi Y, et al. Brain-Hypothermia Study Group. Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: A randomized controlled trial. J Neurotrauma. 2015;32:422–9. doi: 10.1089/neu.2013.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang JY, Xu W, Li WP, Gao GY, Bao YH, Liang YM, et al. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:771–6. doi: 10.1038/sj.jcbfm.9600253. [DOI] [PubMed] [Google Scholar]
- 123.Qiu WS, Liu WG, Shen H, Wang WM, Hang ZL, Zhang Y, et al. Therapeutic effect of mild hypothermia on severe traumatic head injury. Chin J Traumatol. 2005;8:27–32. [PubMed] [Google Scholar]
- 124.Bhalala US, Appachi E, Mumtaz MA. Neurologic injury associated with rewarming from hypothermia: Is mild hypothermia on bypass better than deep hypothermic circulatory arrest? Front Pediatr. 2016;4:104. doi: 10.3389/fped.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lavinio A, Timofeev I, Nortje J, Outtrim J, Smielewski P, Gupta A, et al. Cerebrovascular reactivity during hypothermia and rewarming. Br J Anaesth. 2007;99:237–44. doi: 10.1093/bja/aem118. [DOI] [PubMed] [Google Scholar]
- 126.Suehiro E, Povlishock JT. Exacerbation of traumatically induced axonal injury by rapid posthypothermic rewarming and attenuation of axonal change by cyclosporin A. J Neurosurg. 2001;94:493–8. doi: 10.3171/jns.2001.94.3.0493. [DOI] [PubMed] [Google Scholar]
- 127.Suehiro E, Ueda Y, Wei EP, Kontos HA, Povlishock JT. Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. J Neurotrauma. 2003;20:381–90. doi: 10.1089/089771503765172336. [DOI] [PubMed] [Google Scholar]
- 128.Hogue CW, Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: An evidence-based appraisal of current practices. Anesth Analg. 2006;103:21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 129.Levi AD, Green BA, Wang MY, Dietrich WD, Brindle T, Vanni S, et al. Clinical application of modest hypothermia after spinal cord injury. J Neurotrauma. 2009;26:407–15. doi: 10.1089/neu.2008.0745. [DOI] [PubMed] [Google Scholar]
- 130.Povlishock JT, Wei EP. Posthypothermic rewarming considerations following traumatic brain injury. J Neurotrauma. 2009;26:333–40. doi: 10.1089/neu.2008.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhu SZ, Gu Y, Wu Z, Hu YF, Pan SY. Hypothermia followed by rapid rewarming exacerbates ischemia-induced brain injury and augments inflammatory response in rats. Biochem Biophys Res Commun. 2016;474:175–81. doi: 10.1016/j.bbrc.2016.04.095. [DOI] [PubMed] [Google Scholar]
- 132.Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- 133.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: Neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–88. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 134.Duvdevani R, Roof RL, Fülöp Z, Hoffman SW, Stein DG. Blood-brain barrier breakdown and edema formation following frontal cortical contusion: Does hormonal status play a role? J Neurotrauma. 1995;12:65–75. doi: 10.1089/neu.1995.12.65. [DOI] [PubMed] [Google Scholar]
- 135.Bramlett HM, Furones-Alonso O, Lotocki G, Rodriguez-Paez A, Sanchez-Molano J, Keane RW, et al. Sex differences in XIAP cleavage after traumatic brain injury in the rat. Neurosci Lett. 2009;461:49–53. doi: 10.1016/j.neulet.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: Progesterone plays a protective role. Brain Res. 1993;607:333–6. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- 137.Suzuki T, Bramlett HM, Ruenes G, Dietrich WD. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J Neurotrauma. 2004;21:842–53. doi: 10.1089/0897715041526186. [DOI] [PubMed] [Google Scholar]
- 138.Suzuki T, Bramlett HM, Dietrich WD. The importance of gender on the beneficial effects of posttraumatic hypothermia. Exp Neurol. 2003;184:1017–26. doi: 10.1016/S0014-4886(03)00389-3. [DOI] [PubMed] [Google Scholar]
- 139.Roof RL, Duvdevani R, Stein DG. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restor Neurol Neurosci. 1992;4:425–7. doi: 10.3233/RNN-1992-4608. [DOI] [PubMed] [Google Scholar]
- 140.Goldstein FC, Caveney AF, Hertzberg VS, Silbergleit R, Yeatts SD, Palesch YY, et al. Very early administration of progesterone does not improve neuropsychological outcomes in subjects with moderate to severe traumatic brain injury. J Neurotrauma. 2017;34:115–20. doi: 10.1089/neu.2015.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeng Y, Zhang Y, Ma J, Xu J. Progesterone for acute traumatic brain injury: A Systematic review of randomized controlled trials. PLoS One. 2015;10:e0140624. doi: 10.1371/journal.pone.0140624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Villapol S, Loane DJ, Burns MP. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia. 2017;65:1423–38. doi: 10.1002/glia.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rodríguez-Fanjul J, Durán Fernández-Feijóo C, Lopez-Abad M, Lopez Ramos MG, Balada Caballé R, Alcántara-Horillo S, et al. Neuroprotection with hypothermia and allopurinol in an animal model of hypoxic-ischemic injury: Is it a gender question? PLoS One. 2017;12:e0184643. doi: 10.1371/journal.pone.0184643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chavez-Valdez R, O’Connor M, Perin J, Reyes M, Armstrong J, Parkinson C, et al. Sex-specific associations between cerebrovascular blood pressure autoregulation and cardiopulmonary injury in neonatal encephalopathy and therapeutic hypothermia. Pediatr Res. 2017;81:759–66. doi: 10.1038/pr.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Smith AL, Garbus H, Rosenkrantz TS, Fitch RH. Sex differences in behavioral outcomes following temperature modulation during induced neonatal hypoxic ischemic injury in rats. Brain Sci. 2015;5:220–40. doi: 10.3390/brainsci5020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suehiro E, Koizumi H, Fujisawa H, Fujita M, Kaneko T, Oda Y, et al. Diverse effects of hypothermia therapy in patients with severe traumatic brain injury based on the computed tomography classification of the traumatic coma data bank. J Neurotrauma. 2015;32:353–8. doi: 10.1089/neu.2014.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chesnut RM, Marshall SB, Piek J, Blunt BA, Klauber MR, Marshall LF, et al. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien) 1993;59:121–5. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- 148.Mondello S, Sorinola A, Czeiter E, Vámos Z, Amrein K, Synnot A, et al. Blood-based protein biomarkers for the management of traumatic brain injuries in adults presenting with mild head injury to emergency departments: A Living systematic review and meta-analysis. J Neurotrauma. 2017 doi: 10.1089/neu.2017.5182. doi: 10.1089/neu.2017.5182. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lorente L. Biomarkers associated with the outcome of traumatic brain injury patients. Brain Sci. 2017;7:pii: E142. doi: 10.3390/brainsci7110142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW, et al. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: Clinical article. J Neurosurg. 2012;117:1119–25. doi: 10.3171/2012.9.JNS12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mondello S, Shear DA, Bramlett HM, Dixon CE, Schmid KE, Dietrich WD, et al. Insight into pre-clinical models of traumatic brain injury using circulating brain damage biomarkers: Operation brain trauma therapy. J Neurotrauma. 2016;33:595–605. doi: 10.1089/neu.2015.4132. [DOI] [PubMed] [Google Scholar]
- 152.Bao L, Chen D, Ding L, Ling W, Xu F. Fever burden is an independent predictor for prognosis of traumatic brain injury. PLoS One. 2014;9:e90956. doi: 10.1371/journal.pone.0090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dietrich WD, Bramlett HM. Hyperthermia and central nervous system injury. Prog Brain Res. 2007;162:201–17. doi: 10.1016/S0079-6123(06)62011-6. [DOI] [PubMed] [Google Scholar]
- 154.Diringer MN Neurocritical Care Fever Reduction Trial Group. Treatment of fever in the neurologic intensive care unit with a catheter-based heat exchange system. Crit Care Med. 2004;32:559–64. doi: 10.1097/01.CCM.0000108868.97433.3F. [DOI] [PubMed] [Google Scholar]
- 155.Rincon F, Hunter K, Schorr C, Dellinger RP, Zanotti-Cavazzoni S. The epidemiology of spontaneous fever and hypothermia on admission of brain injury patients to intensive care units: A multicenter cohort study. J Neurosurg. 2014;121:950–60. doi: 10.3171/2014.7.JNS132470. [DOI] [PubMed] [Google Scholar]
- 156.Thompson HJ, Pinto-Martin J, Bullock MR. Neurogenic fever after traumatic brain injury: An epidemiological study. J Neurol Neurosurg Psychiatry. 2003;74:614–9. doi: 10.1136/jnnp.74.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Todd MM, Hindman BJ, Clarke WR, Torner JC, Weeks JB, Bayman EO, et al. Perioperative fever and outcome in surgical patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2009;64:897–908. doi: 10.1227/01.NEU.0000341903.11527.2F. [DOI] [PubMed] [Google Scholar]
- 158.Gaither JB, Galson S, Curry M, Mhayamaguru M, Williams C, Keim SM, et al. Environmental hyperthermia in prehospital patients with major traumatic brain injury. J Emerg Med. 2015;49:375–81. doi: 10.1016/j.jemermed.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 159.Li J, Jiang JY. Chinese head trauma data bank: Effect of hyperthermia on the outcome of acute head trauma patients. J Neurotrauma. 2012;29:96–100. doi: 10.1089/neu.2011.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu CG, Jagid J, Ruenes G, Dietrich WD, Marcillo AE, Yezierski RP, et al. Detrimental effects of systemic hyperthermia on locomotor function and histopathological outcome after traumatic spinal cord injury in the rat. Neurosurgery. 2001;49:152–8. doi: 10.1097/00006123-200107000-00023. [DOI] [PubMed] [Google Scholar]
- 161.Dietrich WD, Alonso O, Halley M, Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: A light and electron microscopic study in rats. Neurosurgery. 1996;38:533–41. doi: 10.1097/00006123-199603000-00023. [DOI] [PubMed] [Google Scholar]
- 162.Hifumi T, Kuroda Y, Kawakita K, Yamashita S, Oda Y, Dohi K, et al. Fever control management is preferable to mild therapeutic hypothermia in traumatic brain injury patients with abbreviated injury scale 3-4: A Multi-center, randomized controlled trial. J Neurotrauma. 2016;33:1047–53. doi: 10.1089/neu.2015.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Titus DJ, Furones C, Atkins CM, Dietrich WD. Emergence of cognitive deficits after mild traumatic brain injury due to hyperthermia. Exp Neurol. 2015;263:254–62. doi: 10.1016/j.expneurol.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sakurai A, Atkins CM, Alonso OF, Bramlett HM, Dietrich WD. Mild hyperthermia worsens the neuropathological damage associated with mild traumatic brain injury in rats. J Neurotrauma. 2012;29:313–21. doi: 10.1089/neu.2011.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Truettner J, Bramlett HM, Dietrich WD. Hyperthermia and mild traumatic brain injury: Effects on inflammation and the cerebral vasculature. J Neurotrauma. 2017 doi: 10.1089/neu.2017.5303. doi: 10.1089/neu.2017.5303. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nybo L, Secher NH, Nielsen B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol. 2002;545:697–704. doi: 10.1113/jphysiol.2002.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nybo L. Brain temperature and exercise performance. Exp Physiol. 2012;97:333–9. doi: 10.1113/expphysiol.2011.062273. [DOI] [PubMed] [Google Scholar]
- 168.Ozgünen KT, Kurdak SS, Maughan RJ, Zeren C, Korkmaz S, Yazici Z, et al. Effect of hot environmental conditions on physical activity patterns and temperature response of football players. Scand J Med Sci Sports. 2010;20(Suppl 3):140–7. doi: 10.1111/j.1600-0838.2010.01219.x. [DOI] [PubMed] [Google Scholar]
- 169.Yokobori S, Bullock R, Gajavelli S, Burks S, Mondello S, Mo J, et al. Preoperative-induced mild hypothermia attenuates neuronal damage in a rat subdural hematoma model. Acta Neurochir Suppl. 2013;118:77–81. doi: 10.1007/978-3-7091-1434-6_13. [DOI] [PubMed] [Google Scholar]
- 170.Tang C, Bao Y, Qi M, Zhou L, Liu F, Mao J, et al. Mild induced hypothermia for patients with severe traumatic brain injury after decompressive craniectomy. J Crit Care. 2017;39:267–70. doi: 10.1016/j.jcrc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 171.Junyun H, Young L, Xiaofeng J. Dihydrocapsaicin-induced hypothermia after asphyxial cardiac arrest in rats. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Ann Conf 2016. 2016:1858–61. doi: 10.1109/EMBC.2016.7591082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gu X, Wei ZZ, Espinera A, Lee JH, Ji X, Wei L, et al. Pharmacologically induced hypothermia attenuates traumatic brain injury in neonatal rats. Exp Neurol. 2015;267:135–42. doi: 10.1016/j.expneurol.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Choi KE, Hall CL, Sun JM, Wei L, Mohamad O, Dix TA, et al. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J. 2012;26:2799–810. doi: 10.1096/fj.11-201822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wei S, Sun J, Li J, Wang L, Hall CL, Dix TA, et al. Acute and delayed protective effects of pharmacologically induced hypothermia in an intracerebral hemorrhage stroke model of mice. Neuroscience. 2013;252:489–500. doi: 10.1016/j.neuroscience.2013.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu D, Shi J, Elmadhoun O, Duan Y, An H, Zhang J, et al. Dihydrocapsaicin (DHC) enhances the hypothermia-induced neuroprotection following ischemic stroke via PI3K/Akt regulation in rat. Brain Res. 2017;1671:18–25. doi: 10.1016/j.brainres.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 176.Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, et al. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia. J Cereb Blood Flow Metab. 2013;33:183–90. doi: 10.1038/jcbfm.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tupone D, Madden CJ, Morrison SF. Highlights in basic autonomic neurosciences: Central adenosine A1 receptor – The key to a hypometabolic state and therapeutic hypothermia? Auton Neurosci. 2013;176:1–2. doi: 10.1016/j.autneu.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang M, Wang H, Zhao J, Chen C, Leak RK, Xu Y, et al. Drug-induced hypothermia in stroke models: Does it always protect? CNS Neurol Disord Drug Targets. 2013;12:371–80. doi: 10.2174/1871527311312030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Katz LM, Frank JE, Glickman LT, McGwin G, Jr, Lambert BH, Gordon CJ. Effect of a pharmacologically induced decrease in core temperature in rats resuscitated from cardiac arrest. Resuscitation. 2015;92:26–31. doi: 10.1016/j.resuscitation.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cao Z, Balasubramanian A, Marrelli SP. Pharmacologically induced hypothermia via TRPV1 channel agonism provides neuroprotection following ischemic stroke when initiated 90 min after reperfusion. Am J Physiol Regul Integr Comp Physiol. 2014;306:R149–56. doi: 10.1152/ajpregu.00329.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Costa AC, Stasko MR, Stoffel M, Scott-McKean JJ. G-protein-gated potassium (GIRK) channels containing the GIRK2 subunit are control hubs for pharmacologically induced hypothermic responses. J Neurosci. 2005;25:7801–4. doi: 10.1523/JNEUROSCI.1699-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]