Abstract
OBJECTIVE:
Mild hypothermia has a protective effect on ischemic stroke, but the mechanisms remain elusive. Here, we investigated microRNA (miRNA) profiles and the specific role of miRNAs in ischemic stroke treated with mild hypothermia.
MATERIALS AND METHODS:
Male adult Sprague Dawley rats were subjected to focal transient cerebral ischemia. Mild hypothermia was induced by applying ice packs around the neck and head of the animals. miRNAs expression profiles were detected in ischemic stroke treated with mild therapeutic hypothermia through miRNA chips. Reverse transcription-polymerase chain reaction (RT-PCR) was used to verify the change of miRNA array. Western blot and adenosine triphosphate (ATP) assay kits were used to detect the changes of protein expression and ATP levels, respectively. miR-15b mimic and its control were injected into the right lateral ventricle 60 min before the induction of ischemia.
RESULTS:
The results showed that mild hypothermia affected miRNAs profiles expression. We verified the expression of miR-15b and miR-598-3p by miRNA RT-PCR. miR-15b mimic inhibited the expression of its target, ADP ribosylation factor-like 2 (Arl2) protein, and decreased ATP levels in PC12 cells. Compared with the control, miR-15b mimic increased the infarct volume and aggravated the neurological function under normothermia or hypothermia treatment. Furthermore, the expression of Arl2 was decreased in the miR-15b mimic group under normothermia or hypothermia treatment.
CONCLUSIONS:
Mild therapeutic hypothermia affected miRNA profiles and protected against cerebral ischemia/reperfusion by inhibiting miR-15b expression in rats. miR-15b may be a potential target for therapeutic intervention in stroke.
Keywords: ADP ribosylation factor-like 2, cerebral ischemia, hypothermia, microRNA, neuroprotection
Introduction
Ischemic stroke is a leading cause of disability and death worldwide.[1] Although numerous studies have been conducted on the underlying mechanisms of ischemic stroke,[2,3,4,5,6] they remain to be elucidated. Moreover, tissue plasminogen activator is still the only effective treatment.[1,7] Hypothermia is known as an effective neuroprotectant,[8,9,10,11] and can be categorized into deep hypothermia (<25°C), moderate hypothermia (30°C–32°C), and mild hypothermia (32°C–35°C).[8,9,10,11] Mild therapeutic hypothermia was shown to be neuroprotective while avoiding serious adverse effects of deep hypothermia, such as shivering and cardiac arrhythmia.[8] Neuroprotective mechanisms of hypothermia may include the reduction of energy failure, decrease of cell death, and promotion of neurogenesis.[8]
microRNAs (miRNAs) are single-stranded, noncoding endogenous RNAs with a length of 19–22 nucleotides, which negatively regulate target mRNA expression by either degradation or translational repression.[12,13,14] miRNAs regulate essential processes during development and adulthood[15,16] and are highly conserved in both plants and animals.[15,16] One miRNA can potentially regulate hundreds of mRNA targets and therefore may be able to affect numerous pathways or cellular processes that involve multiple target genes.[17] miRNAs play important roles as critical regulators of brain development, homoeostasis, and pathological processes.[12,13,14] The previous study reported that cerebral ischemia-induced changes of miRNAs involved in angiogenesis, apoptosis, and neurogenesis.[18] These studies also supported the promising potential for miRNAs as novel biomarkers and new targets for stroke.[12] Although miRNAs control a number of conditions and diseases, miRNA profiles after stroke treatment with mild hypothermia still lack proper investigation.
In this study, miRNA profiles were analyzed in rats with cerebral ischemia treated with mild therapeutic hypothermia. Mild therapeutic hypothermia may protect against cerebral ischemia/reperfusion by inhibiting miR-15b expression in rats, making miR-15b a potential target for the future stroke therapies.
Materials and Methods
Animal model
All animal experiments were approved by the Institutional Animal Care and Use Committee of Capital Medical University and conducted in accordance with the principles outlined in the National Institutes of Health (US) Guide for the Care and Use of Laboratory Animals. Male Sprague Dawley rats weighing 280–310 g suffered from transient ischemia for 2 h. The trachea was intubated after rapid inhalation induction of anesthesia with 5% isoflurane in oxygen, and the lungs were mechanically ventilated with 2% isoflurane in 30% O2/70% N2. Focal ischemia was induced using the intraluminal vascular occlusion method as previously described.[19] Right middle cerebral artery occlusion (MCAO) was performed by inserting 3-0 monofilament nylon suture into the internal carotid artery. For miRNA microarray analysis, sham-operated and MCAO groups were treated with mild hypothermia or normothermia. To ensure the occurrence of ischemia, regional cerebral blood flow was monitored using laser Doppler flowmetry (PeriFlux System 5000, Perimed, Stockholm, Sweden). Blood pressure was monitored through a data acquisition system (MP100A-CE, BIOPAC Systems, Inc., Santa Barbara, CA, USA). All animals were housed in an air-conditioned room at 25°C ± 1°C after recovering from anesthesia. Rectal temperature was measured by a thermocouple probe placed in the rectum and controlled at 37.0°C ± 0.5°C throughout the experiment with a heating lamp and a temperature-regulated heating pad. Brain temperature was measured by means of a thermocouple probe placed close to the skull under the right temporalis. Mild therapeutic hypothermia (33°C ± 0.5°C) was applied by means of ice packs placed around the head/neck and were replaced every hour. Mild therapeutic hypothermia was applied from the time of induction of ischemia for the duration of 3 h.
miRNA microarray
mRNA was isolated from the right cortex using TRIzol (Life Technologies, Carlsbad, CA, USA) and the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA). The absorbance of all samples was measured at 260 nm and 280 nm. miRNA expression profiling was determined using an Affymetrix Rat miRNA Microarray according to the manufacturer's protocol (Affymetrix, Santa Clara, CA, USA). Briefly, 1 μg total RNA was added to the Poly (A)-tailing. FlashTag Biotin Labeling Kits (Genisphere, Hatfield, PA, USA) were used to label miRNA. Labeled RNAs were hybridized with Eukaryotic Hybridization Control Kit (Affymetrix) and Hybridization, Wash and Stain Kit (Affymetrix) according to the manufacturer's protocol. Microarray was scanned using an Affymetrix GeneChip Scanner (Affymetrix).
miRNA microarray data analysis
The miRNA microarray data were analyzed using a miRNA microarray analysis program obtained from Affymetrix GeneChip Command Console (Affymetrix).
Quantitative reverse transcription-polymerase chain reaction validation of miRNA expression
Total RNA was extracted from brain tissues using TRIzol (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Then, 1.5% formaldehyde denaturing agarose gel electrophoresis was used to detect the quantity of total RNA. RNA was reverse-transcribed to cDNA with the stem-loop RT primer using the M-MLV reverse transcriptase (Promega, Madison, WI, USA). Quantitative polymerase chain reaction (PCR) was carried out using AmpliTaq Gold Enzyme (Applied Biosystems, Austin, TX, USA). The miRNA universal sense primer was 5′-GTGCAGGGTCCGAGGT-3′. The RT primer of rno-miR-15b was 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGTAAA-3′; antisense primer of rno-miR-15b was 5′-ACGTAGCAGCACATCATGGTTT-3′; RT primer of rno-miR-598-3p was 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTAACGA-3′; antisense primer of rno-miR-598-3p was 5′-TCTACGTCATCGTCGTCATCG-3′. U6 was amplified as the internal control by forward primer 5′-CTCGCTTCGGCAGCACA-3′, and reverse primer 5′-AACGCTTCACGAATTTGCGT-3′. Reverse transcription (RT) reactions were incubated for 10 min at 16°C, 30 min at 37°C, and 5 min at 65°C. The real-time PCR protocol was used as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, 60°C for 30 s, and 70°C for 30 s.
Cells culture and oligonucleotide transfection
PC12 cells were incubated in RPMI 1640 (Gibco, Life Technologies, Gaithersburg, MD, USA) with 10% horse serum (Gibco), 5% fetal bovine serum (Gibco), and 100 U/ml penicillin/streptomycin (Life Technologies). Cells were maintained at 37°C and 5% CO2. miR-15b mimic and control were purchased from Life Technologies. PC12 cells were inoculated at a density of 3.5 × 105 per well in six-well plates and were transfected using Lipofectamine RNAiMAX (Life Technologies) when they reached 60% confluence. Cells were collected at 24, 48, and 72 h after miRNA transfection. Three independent replications were performed for all experiments.
Western blot analysis
Cells were collected at 0, 24, 48, and 72 h after miRNA transfection, and tissues were collected 24 h after ischemia. Cells and tissues were lysed in lysis buffer (10 mM Tris–HCl, 150 mM NaCl, 600 mM NP-40, 5 mM EDTA, and 2% TritonX100). The lysates were cleared by centrifugation and boiled with sodium dodecyl sulfate (SDS)-sample buffer. Protein concentration was determined using a bicinchoninic acid assay protein assay kit (Pierce, Rockford, IL, USA). Samples were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes using semi-dry blotting. Protein samples (100 μg per lane) were separated on a 12% SDS-polyacrylamide gel. Rabbit anti-ADP ribosylation factor-like 2 (Arl2) polyclonal antibody was used (1:200 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). The blots were visualized by chemiluminescence (Millipore, Billerica, MA, USA). Antiactin antibody was used as an internal loading control. The relative optical density of protein bands was measured using Image-Pro Plus Analysis Software (Media Cybernetics, Inc., Rockville, MD, USA).
Infarct volume analysis
Infarct volume was determined by 2,3,5-triphenyltet-razolium chloride (TTC) as previously described.[20] Six coronal brain sections (2-mm thick) were sliced using a brain matrix. Coronal sections were incubated in a 2% TTC-saline solution for 30 min at 37°C, and then, fixed in 4% paraformaldehyde/phosphate-buffered saline. TTC staining patterns were photographed at 24 h after fixation and were then analyzed using Image-Pro Plus Analysis Software.
Neurological function assessment
Neurological functional deficits were assessed at 24 h after reperfusion in a blind fashion as previously described.[21,22] Neurological functions were graded on a scale from 0 to 12 (normal score, 0; maximal score, 12).
Measurement of cellular and tissular adenosine triphosphate
The amount of adenosine triphosphate (ATP) in the cell lysates was measured using an ATP assay kit (Beyotime Biotechnology, China) in accordance with the manufacturer's instructions.
Intracerebroventricular injection
miRNA mimics were purchased from Life Technologies. Seventy adult male Sprague Dawley rats were randomly assigned into four groups: 37°C + control mimic group (n = 17), 37°C + miR-15b mimic group (n = 17), 33°C + control mimic group (n = 18), and 33°C + miR-15b mimic group (n = 18). Intracerebroventricular injection was performed 60 min before cerebral ischemia as previously described.[23] The coordinates from bregma and pial surface from the tip of the injection needle were anteroposterior = −0.8 mm; mediolateral = +1.5 mm; dorsoventral = −3.2 mm. A volume of 10 μM miRNA in a total volume of 10 μl including 2 μl Lipofectamine 2000 was injected 30 min before MCAO surgery.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 software (GraphPad Software Inc., La Jolla, CA, USA). All data are reported as mean ± standard error of mean. The significance of difference was assessed by one-way analysis of variance and Tukey's multiple comparisons test. Statistical significance was set at a value of P < 0.05.
Results
Total RNA quality
Total RNA was extracted from the ipsilateral cortex of MCAO rats treated with normothermia and mild hypothermia. The quality of total RNA was evaluated by the A260/A280 ratio, which was in the range of 1.8–2.0 (data not shown). To detect the RNA integrity, formaldehyde denaturing gel electrophoresis was performed. The results showed that the 28S and 18S bands were bright and that 28S: 18S RNA bands were greater than or close to 1:1 [Figure 1a]. These data confirmed that the quality of RNA reached the requirements of miRNA array analysis.
Figure 1.
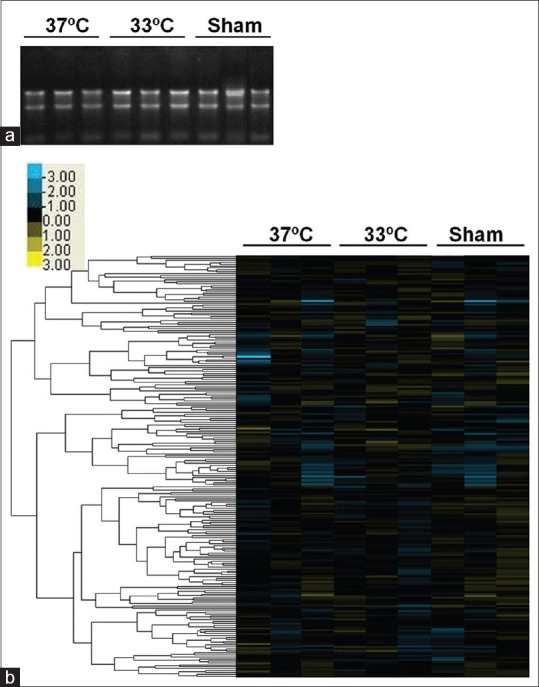
Effect of mild hypothermia on microRNA expression after cerebral ischemia. middle cerebral artery occlusion animals were subjected to transient cerebral ischemia for 2 h, with 3 h of normothermia or hypothermia treatment from the induction of cerebral ischemia and were sacrificed at 24 h after cerebral ischemia. (a) The quality of total RNA extracted from the cortex was confirmed by formaldehyde gel electrophoresis. (b) Hierarchical clustering analysis of sham-operated, cerebral ischemia treated with mild hypothermia or normothermia groups at 24 h after ischemia. Heatmap coloring indicates relative signal intensities related to microRNA expression levels. The blue box represents downregulation, yellow represents upregulation, and black represents no change. n = 3 rats per group. 37°C, normothermia; 33°C, mild hypothermia
Microarray analysis
The hierarchical clustering was shown as Figure 1b. Selective upregulation (>1.5-fold) or downregulation (<0.7-fold) were determined by comparisons with the control and hypothermia group. In our screen of the miRNA transcriptome, we found that two miRNAs, rno-miR-15b (fold change: 0.6654) and rno-miR-598-3p (fold change: 0.5693) were significantly decreased at 24 h after cerebral ischemia.
Quantitative polymerase chain reaction validation
To validate the expression of miRNAs in stroke treated with mild hypothermia, we detected the expression of rno-miR-15b and rno-miR-598-3p by RT-PCR. As shown in Figure 2a and b, rno-miR-15b and rno-miR-598-3p were downregulated at 24 h after cerebral ischemia treatment with mild hypothermia.
Figure 2.
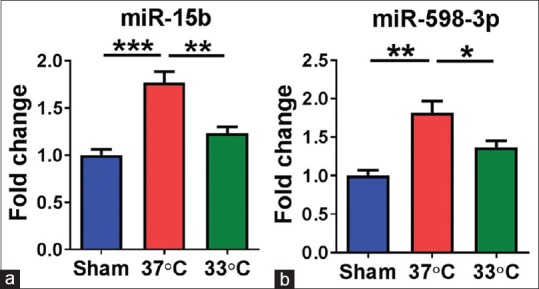
Mild hypothermia changed the expression of miR-15b. (a) And miR-598-3p (b) n = 4 per group. 37°C, normothermia; 33°C, mild hypothermia. Data are expressed as mean ± standard error of mean *P < 0.05, **P < 0.01, ***P < 0.001, by one-way analysis of variance and Tukey's test
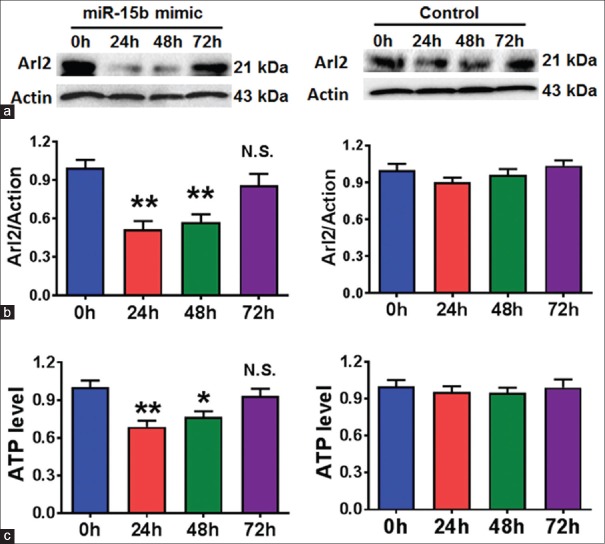
miR-15b overexpression inhibited the expression of its target ADP-ribosylation factor-like 2 and adenosine triphosphate levels in PC12 cells
To investigate the effect of miR-15b on its target Arl2 and ATP levels,[24] miR-15b mimic and control were transfected to PC12 cells. As shown in Figure 3a and b, miR-15b mimic decreased the expression of Arl2 at 24 h and 48 h after transfection (P < 0.01 and P < 0.01, respectively). Moreover, miR-15b mimic attenuated ATP levels in PC12 cells at 24 h and 48 h after transfection (P < 0.01 and P < 0.05, respectively).
Figure 3.
miR-15b inhibited ADP ribosylation factor-like 2 expression and decreased adenosine triphosphate levels in PC12 cells. (a) Representative Western blot of ADP ribosylation factor-like 2 after transfection with miR-15b mimic or control for 24, 48 and 72 h. β-Actin served as a loading control. (b) Statistical analysis of Western blot. miR-15b mimic led to downregulation of ADP ribosylation factor-like 2 protein levels in PC12 cells. n = 4 per group. (c) Cellular adenosine triphosphate levels in different treatment groups. 37°C, normothermia; 33°C, mild hypothermia. Data are expressed as mean ± standard error of mean *P < 0.05, **P < 0.01, by one-way analysis of variance and Tukey's test
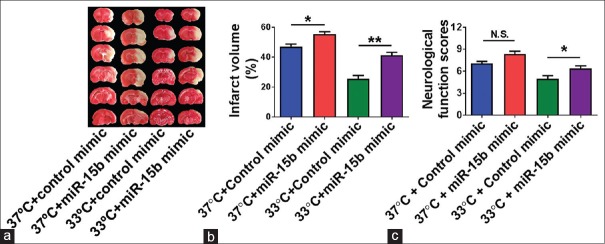
miR-15b overexpression increased infarct volume and aggravated functional outcomes in cerebral ischemia of rats treated with or without mild therapeutic hypothermia
To assess the effect of miR-15b on cerebral ischemia and mild hypothermia-mediated neuroprotection, miR-15b mimic or control miRNA was injected into the ipsilateral ventricle of MCAO rats treated with normothermia and mild hypothermia 60 min before the beginning of ischemia. TTC staining and neurological severity assessment were performed at 24 h after reperfusion. As shown in Figure 4a and b, infarct volume in the miR-15b mimic plus normothermia group was increased significantly compared to the control mimic + normothermia group (P < 0.05). miR-15b mimic aggravated the neurological function compared to control mimic at normothermia [Figure 4c]. The 33°C + miR-15b mimic group had increased infarct volume (P < 0.01) [Figure 4a and b] and aggravated neurological function (P < 0.05) [Figure 4c] compared with the 33°C + control mimic group.
Figure 4.
The effect of miR-15b and hypothermia plus miR-15b on rat cerebral ischemic injury. (a) Representative TTC staining of brain slices in different groups at 24 h after reperfusion. (b) Statistical analysis of infarct volume in different treatment groups. n = 9–10 per group (c) Statistical analysis of neurological function scores in different groups. n = 17–18 per group. 37°C, normothermia; 33°C, mild hypothermia. N. S., no significance. *P < 0.05, **P < 0.01, by one-way analysis of variance and Tukey's test
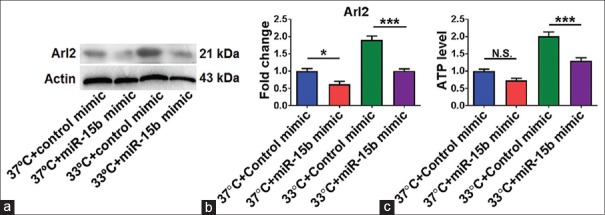
miR-15b overexpression inhibited the expression of its target ADP ribosylation factor-like 2 and ATP levels in middle cerebral artery occlusion rats under normothermia or hypothermia
To further explore the role of miR-15b on stroke treated with mild hypothermia in vivo, Western blot was performed to evaluate the protein level of its target Arl2 and tissular ATP levels using an ATP assay kit. As shown in Figure 5a and b, the expression level of Arl2 was decreased significantly in the 37°C + miR-15b mimic group compared with the 37°C + control mimic group (P < 0.05). Tissular ATP levels had a decreased trend in the 37°C + miR-15b mimic group, but did not reach statistical significance (P = 0.086) [Figure 5c]. The Arl2 and ATP expression levels in the 33°C + miR-15b mimic group were lower than in the 33°C + control mimic group (both P < 0.001) [Figure 5].
Figure 5.
miR-15b inhibited ADP ribosylation factor-like 2 expression and decreased adenosine triphosphate levels in middle cerebral artery occlusion mice treated with mild hypothermia. (a) Representative Western blot of ADP ribosylation factor-like 2. (b) Statistical analysis of Western blot. n = 4 per group. (c) adenosine triphosphate levels in different treatment groups. 37°C, normothermia; 33°C, mild hypothermia. Data are expressed as mean ± standard error of mean N. S., no significance. *P < 0.05, ***P < 0.001, by one-way ANOVA and Tukey's test
Discussion
In this study, we investigated the miRNA profile following cerebral ischemia treated with mild therapeutic hypothermia and identified specific miRNA changes at 24 h after ischemia. Our results demonstrate that mild therapeutic hypothermia protected the brain against cerebral ischemia/reperfusion injury by downregulation of miR-15b in rats.
miRNAs have been proposed as novel biomarkers and therapeutic targets for stroke.[25] Although we only detected a small amount of differentially expressed miRNAs, we verified the expression of two miRNAs, rno-miR-598 and rno-miR-15b. A recent study reported that miR-598 inhibited Notch2 expression[26] while Notch2 may regulate ischemia/reperfusion-associated production of injurious reactive oxygen species, cell death, and inflammation.[27,28] However, the exact role of miR-598 after stroke is still unknown and needs to be further explored. In a similar study in cardiac myocytes, miR-15b was downregulated and modulated cellular ATP levels through Arl2.[24] miR-15b has many mRNA targets, such as Bcl-2, interleukin-15, and Cyclin D1, which are related to apoptosis, inflammation, and cell growth.[29,30,31] Thus, the destructive role of miR-15b after stroke may depend on several mRNA targets and may not be confined to Arl2.
As a member of the ARF family and RAS superfamily of regulatory GTPases,[32] Arl2 is highly conserved in eukaryotes and highly expressed in the brain.[33] Arl2 is associated with mitochondrial functions such as mitochondrial morphology, motility, and maintenance of ATP levels.[32] In addition, Arl2 plays a vital role in microtubule dynamics,[34] apoptosis,[35] cell cycle progression,[36] asymmetric division of neural stem cells,[37] and survival of neural progenitor cells.[35]
Brain tissues demand high energy to sustain homeostasis.[38] Cerebral ischemia leads to mitochondrial dysfunction, ATP depletion, cytoskeletal disruption, and ultimately necrosis or apoptosis.[39,40] ATP depletion occurs within a very short period in cerebral ischemia and triggers plasma membrane depolarization.[38,39] Mild therapeutic hypothermia has a neuroprotective effect on stroke by lowering the metabolic rate and energy consumption, such as prevention of ATP depletion.[41] Our results showed that mild hypothermia may maintain relatively high levels of ATP and that miR-15b transfection decreased ATP levels after stroke treatment with mild hypothermia. A previous study showed that ATP treatment has a striking detrimental impact on cerebral ischemia by inducing mild hypothermia,[42] which contradicts our results. In fact, mild hypothermia facilitated ATP recovery following cerebral ischemia in gerbils.[43] The previous studies reported that cerebral ischemia led to a decrease in intracellular ATP levels, but an increase in extracellular levels.[44,45,46] In the present study, we only detected cellular ATP levels and did not distinguish between extra- and intracellular ATP locations. Thus, the relationship between mild hypothermia and intracellular and extracellular ATP levels should be investigated in more detail.
Several limitations of our study deserve mentioning. First, miR-15b inhibitor was not applied to detect the role of miR-15b, which may provide neuroprotection after stroke. Second, because sham-operated groups treated with normothermia or hypothermia do not differ in apoptosis and the expression level of many proteins related to energetic metabolism,[47,48,49] sham-operated groups treated with hypothermia were not included as a control group. Third, our study did not provide a direct causal relationship between the observed changes in miRNA expression and ATP levels. It is well known that mild hypothermia therapy can reduce the volume of cerebral infarction and improve the neurological function after stroke, but the effect size of the differentially expressed miRNAs is too small. Thus, further studies are needed to clarify the reason for the small number of differentially expressed miRNAs in cerebral ischemia treated with mild therapeutic hypothermia.
Conclusions
In summary, we provide a dataset of miRNA expression after stroke treatment with mild hypothermia and normothermia. Mild therapeutic hypothermia protects against cerebral ischemia/reperfusion by inhibiting miR-15b expression, which may serve as a novel target for stroke therapy.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Grant no. 81471209, 81641055, 81000504, 81171241), the National Natural Science Foundation for Distinguished Young Scholars (Grant no. 81325007), Beijing Natural Science Foundation (Grant no. 7132112), the Distinguished Professor of Cheung Kong Scholars program (Grant no. T2014251), and the Beijing Municipal Administration of Hospitals’ Mission Plan (Grant no. SML20150802).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014;5:442–53. doi: 10.1007/s12975-014-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W, Yang S. Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies. Brain Circ. 2016;2:153–63. doi: 10.4103/2394-8108.195279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Bryant Z, Vann KT, Xiong ZG. Translational strategies for neuroprotection in ischemic stroke – Focusing on acid-sensing ion channel 1a. Transl Stroke Res. 2014;5:59–68. doi: 10.1007/s12975-013-0319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadhav AP, Jovin TG. Endovascular therapy for acute ischemic stroke: The standard of care. Brain Circ. 2016;2:178–82. doi: 10.4103/2394-8108.195283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang YH, Ma YY, Zhang ZJ, Wang YT, Yang GY. Opportunities and challenges: Stem cell-based therapy for the treatment of ischemic stroke. CNS Neurosci Ther. 2015;21:337–47. doi: 10.1111/cns.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chau M, Zhang J, Wei L, Yu SP. Regeneration after stroke: Stem cell transplantation and trophic factors. Brain Circ. 2016;2:86–94. doi: 10.4103/2394-8108.186279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahr MM, Luijckx GJ, Vroomen PC, van der Zee DJ, Buskens E. The chain of care enabling tPA treatment in acute ischemic stroke: A comprehensive review of organisational models. J Neurol. 2013;260:960–8. doi: 10.1007/s00415-012-6647-7. [DOI] [PubMed] [Google Scholar]
- 8.Choi HA, Badjatia N, Mayer SA. Hypothermia for acute brain injury – Mechanisms and practical aspects. Nat Rev Neurol. 2012;8:214–22. doi: 10.1038/nrneurol.2012.21. [DOI] [PubMed] [Google Scholar]
- 9.Darwazeh R, Yan Y. Mild hypothermia as a treatment for central nervous system injuries: Positive or negative effects. Neural Regen Res. 2013;8:2677–86. doi: 10.3969/j.issn.1673-5374.2013.28.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han Z, Liu X, Luo Y, Ji X. Therapeutic hypothermia for stroke: Where to go? Exp Neurol. 2015;272:67–77. doi: 10.1016/j.expneurol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Yenari MA. Hypothermia for treatment of stroke. Brain Circ. 2015;1:14–25. [Google Scholar]
- 12.Ouyang YB, Stary CM, Yang GY, Giffard R. MicroRNAs: Innovative targets for cerebral ischemia and stroke. Curr Drug Targets. 2013;14:90–101. doi: 10.2174/138945013804806424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saugstad JA. MicroRNAs as effectors of brain function. Stroke. 2013;44:S17–9. doi: 10.1161/STROKEAHA.113.000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saugstad JA. Non-coding RNAs in stroke and neuroprotection. Front Neurol. 2015;6:50. doi: 10.3389/fneur.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 16.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 18.Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, et al. MicroRNAs in stroke pathogenesis. Curr Mol Med. 2011;11:76–92. doi: 10.2174/156652411794859232. [DOI] [PubMed] [Google Scholar]
- 19.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–22. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y, Chen F, Ni Y, Marchal G, Collen D, Nagai N, et al. Microplasmin reduces ischemic brain damage and improves neurological function in a rat stroke model monitored with MRI. Stroke. 2004;35:2402–6. doi: 10.1161/01.STR.0000140628.00927.1a. [DOI] [PubMed] [Google Scholar]
- 21.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H, et al. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 22.De Ryck M, Van Reempts J, Borgers M, Wauquier A, Janssen PA. Photochemical stroke model: Flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20:1383–90. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke. 2009;40:1482–9. doi: 10.1161/STROKEAHA.108.531509. [DOI] [PubMed] [Google Scholar]
- 24.Nishi H, Ono K, Iwanaga Y, Horie T, Nagao K, Takemura G, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem. 2010;285:4920–30. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koutsis G, Siasos G, Spengos K. The emerging role of microRNA in stroke. Curr Top Med Chem. 2013;13:1573–88. doi: 10.2174/15680266113139990106. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Zhang H, Chen Y, Qiao G, Jiang W, Ni P, et al. MiR-598 inhibits metastasis in colorectal cancer by suppressing JAG1/Notch2 pathway stimulating EMT. Exp Cell Res. 2017;352:104–12. doi: 10.1016/j.yexcr.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Meng S, Su Z, Liu Z, Wang N, Wang Z. Rac1 contributes to cerebral ischemia reperfusion-induced injury in mice by regulation of Notch2. Neuroscience. 2015;306:100–14. doi: 10.1016/j.neuroscience.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Huang R, Zhou Q, Veeraragoo P, Yu H, Xiao Z. Notch2/Hes-1 pathway plays an important role in renal ischemia and reperfusion injury-associated inflammation and apoptosis and the γ-secretase inhibitor DAPT has a nephroprotective effect. Ren Fail. 2011;33:207–16. doi: 10.3109/0886022X.2011.553979. [DOI] [PubMed] [Google Scholar]
- 29.Shi H, Sun BL, Zhang J, Lu S, Zhang P, Wang H, et al. MiR-15b suppression of Bcl-2 contributes to cerebral ischemic injury and is reversed by sevoflurane preconditioning. CNS Neurol Disord Drug Targets. 2013;12:381–91. doi: 10.2174/1871527311312030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L, Liu T, Zhang M, Guo Y, Song C, Song D, et al. MiR-15b is downregulated in myasthenia gravis patients and directly regulates the expression of interleukin-15 (IL-15) in experimental myasthenia gravis mice. Med Sci Monit. 2015;21:1774–80. doi: 10.12659/MSM.893458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun G, Shi L, Yan S, Wan Z, Jiang N, Fu L, et al. MiR-15b targets cyclin D1 to regulate proliferation and apoptosis in glioma cells. Biomed Res Int 2014. 2014 doi: 10.1155/2014/687826. 687826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman LE, Zhou CJ, Mudigonda S, Mattheyses AL, Paradies E, Marobbio CM, et al. The ARL2 GTPase is required for mitochondrial morphology, motility, and maintenance of ATP levels. PLoS One. 2014;9:e99270. doi: 10.1371/journal.pone.0099270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharer JD, Shern JF, Van Valkenburgh H, Wallace DC, Kahn RA. ARL2 and BART enter mitochondria and bind the adenine nucleotide transporter. Mol Biol Cell. 2002;13:71–83. doi: 10.1091/mbc.01-05-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beghin A, Honore S, Messana C, Matera EL, Aim J, Burlinchon S, et al. ADP ribosylation factor like 2 (Arl2) protein influences microtubule dynamics in breast cancer cells. Exp Cell Res. 2007;313:473–85. doi: 10.1016/j.yexcr.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Jiang H, Gu J, Tang Y, Shen N, Jin Y, et al. MicroRNA-195 targets ADP-ribosylation factor-like protein 2 to induce apoptosis in human embryonic stem cell-derived neural progenitor cells. Cell Death Dis. 2013;4:e695. doi: 10.1038/cddis.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou C, Cunningham L, Marcus AI, Li Y, Kahn RA. Arl2 and Arl3 regulate different microtubule-dependent processes. Mol Biol Cell. 2006;17:2476–87. doi: 10.1091/mbc.E05-10-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen K, Koe CT, Xing ZB, Tian X, Rossi F, Wang C, et al. Arl2- and Msps-dependent microtubule growth governs asymmetric division. J Cell Biol. 2016;212:661–76. doi: 10.1083/jcb.201503047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llorente-Folch I, Rueda CB, Pardo B, Szabadkai G, Duchen MR, Satrustegui J, et al. The regulation of neuronal mitochondrial metabolism by calcium. J Physiol. 2015;593:3447–62. doi: 10.1113/JP270254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baxter P, Chen Y, Xu Y, Swanson RA. Mitochondrial dysfunction induced by nuclear poly(ADP-ribose) polymerase-1: A treatable cause of cell death in stroke. Transl Stroke Res. 2014;5:136–44. doi: 10.1007/s12975-013-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoler O, Fleidervish IA. Functional implications of axon initial segment cytoskeletal disruption in stroke. Acta Pharmacol Sin. 2016;37:75–81. doi: 10.1038/aps.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andresen M, Gazmuri JT, Marín A, Regueira T, Rovegno M. Therapeutic hypothermia for acute brain injuries. Scand J Trauma Resusc Emerg Med. 2015;23:42. doi: 10.1186/s13049-015-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Li W, Niu G, Leak RK, Chen J, Zhang F. ATP induces mild hypothermia in rats but has a strikingly detrimental impact on focal cerebral ischemia. J Cereb Blood Flow Metab. 2013;33:e1–e10. doi: 10.1038/jcbfm.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura T, Sako K, Tanaka K, Kusakabe M, Tanaka T, Nakada T, et al. Effect of mild hypothermia on energy state recovery following transient forebrain ischemia in the gerbil. Exp Brain Res. 2002;145:83–90. doi: 10.1007/s00221-002-1095-8. [DOI] [PubMed] [Google Scholar]
- 44.Jurányi Z, Sperlágh B, Vizi ES. Involvement of P2 purinoceptors and the nitric oxide pathway in [3H] purine outflow evoked by short-term hypoxia and hypoglycemia in rat hippocampal slices. Brain Res. 1999;823:183–90. doi: 10.1016/s0006-8993(99)01169-5. [DOI] [PubMed] [Google Scholar]
- 45.Melani A, Turchi D, Vannucchi MG, Cipriani S, Gianfriddo M, Pedata F, et al. ATP extracellular concentrations are increased in the rat striatum during in vivo ischemia. Neurochem Int. 2005;47:442–8. doi: 10.1016/j.neuint.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;101:1400–13. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X, Wen S, Zhao S, Yan F, Zhao S, Wu D, et al. Mild therapeutic hypothermia protects the brain from ischemia/reperfusion injury through upregulation of iASPP. Aging Dis. doi: 10.14336/AD.2017.0703. ahead of print. Available from: http://www.aginganddisease.org/EN/10.14336/AD.2017.0703 . [DOI] [PMC free article] [PubMed]
- 48.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, et al. AKT contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab. 2007;27:1879–94. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]