Abstract
Background:
Mandating a reduction in the nicotine content of cigarettes to a minimally addictive level could dramatically reduce smoking rates in the US. However, little is known about the effects of reduced nicotine content cigarettes in adolescents.
Methods:
Following overnight abstinence, adolescent daily smokers (ages 15–19, n = 50) reported on their craving, withdrawal, and positive and negative affect pre- and post- ad lib smoking of one cigarette containing varying nicotine content (15.8, 5.2, 1.3 and 0.4 mg/g of tobacco) in the laboratory and reported their subjective evaluations of each cigarette. Carbon monoxide (CO) boost from pre- to post-cigarette was calculated to determine if lower-nicotine cigarettes led to differential acute changes in toxicant exposure.
Results:
All four nicotine cigarette types significantly reduced abstinence-induced craving, withdrawal, and negative affect (all p’s<.05). Mixed models evaluating the effect of nicotine content, with nicotine dependence level and gender included as covariates, revealed a significant effect of nicotine content on craving and subjective evaluations: higher nicotine content resulted in greater reductions in craving and increases in both positive and negative subjective evaluations. There were no significant effects of nicotine dose on withdrawal symptoms, negative affect, or CO boost.
Conclusions:
These results suggest that lower nicotine cigarettes might result in reduced abuse liability compared to higher nicotine content cigarettes due to reduced positive subjective effects, while still reducing withdrawal, in adolescents. These results highlight the potential feasibility of this policy approach and support continued research on how a nicotine reduction policy may affect adolescent smoking patterns.
Keywords: Adolescent, Smoking, Regulatory science, Nicotine, Nicotine reduction
1. Introduction
Cigarette smoking is one of the leading causes of preventable death worldwide (WHO, 2015), and smokers on average lose ten years of life expectancy compared to nonsmokers (Thun et al., 2013). The vast majority of adult cigarette smokers begin smoking in adolescence (USDHHS, 2013) and early smoking onset is correlated with increased dependence and greater risk for smoking-related disease and death (Jha etal., 2013; Kendler et al., 2013; Marshall et al., 2006). As of 2016, 18.2% of high schoolers in the United States had ever tried cigarettes (Johnston et al., 2017). While recent data indicate that adolescent daily smoking is at its lowest recorded levels (Johnston et al., 2017), current rates of use remain unacceptably high, particularly in certain subgroups of vulnerable youth. Given the potential toll of cigarettes on youth, novel public health policy initiatives that can reduce smoking in all adolescents are warranted.
With the 2009 passage of the Family Smoking Prevention and Tobacco Control Act (FSPTCA), the Food and Drug Administration (FDA) acquired regulatory jurisdiction over tobacco products, including the authority to reduce, although not eliminate, nicotine in cigarettes to a minimally-addictive level (United States Congress, 2009). Recently the FDA announced its intention to pursue such a policy (Gottlieb and Zeller, 2017). Reducing levels of nicotine to below the threshold that can maintain addiction has the potential to impact the pattern of cigarette smoking over the lifetime (Benowitz and Henningfield, 2013). For first-time smokers, very low nicotine content (VLNC; 2.5mg/g nicotine or less) cigarettes may be less addictive, which may result in fewer youth who experiment transitioning from occasional to daily smoking; for young and adult current smokers, VLNC cigarettes could facilitate abstinence from smoking (Donny et al., 2015).
As discussion of a nicotine reduction policy for cigarettes moves forward, it is important to consider potential consequences for current smokers. Two important considerations are the potential for discomfort from acute nicotine withdrawal that smokers might experience when nicotine levels are reduced, and the potential abuse liability of VLNC cigarettes that would minimize progression and facilitate cessation of smoking. Among adult smokers, VLNC cigarettes have been shown to acutely reduce withdrawal symptoms, craving, and negative affect in the laboratory, indicating that withdrawal is alleviated by VLNC cigarettes in adults (Butschky et al., 1995; Higgins et al., 2017). Effects of VLNC cigarettes on withdrawal may be due, in part, to the conditioned reinforcing effects of sensorimotor smoking cues that have been repeatedly paired with nicotine delivery (Rose and Levin et al., 1991).
Abuse liability can be measured by asking about subjective effects of cigarette use, as positive subjective evaluations may indicate greater abuse liability (Schoedel and Sellers, 2008). Adults show dose-dependent effects of nicotine content on subjective responses to cigarettes under acute smoking conditions. For example, when comparing three nicotine doses in cigarettes (11.4–12.8mg/g 5.7–5.8mg/g, and 0.4mg/g) in a single laboratory session, adults rated higher-nicotine content research cigarettes as having significantly more psychological reward, satisfaction, enjoyment of sensations, and craving reduction than VLNC cigarettes (Hatsukami et al., 2013). In another recent study comparing acute responses to several doses of nicotine (15.8, 5.2, 2.4 and 0.4mg/g) in research cigarettes in the laboratory, VLNC cigarettes were rated as less satisfying than normal-nicotine content (NNC) cigarettes; similarly, higher dose was associated with greater reinforcing efficacy both when measured behaviorally and using hypothetical measures (Higgins et al., 2016, 2017).
However, relatively little is known about the potential effects of reducing nicotine content in cigarettes, including the potential for VLNC cigarettes to alleviate withdrawal and the abuse liability of these cigarettes for adolescent smokers. Compared to adults, adolescents are more likely to be lighter or intermittent smokers (Colby et al., 2000a; Mermelstein et al., 2002); however, they tend to be less responsive to empirically-supported cessation interventions (Colby and Gwaltney, 2007). Like adult smokers, adolescent smokers experience significant negative effects of smoking abstinence such as withdrawal symptoms, craving to smoke, and increased negative affect, and these effects are reversed with smoking reinstatement (Bidwell et al., 2013; Colby et al., 2010). Furthermore, given their shorter smoking histories, adolescents may be less sensitive than adults to the effects of smoking-associated sensorimotor cues. Thus, the conditioned reinforcing effects of VLNC cigarettes, which effectively buffer the effects of nicotine reduction on withdrawal symptoms and craving in adults (e.g., Higgins et al., 2017), may be less effective at buffering these effects in adolescents.
In one laboratory study of adolescent smokers age 15–18, both nicotine-containing and denicotinized cigarettes (0.06mg/g of nicotine) acutely reduced negative affect, and this effect was stronger in participants with greater nicotine dependence (Kassel et al., 2007a,b). In a recent double-blind, within-subjects study with young adult smokers (ages 18–25; M age=22), responses to four doses (corresponding to approximately 15.8, 5.2, 1.3 and 0.4mg/g of tobacco) of nicotine in research cigarettes were compared. Young adult smokers rated the highest nicotine content cigarettes as more satisfying than the lower doses; however, there were no significant effects of nicotine dose on withdrawal and craving (Faulkner et al., 2017). To date no studies have compared effects of different doses of nicotine in cigarettes on craving, withdrawal symptoms or subjective effects indicative of abuse liability in adolescents, and basic questions about the effects of nicotine in cigarettes in adolescents are unanswered.
The aim of this study was to investigate the effects of varying nicotine content PROOF in cigarettes (15.8, 5.2, 1.3 and 0.4mg/g of tobacco) on: (1) abstinence-induced withdrawal symptoms, craving, and negative affect; and (2) positive and negative subjective evaluations of cigarettes in adolescent daily cigarette smokers, using a within-subjects experimental design, we also explored differences in carbon monoxide (CO) boost pre-to post-smoking as an exploratory aim to determine if reducing the level of nicotine would lead to acute changes in toxicant exposure, which may occur if adolescents were to inhale more deeply or more frequently at lower doses (Kassel et al., 2007 a,b). The four nicotine conditions were tested in four separate laboratory sessions, in counterbalanced order, following overnight abstinence. We chose these doses to provide a control dose (15.8mg/g) that contains roughly the same amount of nicotine as a commercial cigarette; a dose (5.2mg/g) that was hypothesized to be above the reinforcing threshold; and two doses below the hypothesized threshold (1.3mg/g and 0.4mg/g) to determine if differential effects on behavior were evident at these very low levels in this population (Donny et al., 2015). The lowest dose tested results in a nicotine yield of ~0.04mg, which is effectively denicotonized; however, because the FDA does not have the authority to reduce nicotine content in cigarettes to zero (US Congress, 2009), testing the effects of a completely nicotine-free cigarette would not be informative for tobacco regulation.
2. Materials and methods
2.1. Recruitment, screening, and consent
Participants were recruited from Rhode Island and surrounding areas via Craigslist, Facebook, Instagram, local bus advertisements, and informational tables set up at schools during lunchtime and at various other community events. Interested adolescents called the research office and were read a short script with information about the study. If they remained interested in participating, they were asked to complete a brief, confidential telephone screening interview to establish eligibility. If adolescents were recruited in-person, they had the option to self-administer an identical screening questionnaire using an iPad.
The screening questionnaire asked about demographics, past six-month cigarette use, past 30 day frequency of other tobacco/nicotine, alcohol, and other drug use, and plans to quit smoking. If the participant met eligibility criteria and remained interested in participating in the study, the study staff asked permission to contact the participant’s parent (minors only) and asked for contact information. Research staff then called the parent and described the study; if the parent verbally consented to their child’s participation, a parental consent form was mailed to the parent to sign (and one to keep). Minor participants were required to bring the signed consent form to their first session to participate. During the initial baseline session, minor participants completed an informed assent process and signed a written assent form. Participants over 18 provided their own written consent. All study procedures were approved by the Brown University Institutional Review Board.
2.2. Eligibility criteria
To be eligible at screening, adolescents were required to be 15–19 years old, to have smoked at least one cigarette per day on 28 of the last 30 days determined by the Timeline Followback and to be self-reported established daily smokers (for at least the last six months) assessed by the Tobacco Use History Questionnaire (see Section 2.7 below). Participants had to be able to read and write in English. Those who were pregnant, seeking treatment for smoking, planning to quit in the next 30 days, or reported daily alcohol or drug use were excluded (to maximize generalizability to typical adolescent smokers, daily marijuana use was permitted; recent research has shown that the majority of adolescent smokers also use marijuana frequently (Rubinstein et al., 2014)). Those who reported past 30-day suicide plan or attempt were excluded after speaking with the study clinician. At the baseline session, all of the above criteria were re-assessed in person, and pregnancy tests were administered to female participants. Participants were excluded for failing to meet any of the above criteria. In addition, participants were required to provide a breath CO of greater than 6ppm. If they did not, their urine was checked via NicAlert for the presence of cotinine (reading of 3 or higher, indicative of recent smoking, required to participate).
2.3. Research cigarettes
All cigarettes tested were Spectrum research cigarettes (22nd Century Group, Inc.), which are produced for the National Institute on Drug Abuse (NIDA; NOT-DA-14-004). The four doses tested in the current study contained 15.8, 5.2, 1.3 and 0.4mg of nicotine per gram of tobacco, and all tar yields were 9±1.5mg. These correspond to roughly 0.8mg, 0.26mg, 0.07mg and 0.03mg yield of nicotine per cigarette. All doses of research cigarettes were available in menthol and nonmenthol versions, and participants were assigned research cigarettes that corresponded to the menthol status of their usual brand.
2.4. Baseline session
At the initial session, participants completed a battery of interviewer- and self-administered questionnaires and behavioral measures (described below). If participants remained eligible after this visit, their next four experimental sessions were scheduled.
2.5. Experimental sessions
2.5.1. Abstinence criteria
Before each of the four experimental sessions, participants were asked to abstain from smoking cigarettes and (if applicable) marijuana, beginning at 10PM on the night prior to the study session. Abstinence was confirmed via a breath CO level ≤6ppm or a 50% or greater decrease from their baseline CO reading; if participants did not meet the CO criteria, the instructions were explained again and the session was rescheduled. Participants were also asked to sign a written statement that they had not smoked cigarettes or marijuana since 10PM the night before, and had not consumed caffeine in the last hour (as caffeine consumption could interfere with perception of nicotine withdrawal symptoms; Swanson et al., 1994; Treloar et al., 2014).
2.5.2. Procedure
At all experimental sessions, participants completed a battery of pre-cigarette assessments (described below) and were then instructed to smoke a single research cigarette through a smoking topography measurement device.1 The order of dose across sessions was counter-balanced. Within five minutes after finishing the cigarette, participants completed the post-cigarette assessments.
2.6. Compensation
Participants were compensated $25 for completing the in-person screening/baseline session, regardless of eligibility, and $35 for each experimental session completed. A completion bonus of $100 was given to participants who complete all four sessions. The total possible compensation was $265.
2.7. Baseline measures
Expired breath CO level, a reliable and valid assessment of recent smoking, was measured using a Smokerlyzer ED50 CO meter (Bedfont Instruments). Baseline saliva samples were analyzed (Salimetrics, LLC) for cotinine, a sensitive and specific measure of nicotine exposure (Hukkanen et al., 2005; Scherer et al., 2007). Participants provided demographic information, including date of birth, sex, grade in school, race and ethnicity, via self-administered questionnaire. Depressive symptoms were measured using the Center for Epidemiological Studies-Depression questionnaire (CES-D; Radloff, 1991). Participants reported their past-month cigarette, alcohol, marijuana and other tobacco use using a Timeline Follow Back (TLFB; Lewis-Esquerr et al., 2005). Baseline dependence was measured via the Modified Fagerström Tolerance Questionnaire (mFTQ), which has been adapted for use in adolescent populations from the Fagerström Test for Nicotine Dependence; items include cigarettes smoked per day, how soon after waking the participant smoked, and whether they find it difficult to abstain from smoking when they are not able to smoke (Heatherton et al., 1991; Prokhorov et al., 1996).
2.8. Experimental measures
Before and after smoking, nicotine withdrawal symptoms were measured using the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1986), on which individuals rate seven withdrawal symptoms (anger/irritability/frustration, anxiety/nervousness, difficulty concentrating, impatience/restlessness, hunger, depression, cigarette craving) using a 5-point scale (none, slight, mild, moderate, severe); the possible range is 7–35. Craving was assessed via the Brief Questionnaire on Smoking Urges (QSU) on which 10 items are rated on 7-point scales; Factor 1 assesses the positive reinforcement aspects of smoking, including desire to smoke; and Factor 2 assesses the negative reinforcing aspects of smoking, including relief from withdrawal (Cox et al., 2001; Tiffany and Drobes, 1991); the possible range is 1 to 7. Negative affect was assessed via the Positive and Negative Affect Scale (PANAS), on which 10 positive and 10 negative items are rated on 5-point scales (Watson et al., 1988); the possible range is 10–50. Expired breath CO was also measured before and after smoking each cigarette (CO Boost).
Subjective responses to cigarettes were assessed post-smoking via the modified Cigarette Evaluation Scale (mCES), which contains 12 items rated on a 0–6 point Likert scale from ‘not at all’ to ‘extremely’ (Arger et al., 2017; Cappelleri et al., 2007). The mCES is comprised of several subscales: Smoking Satisfaction (e.g., did you enjoy smoking?), Psychological Reward (e.g., did smoking make you feel less irritable?), Aversion (e.g., did smoking make you nauseous?), Enjoyment of Respiratory Tract Sensations (single item; did you enjoy the sensations in your throat and chest?) and Craving Reduction (single item; did smoking immediately relieve your craving for a cigarette?).
2.9. Data analysis plan
All demographic variables were summarized using descriptive statistics. Pre-and post-smoking measures of craving, withdrawal and negative affect were plotted as means. In all reported analyses, effects of cigarette condition on pre-post difference scores (post- minus pre- score) were tested. Modified CES data were also summarized and plotted using descriptive statistics. For all outcomes, we then used a multi-level modeling approach to examine the effects of nicotine content and included nicotine dependence (mFTQ score, centered on the grand mean) and sex in the models based on studies showing that these variables affect responses to cigarettes varying in nicotine content (Kassel et al., 2007a,b; Perkins and Karelitz, 2015). The first level of the model predicted each outcome as a function of cigarette nicotine yield within-person, while the second level modeled the effects of person-level covariates on both initial value of the outcome and sensitivity to changes in nicotine content. Sex and dependence were included as time-invariant predictors of both initial status and rate of change across nicotine level2. We chose this regression-based approach in order to best model the potential magnitude effects of nicotine in cigarettes, by conceptualizing the effects as a continuous measure rather than a comparison means across doses while robustly modeling both across- and within-subject change (Singer and Willett, 2003). All analyses were conducted using HLM Version 7 for Windows (SSI Inc). Tests of significance were considered significant at p≤.05.
3. Results
A total of 195 adolescents were phone screened and 120 (61.5%) were eligible. Of these, 88 participants (73%) completed an in-person screening/baseline session. Of these 88, 55 were confirmed eligible (62.5%). Of the 55 participants who were eligible at baseline, four withdrew voluntarily prior to the first experimental session, and one participant was withdrawn by the PI for excessive rescheduling; leaving 50 participants who completed all sessions. Characteristics of the final sample can be found in Table 1. Participants did not differ by gender on any demographic variables.
Table 1.
Participant Characteristics by gender (n=50).
| Variable | Female (n = 25) M (SD) | Male (n = 25) M (SD) |
|---|---|---|
| Age | 17.7 (1.0) | 17.7 (1.1) |
| Race | 48% White, 2% Pacific Islander, 16% Black, 8% Asian4% American Indian/ Alaskan Native, 5% mixed race |
60% White, 4% Black, 28% Asian, 4% American Indian/Alaskan Native, 1% mixed race |
| Menthol Status | 48% Menthol | 40% Menthol |
| Average Cigarettes per Day |
7.9 (4.4) | 8.6 (4.8) |
| Modified Fagerström Tolerance Questionnaire score |
4.4 (1.6) | 4.0 (1.5) |
| Salivary cotinine (ng/mL) |
198.4 (144.0) | 240.4 (159.3) |
| CESD score | 12.4 (9.1) | 12.4 (9.7) |
| CO (ppm) | 10.6(7.9) | 11.8 (6.5) |
| Age at First Cigarette in years |
14.2 (2.3) | 14.1 (2.6) |
| Age of Onset of Daily Smoking in years |
16.0 (1.7) | 16.0 (1.7) |
| Number of Participants Reporting any Marijuana use |
13 | 17 |
| Average Marijuana Use (Days out of past 30) Among Those Reporting Use |
10.0 (10.46) | 8.0 (11.8) |
Note. CO refers Carbon Monoxide. CESD refers to the Center for Epidemiological Studie Depression questionnaire score.
3.1. Effects of nicotine dose on withdrawal, craving and negative affect
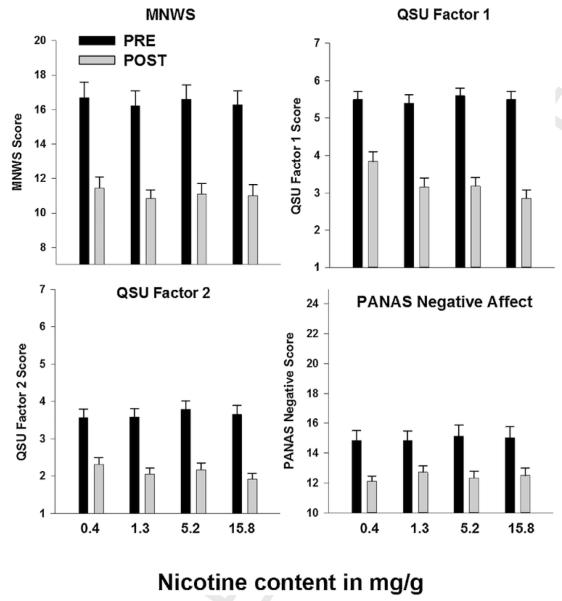
Fig. 1 shows pre- and post-smoking measures of withdrawal symptoms (MNWS), craving (QSU Factor 1 and Factor 2) and negative affect (PANAS negative affect scale).Table 2 shows the results of the mixed models examining the effect of dose on the pre- to post-smoking difference score calculated for each of these outcomes, whereby a negative value indicates a decrease in the symptom after smoking and a higher absolute value corresponds to greater change. CO boost is expressed in parts permillion, and a positive change indicates an increase in CO from pre- to post-smoking. A significant outcome for the intercept factor (initial status) indicates that the intercept of the model was significantly different from 0. The slope refers to the effect of dose, with a significant slope factor indicating that there was a significant change in slope across nicotine yield (Singer and Willett, 2003). For withdrawal (MNWS), negative affect (PANAS NA scale) and CO boost, no main effect of dose was evident. For craving (QSU), however, a main effect of dose was evident for both QSU Factor 1 and Factor 2 such that greater nicotine dose yielded greater reduction in craving (see Fig. 1). There was a significant interaction between baseline dependence (mFTQ) and QSU Factor 2, such that adolescents of both sexes with higher dependence were more likely to show a stronger decrease in craving in response to higher doses of nicotine.
Fig. 1.
Pre- and Post-smoking measures of abstinence symptoms as a function of nicotine yield. Error bars represent standard error of the mean. MNWS refers to the Minnesota Nicotine Withdrawal Scale (possible range 7–35). QSU refers to the Questionnaire on Smoking Urges. PANAS Negative refers to the Positive and Negative Affect Scale measure (possible range 1–7), Negative Affect subscale (possible range 10–50). All pre-post comparisons are significant (p’s<.05).
Table 2.
Multilevel model of abstinence-induced outcomes across dose.
| Predictor | Withdrawal MNWS | Craving QSU F1 | Craving QSU F2 | Negative Affect PANAS | CO Boost ppm | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | B | SE | |
| Intercept | − 4.41 3 | 0.52 | − 1.95 ** | 0.26 | − 1.2 ** | 0.16 | − 1.78 ** | 0.44 | 4.26 ** | 0.49 |
| Baseline Dependence1 | −0.52 | 0.38 | −0.03 | 0.10 | −0.04 | 0.08 | −0.11 | 0.24 | 0.13 | 0.26 |
| Sex2 | −1.75 | 1.09 | −0.10 | 0.36 | −0.27 | 0.28 | −1.90 | 0.83 | −0.29 | 0.78 |
| Slope | ||||||||||
| Dose3 | −0.98 | 1.23 | − 1.3 ** | 0.42 | − 0.59 ** | 0.25 | −1.6 | 1.24 | −0.13 | 0.62 |
| Dependence × Dose | −0.38 | 0.45 | −0.31 | 0.17 | − 0.42 ** | 0.11 | −0.46 | 0.51 | −0.04 | 0.51 |
| Gender × Dose | 2.14 | 1.68 | 0.82 | 0.59 | 0.29 | 0.34 | 2.39 | 1.71 | 1.60 | 1.01 |
Note. All outcomes except CO boost are expressed as difference scores (post-pre smoking score); negative values indicate a decrease from pre- to post-smoking. MNWS refers to the Minnesota Nicotine Withdrawal Scale. QSU F1 and QSU F2 refer to the Questionnaire on Smoking Urges Factor 1 and 2, respectively. PANAS refers to the Positive and Negative Affect Scale. CO boost is presented in parts per million (ppm) from pre- to post-smoking, positive values represent an increase in CO. Statistical significance is denoted by bold text.
Baseline Dependence was mean-centered across participants.
Sex was entered with males as the referent category.
Dose was entered in to the model as nicotine yield per cigarette dose level.
p<.05.
p<.01
3.2. Effects of nicotine dose on subjective responses
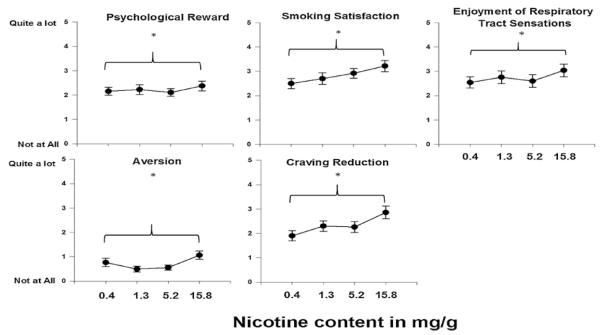
Fig. 2 shows the observed values of the effects of nicotine dose on subscales from the Cigarette Evaluation Scale. Table 3 shows the results of the mixed models examining the effect of dose on each subscale. A main effect of dose was found for all subscales, such that smoking cigarettes with greater nicotine content resulted in more smoking satisfaction, psychological reward, craving reduction, enjoyment of respiratory sensations, and aversion. There was a significant interaction between nicotine dose and sex on enjoyment of respiratory tract sensations, such that males showed greater enjoyment of respiratory sensations at higher nicotine contents than females.
Fig. 2.
Modified Cigarette Evaluation Scale subscale outcomes as a function of nicotine yield. Error bars represent standard error of the mean. Asterisks represent a significant effect of dose.
Table 3.
Multilevel model of the modified Cigarette Evaluation Subscale outcomes across dose.
| Predictor | Psychological Reward | Smoking Satisfaction | Enjoyment of Respiratory Tract Sensations |
Aversion | Craving Reduction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | B | SE | |
| Intercept | 2.23 ** | 0.18 | 2.46 ** | 0.25 | 2.43 ** | 0.31 | 0.46 ** | 0.13 | 2.12 ** | 0.24 |
| Baseline Dependence | 0.17 | 0.10 | 0.02 | 0.12 | −0.03 | 0.13 | 0.07 | 0.07 | −0.08 | 0.12 |
| Sex | −0.27 | 0.28 | 0.19 | 0.38 | 0.23 | 0.43 | 0.17 | 0.22 | −0.21 | 0.35 |
| Slope | ||||||||||
| Dose1 | 0.69 * | 0.32 | 1.44 ** | 0.36 | 1.54 ** | 0.40 | 0.84 ** | 0.28 | 1.55 ** | 0.49 |
| Dependence × Dose | 0.16 | 0.14 | 0.27 | 0.19 | 0.36 | 0.23 | −0.19 | 0.09 | −0.07 | 0.25 |
| Sex × Dose | −0.72 | 0.46 | −1.09 | 0.57 | − 1.82 * | 0.63 | −0.51 | 0.40 | −0.95 | 0.71 |
Note. Statistical significance is denoted by bold text. Baseline Dependence was mean-centered across participants.
Dose entered in to the model as nicotine yield per cigarette.
p<.05.
p<.01.
4. Discussion
As the FDA considers mandating a reduction in the maximum allowable amount of nicotine in cigarettes to make them less addictive (FDA, 2017), basic questions remain about how reducing nicotine levels in cigarettes may affect young people. To our knowledge, this is the first study to directly test the effects of different levels of nicotine in cigarettes on responses to cigarettes in adolescents, with the goal of informing regulatory policy. The current study demonstrated that, in adolescent daily smokers, withdrawal symptoms, craving and negative affect were all reduced by smoking cigarettes containing varying levels of nicotine. We did not find that reductions in withdrawal and negative affect varied significantly with nicotine content, whereas reductions in craving were dose-dependent, with higher doses of nicotine having a greater effect on craving reduction than lower doses. Further, we found that for negative reinforcement aspects of craving, as measured by Factor 2 on the QSU, adolescents with higher dependence showed a greater decrease in craving at higher nicotine levels than those with lower dependence. This indicates that level of dependence may affect the extent to which VLNC cigarettes can alleviate craving for negative reinforcing effects of cigarettes (i.e., craving to smoke in order to reduce withdrawal discomfort) in adolescent smokers. In terms of subjective evaluations, cigarettes with higher nicotine content were rated as more satisfying, more psychologically rewarding, producing greater enjoyment of respiratory sensations, more aversive, and more effective for reducing craving than cigarettes with lower nicotine content. Nicotine content did not affect CO boost, indicating that lower levels of nicotine did not result in compensatory increases in smoke intake.
Our pattern of findings is consistent with recent findings from young and older adult smokers undergoing similar experimental procedures (Faulkner et al., 2017; Higgins et al., 2017). As in those studies, effects were modestly dose dependent, and effects such as withdrawal showed a nonsignificant trend that might be significant in a larger sample and/or with longer exposure. As with these acute studies in young and older adults, our findings should be interpreted in the context of several limitations. The most significant is that this study only compares the effects of acute exposure to each nicotine content in the laboratory. Each participant was presented with each cigarette only once, and under abstinence conditions. No dose information was given (administration was under double-blind conditions), and the doses were not compared concurrently. It is very likely that longer exposure to these cigarettes under naturalistic conditions, as in other clinical trials with adults (Donny et al., 2015), would better approximate how adolescent smoking behavior would change in response to a national policy of nicotine reduction. We currently have such a study underway with adolescents (NCT02587312). Further, in the current study we relied mainly on subjective measures as an index of abuse liability, rather than using a behavioral choice procedure. Future studies can use such paradigms to determine behavioral preference and other indices of abuse liability in this population.
We did not exclude daily marijuana smokers from our sample in order to enhance generalizability due to the high rates of marijuana use in this population (N = 6 participants who reported smoking on 28 out of the last 30 days); however, as we also asked participants to abstain from marijuana use overnight in order to ensure a decreased CO value, this may have differentially impacted their withdrawal reports. The within-subjects nature of the design mitigates this concern, however, as all participants experienced all doses. Another potential limitation is the nature of the sample itself, which included only daily smokers. Compared with adults, adolescents tend to be lighter and more intermittent smokers on average, and therefore the severity of withdrawal across the sample is highly variable, though adolescents do report withdrawal even at low levels of smoking during laboratory abstinence protocols (Colby et al., 2000b). In general, smoking in adolescent samples is more heterogeneous than that of adult samples, as adolescent trajectories of smoking differ both within and across individuals (Hampson et al., 2013; Orpinas et al., 2016). Further, many adolescents are nondaily smokers (Rubinstein et al., 2014); therefore, this study may not generalize to this group; this study also does not inform initiation of use in adolescents. On the other hand, the results of this study are relevant to adolescent smokers who are most at risk for continuing to smoke into adulthood, i.e., those who are daily smokers. Furthermore, we controlled for cigarette dependence in our analyses, and did not find significant interactions between dependence and nicotine content in the current sample, with the exception of the QSU Factor 2 craving subscale as noted above. The heterogeneity of adolescent smoking trajectories highlights the need for research specifically on adolescent populations, as results generated with adult populations cannot always be assumed to generalize to adolescents. As vast majority of adult cigarette smokers begin smoking in adolescence (USDHHS, 2013), it is essential to investigate adolescents’ responses to potential tobacco control policies.
In sum, the current data provide preliminary evidence that nicotine reduction would be unlikely to have differentially adverse effects on adolescent smokers. All doses of nicotine in the research cigarettes, including the VLNC cigarettes, were able to reduce symptoms of withdrawal, craving and negative affect in study participants. Subjectively, adolescents did not rate VLNC cigarettes as very satisfying, suggesting that they may be likely to have a lower abuse liability than traditional cigarettes. Finally, smoking VLNC cigarettes did not result in an acute increase in CO exposure. This acute laboratory study, while not mimicking real-life conditions, is an important first step to establish acute safety and tolerance in this vulnerable population before moving to longer-term exposure studies that will more directly inform FDA policy. These promising results will help regulatory agencies evaluate the feasibility of a nicotine reduction policy and how it may affect adolescent smokers.
Acknowledgments
The authors wish to acknowledge the contributions of Graham DiGuiseppi for his work as a research assistant on the project; and Tim Souza and Sue Sales for implementing data management systems critical to the success of the project.
Role of the funding source
The study was funded by the National Institutes of Health [grant number K01CA189300]. Research reported in this publication was supported by NCI and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. Research cigarettes were supplied by NIDA. Neither NIH nor FDA CTP was involved in the study design, data collection, analysis or interpretation of the data, the writing of the manuscript, or the decision to submit the paper for publication.
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
Contributors
RNC was the principal investigator of the grant which funded this study. RNC analyzed the data and prepared the manuscript first draft. SMC and JWT helped to design the study, aided with data interpretation and manuscript drafting, and also served as co-investigators during data collection. PC served as the study clinician, and edited the final draft of the manuscript. KMJ, SK-S, DKH served as co-mentors on the project, aided with data interpretation and analysis. All authors approve of the submission of the manuscript.
Topography measures not reported due to intermittent equipment failure.
Age was tested but ultimately excluded due to insufficient range and potential multicollinearity with dependence.
References
- Arger CA,Heil SH,Sigmon SC,Tidey JW,Stitzer ML,Gaalema DE,Durand HJ,Bunn JY,Ruggieri EK,Higgins ST Preliminary validity of the modified cigarette evaluation questionnaire in predicting the reinforcing effects of cigarettes that vary in nicotine content. Exp. Clin. Psychopharmacol 2017. 25, 473–478. 10.1037/pha0000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL,Henningfield JE Reducing the nicotine content to make cigarettes less addictive. Tob. Control 2013. 22, Suppl. 1 i14–i17. 10.1136/tobaccocontrol-2012-050860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC,Leventhal AM,Tidey JW,Brazil L,Niaura RS,Colby SM Effects of abstinence in adolescent tobacco smokers: withdrawal symptoms, urge, affect, and cue reactivity. Nicotine Tob. Res 2013. 15, 457–464. 10.1093/ntr/nts155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butschky MF,Bailey D,Henningfield JE,Pickworth WB Smoking without nicotine delivery decreases withdrawal in 12-h abstinent smokers. Pharmacol. Biochem. Behav 1995. 50, 91–96.http://www.ncbi.nlm.nih.gov/pubmed/7700960. [DOI] [PubMed] [Google Scholar]
- Cappelleri JC,Bushmakin AG,Baker CL,Merikle E,Olufade AO,Gilbert DG Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict. Behav 2007. 32, 912–923. 10.1016/j.addbeh.2006.06.028.. [DOI] [PubMed] [Google Scholar]
- Colby SM,Gwaltney CJ Pharmacotherapy for adolescent smoking cessation. JAMA 2007. 298, 2182–2184.http://www.ncbi.nlm.nih.gov/pubmed/18027441. [DOI] [PubMed] [Google Scholar]
- Colby SM,Tiffany ST,Shiffman S,Niaura RS Are adolescent smokers dependent on nicotine? A review of the evidence. Drug Alcohol Depend 2000. 59, Suppl. 1 S83–S95.http://www.ncbi.nlm.nih.gov/pubmed/10773439. [DOI] [PubMed] [Google Scholar]
- Colby SM,Tiffany ST,Shiffman S,Niaura RS Measuring nicotine dependence among youth: a review of available approaches and instruments. Drug Alcohol Depend 2000. 59, Suppl. 1 S23–S39.http://www.ncbi.nlm.nih.gov/pubmed/10773436. [DOI] [PubMed] [Google Scholar]
- Cox LS,Tiffany ST,Christen AG Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res 2001. 3, 7–16. 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Donny EC,Denlinger RL,Tidey JW,Koopmeiners JS,Benowitz NL,Vandrey RG,al’Absi M,Carmella SG,Cincripini PM,Dermody MS,Drobes DJ,Hecht SS,Jensen J,Lane T,Le CT,McClernon FJ,Montoya ID,Murphy SE,Robinson JD,Stitzzer ML,Strasser AA,Tindle H,Hatsukami DK Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med 2015. 373, 1340–1349. 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner P,Ghahremani DG,Tyndale RF,Cox CM,Kazanjian AS,Paterson N,Loftipour S,Hellemann GS,Petersen N,Vigil C,London ED Reduced-nicotine cigarettes in young smokers: impact of nicotine metabolism on nicotine dose effects. Neuropsychopharmacology 2017. 42, 1610–1618. 10.1038/npp.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA) FDA Announces Comprehensive Regulatory Plan to Shift Trajectory of Tobacco-Related Disease, Death 30October 2017. 2017https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm568923.htm. [Google Scholar]
- Gottlieb S,Zeller M A nicotine-focused framework for public health. N. Engl. J. Med 2017. 377, 1111–1114. 10.1056/NEJMp1707409. [DOI] [PubMed] [Google Scholar]
- Hampson SE,Tildesley E,Andrews JA,Barckley M,Peterson M Smoking trajectories across high school: sensation seeking and hookah use. Nicotine Tob. Res 2013. 15, 1400–1408. 10.1093/ntr/nts338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK,Heishman SJ,Vogel RI,Denlinger RL,Roper-Batker AN,Mackowick KM,Jensen J,Murphy SE,Thomas BF,Donny E Dose-response effects of spectrum research cigarettes. Nicotine Tob. Res 2013. 15, 1113–1121. 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF,Kozlowski LT,Frecker RC,Fagerström KO The fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict 1991. 86, 1119–1127.http://www.ncbi.nlm.nih.gov/pubmed193288. [DOI] [PubMed] [Google Scholar]
- Higgins ST,Heil SH,Sigmon SC,Tidey JW,Gaalema DE,Hughes JR,Stitzer ML,Durand H,Bunn JY,Priest JS,Arger CA,Miller ME,Bergeria CL,Davis DR,Streck JM,Reed DD,Skelly JM,Tursi L Addiction potential of cigarettes with reduced nicotine content in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry 2017. 74, 1056. 10.1001/jamapsychiatry.2017.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST,Heil SH,Sigmon SC,Tidey JW,Gaalema DE,Stitzer ML,Durand H,Bunn JY,Priest JS,Arger CA,Miller ME,Bergeria CL,Davis DR,Streck JM,Zvorsky I,Redner R,Vandrey R,Pacek LR Response to varying the nicotine content of cigarettes in vulnerable populations: an initial experimental examination of acute effects. Psychopharmacology 2016. 234, 89–98. 10.1007/s00213-016-4438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR,Hatsukami D Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry 1986. 43, 289. 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hukkanen J,Jacob P,Benowitz NL Metabolism and disposition kinetics of nicotine. Pharmacol. Rev 2005. 57, 79–115. 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Jha P,Ramasundarahettige C,Landsman V,Rostron B,Thun M,Anderson RN,McAfee T,Peto R 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med 2013. 368, 341–350. 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- Johnston LD,O’ malley PM,Miech RA,Bachman JG,Schulenberg JE Monitoring the Future National Survey Results on Drug Use 1975–2016: 2016 Overview: Key Findings on Adolescent Drug Use. The University of Michigan Institute for Social Research, Ann Arbor, Michigan 2017http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2016.pdf. [Google Scholar]
- Kassel JD,Evatt DP,Greenstein JE,Wardle MC,Yates MC,Veilleux JC The acute effects of nicotine on positive and negative affect in adolescent smokers. J. Abnorm. Psychol 2007. 116, 543–553. 10.1037/0021-843X.116.3.543. [DOI] [PubMed] [Google Scholar]
- Kassel JD,Greenstein JE,Evatt DP,Wardle MC,Yates MC,Veilleux JC,Eissenberg T Smoking topography in response to denicotinized and high-yield nicotine cigarettes in adolescent smokers. J. Adolesc. Health 2007. 40, 54–60. 10.1016/j.jadohealth.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kendler KS,Myers J,Damaj MI,Chen X Early smoking onset and risk for subsequent nicotine dependence: a monozygotic co-twin control study. Am. J. Psychiatry 2013. 170, 408–413. 10.1176/appi.ajp.2012.12030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Esquerre JM,Colby SM,Tevyaw TO,Eaton CA,Kahler CW,Monti PM Validation of the timeline follow-back in the assessment of adolescent smoking. Drug Alcohol Depend 2005. 79, 33–43. 10.1016/j.drugalcdep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Marshall L,Schooley M,Ryan H,Cox P,Easton A,Healton C,Jackson K,Davis KC,Homsi G Centers for Disease Control and Prevention Youth tobacco surveillance—United States, 2001–2002. MMWR Morb. Mortal. Wkly. Rep. Surveil. Summ 2006. 55, 1–56.http://www.ncbi.nlm.nih.gov/pubmed/16708059. [PubMed] [Google Scholar]
- Mermelstein R,Colby SM,Patten C,Prokhorov A,Brown R,Myers M,Aldeman W,Hudmon K,McDonald P Methodological issues in measuring treatment outcome in adolescent smoking cessation studies. Nicotine Tob. Res 2002. 4, 395–403. 10.1080/1462220021000018470. [DOI] [PubMed] [Google Scholar]
- Orpinas P,Lacy B,Nahapetyan L,Dube SR,Song X Cigarette smoking trajectories from sixth to twelfth grade: associated substance use and high school dropout. Nicotine Tob. Res 2016. 18, 156–162. 10.1093/ntr/ntv040. [DOI] [PubMed] [Google Scholar]
- Perkins KA,Karelitz JL Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob. Res 2015. 17, 443–448. 10.1093/ntr/ntu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhorov AV,Pallonen UE,Fava JL,Ding L,Niaura R Measuring nicotine dependence among high-risk adolescent smokers. Addict. Behav 1996. 21, 117–127.http://www.ncbi.nlm.nih.gov/pubmed/8729713. [DOI] [PubMed] [Google Scholar]
- Radloff LS The use of the center for epidemiologic studies depression scale in adolescents and young adults. J. Youth Adolesc 1991. 20, 149–166. 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Rose JE,Levin ED Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br. J. Addict 1991. 86, 605–609.http://www.ncbi.nlm.nih.gov/pubmed/1859927. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML,Rait MA,Prochaska JJ Frequent marijuana use is associated with greater nicotine addiction in adolescent smokers. Drug Alcohol Depend 2014. 141, 159–162. 10.1016/j.drugalcdep.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G,Engl J,Urban M,Gilch G,Janket D,Riedel K Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul. Toxicol. Pharmacol 2007. 47, 171–183. 10.1016/j.yrtph.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Schoedel K,Sellers E Assessing abuse liability during drug development: changing standards and expectations. Clin. Pharmacol. Ther 2008. 83, 622–626. 10.1038/sj.clpt.6100492. [DOI] [PubMed] [Google Scholar]
- Singer JD,Willett JB Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence 2003Retrieved from Oxford University Press; Oxford: https://books.google.com/books/about/Applied_Longitudinal_Data_Analysis. html?id=PpnA1M8VwR8C. [Google Scholar]
- Thun MJ,Carter BD,Feskanich D,Freedman ND,Prentice R,Lopez AD et al. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med 2013. 368, 351–364. 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST,Drobes DJ The development and initial validation of a questionnaire on smoking urges. Br. J. Addict 1991. 86, 1467–1476.http://www.ncbi.nlm.nih.gov/pubmed/1777741. [DOI] [PubMed] [Google Scholar]
- United States Congress. Family Smoking Prevention and Tobacco Control Act (H.R. 1256) United States Government Publishing Office; Washington: 2009. [Google Scholar]
- United States Department of Health and Human Services (USDHHS) The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General 2014Office of Public Health; Rockville, MD: https://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. [Google Scholar]
- Watson D,Clark LA,Tellegen A Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol 1988. 54, 1063–1070.http://www.ncbi.nlm.nih.gov/pubmed/3397865. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) WHO Global Report on Trends in Prevalence of Tobacco Smoking 2015. World Health Organization; Geneva: 2015. [Google Scholar]