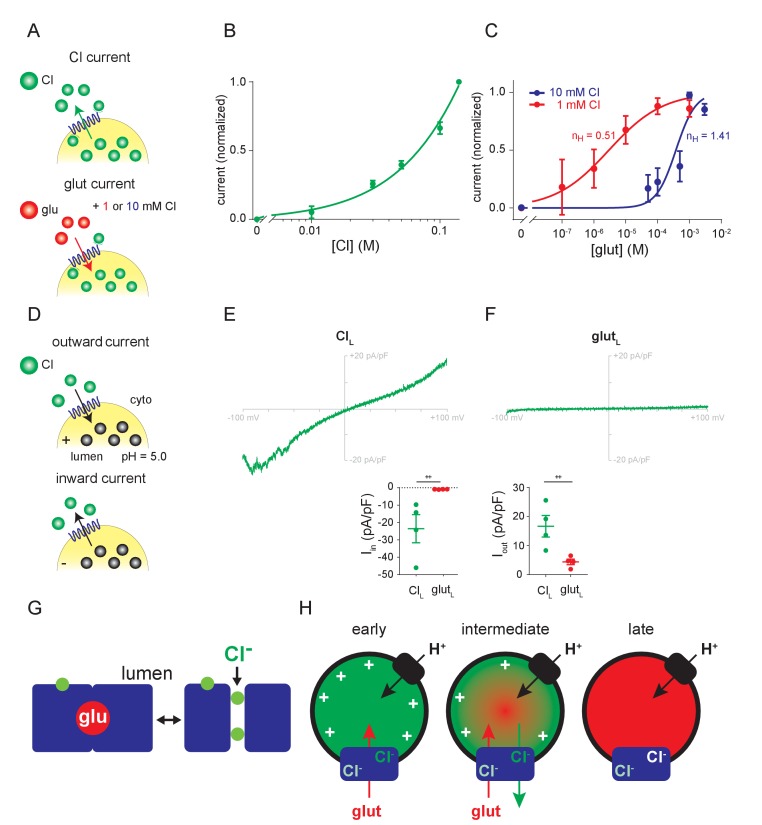
Figure 4. Relationship between Cl- and glutamate conductances.
(A–C) Dose-response of outward currents to external Cl- (B) or glutamate in the presence of either 1 mM or 10 mM external Cl- (C), all at lumenal pH 5.0. The EC50 for glutamate (C) was 376 µM in 10 mM Cl- and 2.95 µM in 1 mM Cl- (p=0.017 by Mann-Whitney, n = 5–6 each). nH, the Hill coefficients for glutamate in different cytoplasmic Cl concentrations. (D–F) Representative recordings and compiled data for endosomes expressing VGLUT1 with either high lumenal Cl- (E) or glutamate (F) in the presence of 140 mM external Cl- (Iin, p=0.029, Iout, p=0.029 both by Mann-Whitney) (++p<0.05 by Mann-Whitney test). (G) Permeation of glutamate by an alternating access mechanism, with the occluded state shown on the left, and permeation by Cl- through a channel (shown on the right). Both anions use a related permeation pathway, but the arginine in TM4 that confers allosteric activation by lumenal Cl- lies outside the permeation pathway. (H) Model for different stages in the filling of synaptic vesicles with glutamate. Immediately after endocytosis (early), the synaptic vesicle contains high concentrations of Cl- which provide the allosteric activation required for anion flux by the VGLUTs (blue). The H+ pump (black) provides the driving force Δψ and cytosolic Cl- allosterically activates rather than inhibits due to the saturating concentration of cytosolic glutamate. As glutamate enters (intermediate), Δψ dissipates and the vesicle acidifies, activating the Cl- conductance and the resulting Cl- efflux maintains the Δψ that drives glutamate uptake. When vesicle filling approaches completion (late), the different permeation mechanisms ensure that efflux of Cl- but not glutamate maintains Δψ while stabilizing the accumulated transmitter.