Introduction
Exposure to traumatic events is common, with 50–70% of individuals experiencing at least one over their lifetime [1]. Of those who report experiencing a traumatic event, between 5–31% meet lifetime criteria for posttraumatic stress disorder [PTSD; [2–4]]. Those who develop PTSD are at risk for adverse outcomes including major depression [5], substance misuse [6], physical health problems ([7, 8], and unemployment and marital instability [9]. These substantial deleterious effects of PTSD reinforce the clinical and public health significance of research aimed at identifying factors that increase risk for PTSD following trauma.
Existing literature suggests that biological processes may underlie risk for PTSD [15] and there is mounting evidence that stress response processes are largely determined by genetic influences [16, 17]. Additionally, evidence from twin [18, 19] and molecular [20] studies suggests that PTSD itself is moderately heritable. However, minimal progress has been made in elucidating its genetic architecture. Although a candidate gene methodology allows researchers to choose polymorphisms using an a priori approach, work in this area has yielded conflicting evidence in terms of the impact of individual SNPs on PTSD [[10, 11]]. Perhaps the most widely studied candidate gene marker in psychiatric genetics, particularly in studies incorporating trauma, is the promoter region of the serotonin transporter gene (5-HTTLPR), with work examining the relation between 5-HTTLPR and PTSD yielding a ‘mixed picture’ [12, 13]. However, meta-analyses examining the promoter region of this gene have recently provided further evidence on the effect of 5-HTTLPR SNPs and VNTRs on PTSD, indicating that those with at least one of the ‘s’ alleles are more vulnerable to adverse environments (e.g. [14–16]).
Some recent research has implicated neurotrophins, a group of proteins that support synaptic plasticity and long-term potentiation (LTP; Chao, 2003), in elevated risk for PTSD. Specifically, BDNF within the neurotrophin family plays a role in modulating synaptic changes, including hippocampal LTP, integral to associative memory formation [17, 18]. The hippocampus also interacts with the amygdala, implicated in the response to affective material, including visual and auditory cues [19]. Therefore, following a traumatic event, some individuals may perceive sensory information in their environment as a cue for danger even when it is not paired with the traumatic event. The result is that these individuals have difficulty discriminating between real danger and non-dangerous cues associated with the traumatic event [20] and typically display increased hyper-reactivity when reminded of the trauma [21].
Candidate studies of PTSD have identified more than 50 genes that exert significant effects [for reviews, see [22, 23]], with some finding a significant effect of the Val66Met BDNF polymorphism on PTSD (e.g., [24]). This polymorphism is functional, as the Met allele is associated with reduced BDNF gene expression [25], impeding the extinction of conditioned fear [26]. Therefore, the Met allele of this SNP may be associated with increased risk for PTSD. Given the mixed research examining BDNF Val66Met and PTSD, and the evidence suggesting that the Met allele is important in fear responses, meta-analyses reconciling this literature are needed.
Two meta-analyses examining the relation between the BDNF Val66Met polymorphism and PTSD have been conducted [27, 28]. The Wang et al. (2015, n=6 studies) paper found no overall effect of the Met allele on PTSD symptoms or correlates. However, when the authors excluded non-trauma exposed controls (i.e., n=3 studies) they found a significant increasing effect of the Met allele on PTSD/correlates. Another more recent meta-analysis [28] also found no overall effect of the Met allele on PTSD (n=9 studies), but found a marginal effect when comparing those with trauma exposure with and without PTSD (n=5 studies). These findings highlight the importance of examining trauma-exposed controls in genetically-informed investigations of PTSD. However, a major limitation in the extant literature is that potential ethnicity and sex differences were not examined. Examination of sex effects, which is critical given that findings that PTSD may be more genetically driven in females [29]. To illustrate, preliminary evidence has shown that estrogen induces synthesis of BDNF in several brain regions [30], which could contribute to an elevated risk for PTSD in females versus males. Given differences in minor allele frequency across ethnic groups, there is also a need to test ethnicity as a moderator. Thus, the current study adds to existing literature by: (1) investigating the Val66Met polymorphism of BDNF on PTSD, including a larger number of studies comparing trauma-exposed controls to those with PTSD (n=11 studies) and (2) testing whether sex and ethnicity potentially moderate the effect of genotype on PTSD.
Method
Ethical Approval
As this study did not involve subjects, no IRB approval was sought.
Search and selection of studies for inclusion
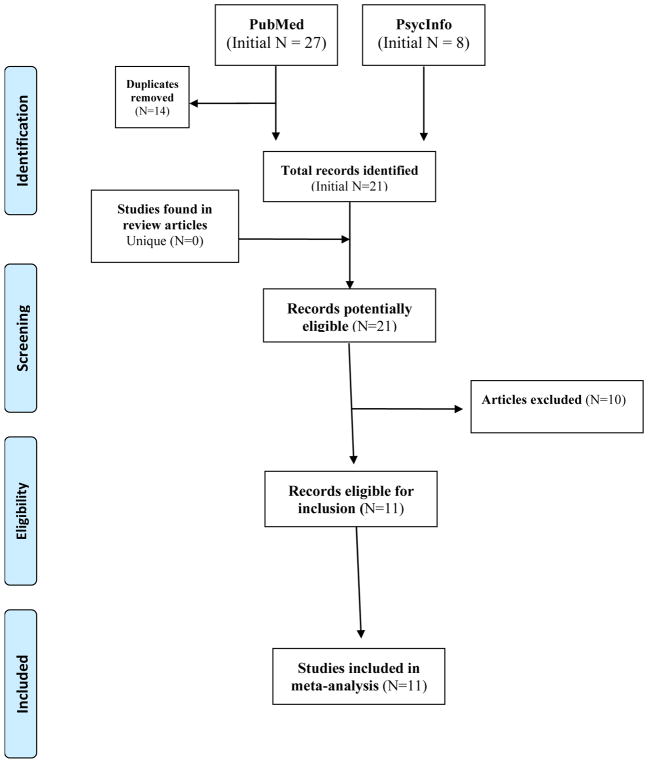
We identified existing studies examining the main effects of BDNF Val66Met polymorphism on risk for PTSD. Potential studies were identified through the PubMed and PsycINFO databases following a two-step search strategy of association studies for 1) Neurotrophins genes broadly and 2) targeted genes more specifically up until March of 2017. This search process was repeated closer in time to the publication of this article (in October 2017) to ensure that no new articles had been missed between March and October of 2017. In step one, search terms were: [posttraumatic stress disorder OR PTSD OR traumatic stress] AND [gene OR genetic] AND [Neurotrophins]. The step two search for specific genes replaced the Neurotrophins search term with a specific gene of interest. Additionally, any other articles identified through two recent PTSD genetics reviews were included ([22, 23]: [BDNF or Brain Derived Neurotrophic Factor OR Brain-Derived Neurotrophic Factor]. The reference sections of these review articles were examined to identify articles missed through the above search strategy. Figure 1 details the search steps and results, yielding 21 unique articles.
Figure 1.
PRISMA-style flow chart showing selection of studies for meta-analysis of Val66Met BDNF SNP and PTSD. Adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097
Screening of search results
The first author screened search results to select studies for possible inclusion. The following inclusion criteria were applied to articles: 1) original research; 2) use of human subjects; 3) association study including the BDNF gene; 4) PTSD as an outcome; and 5) trauma-exposed control group. Only effect sizes resulting from main effects of BDNF Val66Met and PTSD were included. In cases where the criteria were unclear, articles were more thoroughly examined, co-authors were consulted, and a consensus was made. Supplemental material was also reviewed for identification and extraction of relevant data. Of the 21 unique articles initially identified, 11 met these inclusion criteria. See Table 1 for information on included articles.
Table 1.
Included Study Descriptives.
| Study Number | First Author (Year) | Analyzed N | Outcome | Coding |
|---|---|---|---|---|
| 1 | Bruenig (2016) | 257 | PTSD Diagnosis | Additive |
| 2 | Dai (2017) | 175 | PTSD Diagnosis | Dominant |
| 3 | Dretsch (2015) | 689 | PTSD Diagnosis | Additive |
| 4 | Dunn (2014) | 205 | PTSD Symptoms | Additive |
| 5 | Hemmings (2013) | 150 | PTSD Symptoms | Additive |
| 6 | La Greca (2013) | 115 | PTSD Symptoms | Additive |
| 7 | Li (2016) | 524 | PTSD Diagnosis | Dominant |
| 8 | Pivac (2012) | 500 | PTSD Diagnosis | Dominant |
| 9 | Valente (2011) | 144 | PTSD Diagnosis | Additive |
| 10 | Van den Hueval (2016) | 115 | PTSD Diagnosis | Dominant |
| 11 | Zhang (2014) | 461 | PTSD Diagnosis | Additive |
Data extraction and coding
Two review authors (KK and RT) coded each included article based on a pre-determined coding manual that included relevant variables. Information that the two coders extracted from each article included, but was not limited to, each study’s outcome variable (i.e., PTSD diagnosis versus symptoms), coding of alleles (e.g., additive, dominant, or recessive coding), and what/if any variables were controlled for (e.g., sex, age, race/ethnicity). Concerns (e.g., one coder indicated that the authors used additive coding and the other coder indicated dominant coding was used) were resolved throughout the coding process, and changes were made to the coding manual as necessary. Next, data from the coding manual was transferred into an appropriate meta-analysis worksheet, which included space for inputting proper statistics and effect sizes for all studies. Following data extraction and entry, one independent reviewer (TH) checked for agreement across coders. The first author reconciled identified discrepancies.
Effect Size Calculation
An Odds Ratio (OR) and Confidence Interval was calculated for each study to examine whether the Met risk allele was associated with PTSD diagnoses and symptoms. In addition to the OR data, descriptive data, including means and standard deviations or frequency of occurrence, were used to calculate an effect size. When studies reported results from multiple regression analysis, the OR was calculated using the natural exponential of the unstandardized regression coefficient. In cases where no estimate of standard error was reported, the confidence interval was computed using equations provided by [31]. We contacted authors (k=1) to request further information in cases of missing data necessary for effect size computation and all studies that met inclusionary criteria were included in the meta-analysis.
Statistical Analysis
Meta-analyses were conducted using MAc R package on effect size data with combined sexes and ethnicities (k = 11). To account for various modes of inheritance, diagnosis type, duration, sex and ethnic distribution in analyzed studies, we employed a random effect model variance [32]. Homogeneity of the effect size distribution was examined with Cochran’s Q statistic, which investigates the null hypothesis that all studies are evaluating the same effect by summing the squared deviations of each study’s estimate from the overall meta-analytic estimate and weighting each study’s contribution in the same manner as in the meta-analysis. P values are obtained by comparing the statistic with a χ2distribution with k-1 degrees of freedom [33]. In order to test for moderation, the meta-regression function (mareg) in MAc package in the statistical program R was employed.
Some studies explored associations between BDNF and PTSD reported findings from multiple outcomes, for example reporting effect sizes for PTSD severity and diagnosis. In these cases, we prioritized associations with enough data to calculate an OR. When studies reported findings providing enough data to calculate multiple effect sizes, we followed a protocol selected a priori used in a previous meta-analysis [34]. When studies reported raw/descriptive data in addition to parameter estimates for a specific outcome, raw/descriptive data were selected.
Results
Quality Assessment
Studies included in the final analysis (k=11, see Figure 1) met quality standards. Studies either clearly described recruitment processes and inclusion/exclusion criteria in published manuscripts (k =10) or researchers provided details to the current study authors in separate correspondences (k =1). All included studies identified a psychometrically sound instrument or clinical interview used to measure PTSD. Four of the studies reported employing correction for multiple testing (i.e., [10, 35–37]), while the rest did not.
Primary Analyses
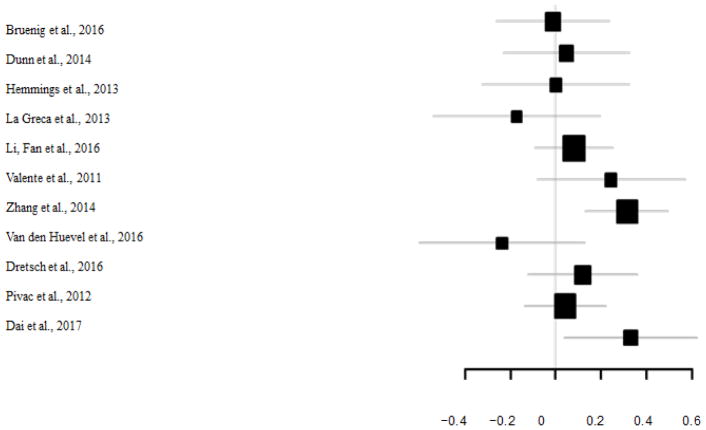
A total of 3,335 participants from 11 samples were included in the meta-analysis examining the effect of variation within the BDNF Val66Met SNP on PTSD. Results indicate a marginally significant effect for the Met allele on risk for PTSD (OR: 1.20, CI: .99–1.26, p=.057). The forest plots depicting effect sizes and their confidence intervals for the included studies can be seen in Figure 2. It should also be noted that one of the studies (Zhang et al., 2014) evidenced an effect size of 3.31. After omitting the Zhang et al. (2014) article, the marginally significant effect became non-significant (OR: 1.10, CI: .97–1.24, NS). The homogeneity of variance analyses suggests no significant between study variability in this effect either in the full sample of studies (Qp=0.11) or when excluding Zhang et al., (2014) (Qp=0.39). Thus, we fail to demonstrate between study variation. Additionally, we found a non-significant moderating effect of gender within the full sample (β: .0002, NS) or when the Zhang et al., (2014) article is excluded (β: −.0002, NS). We also found that ethnicity did not moderate the effect in the full sample, or excluding the Zhang et al., (2014) article (β’s for percent African-American, with [−.0002, NS] and without this article [−.0003, NS] ; β’s for percent European American, with [.0002, NS] and without this article [−.0001, NS] ; β’s for percent Asian, with [.0007, NS] and without this article [.0005, NS]). Publication bias analyses were not conducted because the overall p-value did not meet conventional standards for significance (i.e., p<.05), so no unreported null studies would be a needed for a non-significant effect.
Figure 2.
Forest plot depiction of effect sizes and confidence intervals.
Discussion
Given the conflicting candidate gene research examining BDNF Val66Met and PTSD, a meta-analysis was conducted to examine the relation between this polymorphism and PTSD. The Met allele, hypothesized to be related to PTSD risk, is associated with reduced BDNF gene expression [25], which impedes the extinction of conditioned fear [26]. Consistent with the mixed findings in prior candidate gene studies of Val66Met and PTSD, our meta-analysis does not provide support for a significant effect of BDNF Val66Met on PTSD. The overall p-value in the meta-analysis was marginally significant, and the hint of an effect is being driven by one study with a large z-score [10], as nine of the 11 studies we included in this meta-analysis suggested no effect of BDNF on PTSD. Additionally, none of the eight GWAS of PTSD found BDNF to be a genome-wide significant hit [38–45]. Candidate gene studies and GWAS select SNPs for inclusion in discrepant ways (i.e., one SNP chosen a priori based on functional literature versus agnostic tests across the entire genome) and apply different p-value thresholds (i.e., p<.05 and p < 5 × 10−8). This meta-analysis reconciles the discrepant candidate gene findings, supporting the findings of GWAS in this area, specifically that there is not a significant direct effect of Val66Met BDNF on PTSD.
The present investigation further extended published literature by examining heterogeneity across findings, and whether we might detect sex or ethnicity as a potential moderator in the relation between BDNF and PTSD. Ethnic differences in the minor allele frequency have of this variant have been demonstrated in the literature, but tests of ethnic differences in the impact of this polymorphism on PTSD have been largely ignored. Sex has been shown to influence likelihood of PTSD diagnosis and preliminary work suggested that these genetic influences were stronger in women than men [29]. However, other work on BDNF by sex interactions to predict related phenotypes has been mixed [46, 47]. Our analyses suggest that there is no significant moderation by ethnicity or sex, meaning that the findings do not differ for men and women, or across ethnic groups. It should be noted that of the participants from studies included in this meta-analysis, four (including Zhang et al., 2014) of the 11 articles having 89% or more male participants, which may have impacted our not finding a significant sex moderation effect.
This study is not without its limitations. Specifically, our meta-analysis included analyses of candidate gene studies which provide sparse coverage of genes, include small samples with low power, and are heterogeneous in terms of quality control approaches (e.g., lack of genomic control for ancestry). Additionally, there were a number of limitations common to meta-analytic approaches. Specifically, studies used varied methods of sampling, different ancestral groups were represented with varying treatment of that heterogeneity, and studies differed on the type of phenotype they measured (i.e., diagnosis versus clinical symptoms). Thus, the current study findings are limited by these inconsistencies across currently available studies.
However, this study expands previous meta-analytic research by including only trauma-exposed controls, exclusively examining individuals with PTSD, and including the largest number of studies (k=11). The present meta-analytic investigation did not support a significant association between the Val66Met marker of BDNF and PTSD. Additionally, our meta-analysis of published studies and prior meta-analyses investigating the effect of BDNF on PTSD suggests that it may be beneficial for future research to utilize more precise phenotypic measurements, include measures of epigenetic influences, or perhaps examine interactions between BDNF and other markers (rather than simply examining a single marker) in predicting PTSD risk.
Acknowledgments
Funding provided by: R01MH105379 (PI Nugent); R01MH108641 (PI Nugent); MH020030-17 (Sheerin, Lind).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References Cited
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Kulka R, et al. Trauma and the Vietnam War Generation: Report of the findings from the National Vietnam Veterans Readjustment Study. New York: Brunner/ Mazel; 1990. [Google Scholar]
- 3.Breslau N, Kessler RC, Peterson EL. PTSD assessment with a structured interview: reliability and concordance with a standardized clinical interview. International Journal of Methods in Psychiatric Research. 1998;7(121–127) [Google Scholar]
- 4.Adams RE, Boscarino JA. Predictors of PTSD and delayed PTSD after disaster: the impact of exposure and psychosocial resources, (in eng) J Nerv Ment Dis. 2006 Jul;194(7):485–93. doi: 10.1097/01.nmd.0000228503.95503.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: The posttraumatic stress disorder-major depression connection. Biological Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- 6.Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Archives of General Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- 7.Simpson TL. Women’s treatment utilization and its relationship to childhood sexual abuse history and lifetime PTSD. Substance Abuse. 2002;23(1):17–30. doi: 10.1080/08897070209511472. [DOI] [PubMed] [Google Scholar]
- 8.Zayfert C, Dums AR, Ferguson RJ, Hegel MT. Health functioning impairments associated with posttraumatic stress disorder, anxiety disorders, and depression. The Journal of Nervous and Mental Disease. 2002;190(4):233–240. doi: 10.1097/00005053-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. Journal of Clinical Psychiatry. 2000;61:4–12. [PubMed] [Google Scholar]
- 10.Zhang L, et al. PTSD risk is associated with BDNF Val66Met and BDNF overexpression, (in eng) Mol Psychiatry. 2014 Jan;19(1):8–10. doi: 10.1038/mp.2012.180. [DOI] [PubMed] [Google Scholar]
- 11.Valente N, et al. Candidate-gene approach in posttraumatic stress disorder after urban violence: Association analysis of the genes encoding serotonin transporter, dopamine transporter, and BDNF. Journal of Molecular Neuroscience. 2011;44(1):59–67. doi: 10.1007/s12031-011-9513-7. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans, (in eng) PLoS One. 2015;10(3):e0119998. doi: 10.1371/journal.pone.0119998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacic Petrovic Z, Nedic Erjavec G, Nikolac Perkovic M, Peraica T, Pivac N. No association between the serotonin transporter linked polymorphic region polymorphism and severity of posttraumatic stress disorder symptoms in combat veterans with or without comorbid depression, (in eng) Psychiatry Res. 2016 Oct 30;244:376–81. doi: 10.1016/j.psychres.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Navarro-Mateu F, Escamez T, Koenen KC, Alonso J, Sanchez-Meca J. Meta-analyses of the 5-HTTLPR polymorphisms and post-traumatic stress disorder (in eng) PLoS One. 2013;8(6):e66227. doi: 10.1371/journal.pone.0066227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits, (in eng) Am J Med Genet B Neuropsychiatr Genet. 2004 May 15;127b(1):85–9. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- 16.van Ijzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies, (in eng) Transl Psychiatry. 2012 Aug 7;2:e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus, (in eng) Nature. 1993 Jan 7;361(6407):31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 18.Martin PD, Shapiro ML. Disparate effects of long-term potentiation on evoked potentials and single CA1 neurons in the hippocampus of anesthetized rats, (in eng) Hippocampus. 2000;10(3):207–12. doi: 10.1002/1098-1063(2000)10:3<207::AID-HIPO1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit, (in eng) Nat Neurosci. 2013 Mar;16(3):332–9. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes JP, et al. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD, (in eng) J Psychiatr Res. 2011 May;45(5):660–9. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity, (in eng) Mol Endocrinol. 2001 Oct;15(10):1748–57. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 22.Voisey J, Young RM, Lawford BR, Morris CP. Progress towards understanding the genetics of posttraumatic stress disorder, (in Eng) J Anxiety Disord. 2014 Dec;28(8):873–83. doi: 10.1016/j.janxdis.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Almli LM, Fani N, Smith AK, Ressler KJ. Genetic approaches to understanding post-traumatic stress disorder, (in eng) Int J Neuropsychopharmacol. 2014 Feb;17(2):355–70. doi: 10.1017/S1461145713001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pivac N, et al. The association between brain-derived neurotrophic factor Val66Met variants and psychotic symptoms in posttraumatic stress disorder, (in eng) World J Biol Psychiatry. 2012 Apr;13(4):306–11. doi: 10.3109/15622975.2011.582883. [DOI] [PubMed] [Google Scholar]
- 25.Hsu PK, Xu B, Mukai J, Karayiorgou M, Gogos JA. The BDNF Val66Met variant affects gene expression through miR-146b, (in eng) Neurobiol Dis. 2015 May;77:228–37. doi: 10.1016/j.nbd.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory?, (in eng) Neurobiol Learn Mem. 2008 Mar;89(3):312–23. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T. Does BDNF Val66Met Polymorphism Confer Risk for Posttraumatic Stress Disorder?, (in eng) Neuropsychobiology. 2015;71(3):149–53. doi: 10.1159/000381352. [DOI] [PubMed] [Google Scholar]
- 28.Bruenig D, et al. A Case-Control Study and Meta-Analysis Reveal BDNF Val66Met Is a Possible Risk Factor for PTSD (in eng) Neural Plast. 2016;2016:6979435. doi: 10.1155/2016/6979435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan LE, et al. Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability (in eng) Mol Psychiatry. 2017 Apr 25; doi: 10.1038/mp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases, (in eng) Front Neuroendocrinol. 2006 Dec;27(4):404–14. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman DG, Bland JM. How to obtain the confidence interval from a P value, (in eng) Bmj. 2011 Aug 8;343:d2090. doi: 10.1136/bmj.d2090. [DOI] [PubMed] [Google Scholar]
- 32.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327(7414):557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lind MJ, et al. Association of Posttraumatic Stress Disorder With rs2267735 in the ADCYAP1R1 Gene: A Meta-Analysis, (in eng) J Trauma Stress. 2017 Aug;30(4):389–398. doi: 10.1002/jts.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn EC, et al. Interaction between genetic variants and exposure to Hurricane Katrina on post-traumatic stress and post-traumatic growth: a prospective analysis of low income adults, (in eng) J Affect Disord. 2014 Jan;152–154:243–9. doi: 10.1016/j.jad.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Heuvel L, Suliman S, Malan-Muller S, Hemmings S, Seedat S. Brain-derived neurotrophic factor Val66met polymorphism and plasma levels in road traffic accident survivors, (in eng) Anxiety Stress Coping. 2016 Nov;29(6):616–29. doi: 10.1080/10615806.2016.1163545. [DOI] [PubMed] [Google Scholar]
- 37.Dretsch MN, et al. Brain-derived neurotropic factor polymorphisms, traumatic stress, mild traumatic brain injury, and combat exposure contribute to postdeployment traumatic stress, (in eng) Brain Behav. 2016 Jan;6(1):e00392. doi: 10.1002/brb3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almli LM, et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces, (in eng) Am J Med Genet B Neuropsychiatr Genet. 2015 Jul;168b(5):327–36. doi: 10.1002/ajmg.b.32315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashley-Koch AE, et al. Genome-wide association study of posttraumatic stress disorder in a cohort of Iraq-Afghanistan era veterans, (in eng) J Affect Disord. 2015 Sep 15;184:225–34. doi: 10.1016/j.jad.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guffanti G, et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women (in eng) Psychoneuroendocrinology. 2013 Dec;38(12):3029–38. doi: 10.1016/j.psyneuen.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logue MW, et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus, (in eng) Mol Psychiatry. 2013 Aug;18(8):937–42. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nievergelt CM, et al. Genomic predictors of combat stress vulnerability and resilience in US Marines: A genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 43.Stein MB, et al. Genome-wide Association Studies of Posttraumatic Stress Disorder in 2 Cohorts of US Army Soldiers. JAMA psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf EJ, Rasmusson AM, Mitchell KS, Logue MW, Baldwin CT, Miller MW. A genome-wide association study of clinical symptoms of dissociation in a trauma-exposed sample, (in eng) Depress Anxiety. 2014 Apr;31(4):352–60. doi: 10.1002/da.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie P, Kranzler HR, Yang C, Zhao H, Farrer LA, Gelernter J. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder, (in eng) Biol Psychiatry. 2013 Nov 01;74(9):656–63. doi: 10.1016/j.biopsych.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shalev I, et al. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions, (in eng) Psychoneuroendocrinology. 2009 Apr;34(3):382–8. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 47.van Oostrom I, et al. Interaction between BDNF Val66Met and childhood stressful life events is associated to affective memory bias in men but not women, (in eng) Biol Psychol. 2012 Jan;89(1):214–9. doi: 10.1016/j.biopsycho.2011.10.012. [DOI] [PubMed] [Google Scholar]