Abstract
The role for kidney TLR9 in ischemic acute kidney injury (AKI) remains unclear. Here, we tested the hypothesis that renal proximal tubular TLR9 activation exacerbates ischemic AKI by promoting renal tubular epithelial apoptosis and inflammation. To test this hypothesis, we generated mice lacking TLR9 in renal proximal tubules (TLR9fl/fl PEPCK Cre mice). Contrasting previous studies in global TLR9KO mice, mice lacking renal proximal tubular TLR9 were protected against renal ischemia reperfusion (IR) injury with reduced renal tubular necrosis, inflammation (decreased pro-inflammatory cytokine synthesis and neutrophil infiltration) and apoptosis (decreased DNA fragmentation and caspase activation) when compared to wild type (TLR9fl/fl) mice. Consistent with this, a selective TLR9 agonist ODN-1668 exacerbated renal IR injury in TLR9fl/fl mice but not in renal proximal tubular TLR9 null mice. Furthermore, in cultured human and mouse proximal tubule cells, TLR9 selective ligands induced NFκB activation, pro-inflammatory cytokine mRNA synthesis as well as caspase activation. We further confirm here that global TLR9 deficiency had no impact on murine ischemic AKI. Taken together, our studies show that renal proximal tubular TLR9 activation exacerbates ischemic AKI by promoting renal tubular inflammation, apoptosis as well as necrosis after IR via NFκB and caspase activation. Our studies further suggest complex nature of TLR9 activation as renal tubular epithelial TLR9 promote cell injury and death whereas TLR9 signaling in other cell types may promote cytoprotective effects.
Keywords: Apoptosis, inflammation, ischemia and reperfusion injury, necrosis, NFκB, neutrophil
Introduction
Acute kidney injury (AKI) is a major clinical problem (1). Renal ischemia and reperfusion (IR) injury is a leading cause of AKI and patients undergoing major surgical procedures (cardiac, vascular or liver transplantation) have ~50–80% chance of developing ischemic AKI (2, 3). Renal IR results in proximal tubular necrosis and apoptosis with rapid upregulation of pro-inflammatory cytokines and chemokines that causes influx of inflammatory leukocytes into the renal parenchyma (4–6). Unfortunately, despite nearly 7 decades of research, there is no effective preventive measures or therapy for ischemic AKI (7, 8).
Toll-like receptors (TLRs) are pattern recognition receptors critical for regulating innate as well as adaptive immunity and play important roles in protecting against microbial invasion (9–11). Of 13 identified TLRs for mice and 11 for humans (9, 10), TLRs can be classified into cell surface TLRs (TLR1, 2, 4, and 6 that recognize bacterial or fungal products) and intracellular TLRs (TLR 3, 7, 8 and 9 which recognize DNA and RNA products) (12). Numerous endogenous ligands can also activate TLRs including histones, high mobility group box 1, heat shock proteins and mitochondrial DNA – all of these endogenous damage associated pattern ligands (DAMPs) are rapidly released after IR injury. Indeed, previous studies showed that cell surface TLR4 and TLR2 play important roles in liver and kidney IR injury (12–15).
TLR9 is a cytosolic receptor for un-methylated cytosine-phosphate-guanosine (CpG) deoxyribonucleic acid found in microbial DNA and DNA viruses (12, 16). Directly relevant to ischemic tissue injury, TLR9 also recognizes endogenous mitochondrial DNA products released from injured cells to trigger MyD88-dependent NFκB-mediated gene transcription leading to inflammation and apoptosis (16–19). Indeed, TLR9 activation plays a critical role in hepatic IR injury (16, 18). Furthermore, mitochondrial DNA released after trauma causes neutrophil extracellular trap (NET) formation (20). Recent studies suggest that NET formed by DAMPs exacerbates sterile liver IR injury in mice (21).
However, the role for kidney TLR9 in ischemic AKI remains unclear. Previous studies suggest TLR9 does not play a role in ischemic AKI as mice deficient in TLR9 mice were not protected against ischemic AKI (22, 23). However, TLR9 has diverse effects depending on cell types and organs studied (12). Indeed, TLR9 activation induces inflammation in hepatic IR and plays a role in septic AKI (17, 18, 24, 25). In contrast, TLR9 induces cytoprotective signaling in immune, cardiac and neuronal cells (26–28). In addition, global TLR9 deficiency may lead to compensatory changes in multiple non-renal tissue and cell types (29). Taken together, global TLR9 deficiency may not allow for direct examination of TLR9 in ischemic AKI. Therefore, we generated mice with proximal specific deletion of TLR9 and tested the hypothesis that selective renal proximal tubular TLR9 activation exacerbates ischemic AKI by promoting renal tubular epithelial apoptosis and inflammation.
Methods
Generation of mice with renal proximal tubule cell specific TLR9 deficiency
We bred mice with floxed TLR9 gene (TLR9fl/fl mice on a C57BL/6 background (30)) with mice that express Cre-recombinase selectively in proximal tubular epithelia (Cre recombinase under the control of the phosphoenolpyruvate carboxykinase promoter or PEPCK Cre – generated by Dr. Volker Haase, Vanderbilt University) (31). PEPCK Cre mice were obtained through the laboratory of Dr. Holger Eltzschig (University of Colorado School of Medicine) after they were backcrossed with C57BL/6 strain for several generations. This approach allowed us to generate sibling mice with proximal tubule-specific deletion of TLR9 (TLR9fl/fl PEPCK Cre mice) or wild type (TLR9fl/fl) mice. At least 4 generations of sibling breeding in our laboratory resulted in mice used in this study. Tail PCR with PEPCK Cre and TLR9 loxP-specific primers (Table 1) confirmed the genotypes of proximal tubule cell specific TLR9 null mice and the control wild type mice (TLR9fl/fl) generated from breeding.
Table 1.
Primers used in genotyping to amplify mouse genomic DNAs based on published GenBank sequences for mouse. Annealing temperatures used for each primer are also provided.
| Primers | Sequence (Sense/Antisense) | Annealing Temp (°C) |
|---|---|---|
| TLR9 FLOX | 5′-CAAGGAGAATCCAGGAGGCTAGTG-3′ 5′-GGAGAACCTGTGAGAGCCAGG-3′ |
59 °C |
| PEPCK Cre | 5′-TGGGCGGCATGGTGCAAGTT-3′ 5′-CGGTGCTAACCAGCGTTTTC-3′ |
60 °C |
Confirmation of renal proximal tubular TLR9 deletion in TLR9fl/fl PEPCK Cre mice
Paraffin embedded kidney tissues were cut at 5 μm, deparaffinized, and rehydrated in graded ethanol series. Endogenous peroxidase was inhibited using 3% hydrogen peroxide in methanol for 30 min, and then the sections were autoclaved in sodium citrate buffer (10 mM, pH 6) at 120 °C for 10 min. The sections were incubated with 3% BSA in PBS for 60 min and then incubated overnight at 4°C with rabbit monoclonal anti-TLR9 antibody (1:100; 1% BSA in PBS; Abcam, Cambridge, MA). After washing 3 times with PBS buffer, the kidney sections were incubated with Alexa 594-conjugated goat anti-rabbit secondary antibody (1:100; 1% BSA in PBS; Abcam, Cambridge, MA). To stain brush border of proximal tubules, kidney sections were also stained using Phaseolus vulgaris leucoagglutinin (PHA-L) lectin conjugated to a green fluorescent dye according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA) as described previously (32). PHA-L lectin binds avidly to the brush border of proximal tubules (33).
We also confirmed selective deletion of renal proximal tubular TLR9 in TLR9fl/fl PEPCK Cre mice by measuring TLR9 mRNA in isolated proximal tubule cells (34) as well as bone-marrow cells, small intestine and spleen with RT-PCR as described previously (35, 36). Primer design was based on published GenBank sequences. To control for RNA loading, GAPDH mRNA expression was also measured.
Renal IR injury in mice
After Columbia University Institutional Animal Care and Use Committee approval, 8–10 week old male TLR9 proximal tubule deficient (TLR9fl/fl PEPCK Cre) mice or control wild type (TLR9fl/fl) mice weighing 20–25g were anesthetized with pentobarbital i.p. (Sigma, St Louis, MO: 50 mg/kg body weight or to effect). Mice were then subjected to right nephrectomy and 30 min left renal ischemia as described previously (37, 38). A separate cohort of TLR9fl/fl mice or renal tubular TLR9 null mice were pretreated with a selective TLR9 agonist (1 mg/kg ODN-1668 or control ODN, Invivogen, San Diego, CA) and subjected to 20 min renal IR injury. Some mice were treated with vehicle for ODN (saline) before 20 min renal ischemia. To determine the effects of global TLR9 deletion in ischemic AKI, we also subjected TLR9 knockout mice (TLR9KO, C57BL/6JTlr9M7Btlr/Mmjax) and their wild type controls (C57BL/6J mice (B6)) to 30 min renal IR injury (Jackson Labs, Bar Harbor, ME). For pain management, all mice received 0.5–1 mg/kg s.c. buprenorphine SR prior to surgery. Sham-operated animals underwent anesthesia followed by laparotomy, right nephrectomy, bowel manipulations and wound closure without renal ischemia. Body temperature of all mice were sustained at ~37°C using a surgical heating pad during surgery as well as during recovery from anesthesia.
Kidney TLR9 protein expression after sham-surgery and renal IR injury
Paraffin embedded kidney sections were stained rabbit monoclonal anti-TLR9 antibody as described above. The sections were subsequently incubated with anti-rabbit secondary antibody (1:100, 2% goat serum in PBS; Vector Laboratories) for 1 h, washed, and incubated with avidin-biotin complex (ABC kit; Vector Laboratories). The sections were developed with 3, 3′-diaminobenzidine (DAB). Negative controls were performed on serial sections by omitting primary antibody and by using non-specific isotype control primary antibody. Kidney TLR9 expression was quantified from 3–5 randomly chosen 200× microscope image fields surrounding corticomedullary junction as described by Ruifrok et al. (39).
Measurement of renal injury after IR injury
Twenty-four hrs after renal IR injury, we measured plasma BUN and creatinine using an enzymatic creatinine reagent kit (Thermo Fisher Scientific, Waltham, MA). This creatinine measurement method limits the interferences from mouse plasma chromagens known to occur in the Jaffe method. We also performed qRT-PCR for kidney NGAL mRNA from mice subjected to sham-surgery or to renal IR injury. NGAL is an early and sensitive marker of renal tubular injury (40).
Histological detection of kidney injury
Twenty-four hrs after renal IR injury, kidney hematoxylin-eosin (H&E). Kidney H&E sections post renal IR surgery or sham surgery were blindly assessed using a grading scale of kidney necrotic IR injury to the proximal tubules (0–4, Renal Injury Score) as outlined by Khalid et al. (41). In brief, no damage = 0; loss of brush border in less than 25% of tubular cells = 1; loss of brush border in more than 25% of tubular cells = 2; necrosis up to 60% of tubular cells = 3 and necrosis in more than 60% of tubular cells = 4.
Detection of kidney tubular apoptosis
We utilized 2 independent assays to assess kidney apoptosis after IR –with TUNEL staining as well as detection of caspase 3 and caspase 8 fragmentation by immunoblotting. Twenty four hrs after sham surgery or renal IR injury, TUNEL staining detected fragmented DNA as described (42) using a commercially available kit (Roche, Indianapolis, IN). Apoptotic terminal deoxynucleotidyl transferase 2′-deoxyuridine-5′-triphosphate nick end labeling positive cells were quantified in 5–7 randomly chosen 100× microscope images fields in the corticomedullary junction and results were expressed as apoptotic cells counted per 100× field. Kidney caspase 3 and caspase 8 immunoblotting were performed as described previously (43). Primary antibodies for cleaved mouse caspase 3 and caspase 8 were from Cell Signaling Technology (Danvers, MA).
Detection of kidney neutrophil infiltration
Kidney neutrophil infiltration after IR injury was detected with immunohistochemistry using rat anti-mouse Ly6B monoclonal antibody (AbD Serotec, Raleigh, NC) as described (36, 44). Primary IgG2a antibody (MCA1212, AbD Serotec, Raleigh, NC) was utilized as a negative isotype control. Quantification of kidney infiltrating neutrophils was performed using 5–7 randomly chosen 200× microscope image fields (corticomedullary junction for kidney neutrophils) and results were expressed as neutrophils counted per 200× field.
Q-RTPCR for pro-inflammatory cytokine and chemokine mRNA expression
Renal inflammation after IR was also assessed by measuring pro-inflammatory mRNA markers including IL-6, IL-8, intercellular adhesion molecule-1 (ICAM-1), monocyte chemoattractive protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), and tumor necrosis factor-α (TNF-α) quantitative RT-PCR as described previously with primers listed in Table 2 (35, 36). Primer design was based on published GenBank sequences. To confirm equal RNA loading, GAPDH mRNA expression was also measured.
Table 2.
Primers used in quantitative reverse transcription polymerase chain reactions to amplify mouse cDNAs based on published GenBank sequences. Annealing temperatures used for each primer are also provided.
| Primers | Sequence (Sense/Antisense) | Annealing Temp (°C) |
|---|---|---|
| mouse TLR9 | 5′-CCAGTTTGTCAGAGGGAGCC-3′ 5′-GGACAGGTGGACGAAGTCAG-3′ |
66 °C |
| mouse TNF-α | 5′-TACTGAACTTCGGGGTGATTGGTCC-3′ 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′ |
65 °C |
| mouse MCP-1 | 5′-ACCTGCTGCTACTCATTCAC-3′ 5′-TTGAGGTGGTTGTGGAAAAG-3′ |
60 °C |
| mouse MIP-2 | 5′-CCAAGGGTTGACTTCAAGAAC-3′ 5′-AGCGAGGCACATCAGGTACG-3′ |
60 °C |
| mouse IL-8 | 5′-CAATGAGCTGCGCTGTCAGTG-3′ 5′-CTTGGGGACACCTTTTAGCATC-3′ |
60 °C |
| mouse IL-6 | 5′-CCGGAGAGGAGACTTCACAG-3′ 5′-GGAAATTGGGGTAGGAAGGA-3′ |
62 °C |
| mouse NGAL | 5′-CACCACGGACTACAACCAGTTCGC-3′ 5′-TCAGTTGTCAATGCATTGGTCGGTG-3′ |
66 °C |
| mouse ICAM-1 | 5′-TGTTTCCTGCCTCTGAAGC-3′ 5′-CTTCGTTTGTGATCCTCCG-3′ |
60 °C |
| human TNF-α | 5′-CGGGACGTGGAGCTGGCCGAGGAG-3′ 5′-CACCAGCTGGTTATCTCTCAGCTC-3′ |
68 °C |
| human IL-8 | 5′-TCTGCAGCTCTGTGTGAAGG-3′ 5′-ATTGCATCTGGCAACCCTAC-3′ |
64 °C |
| human ICAM-I | 5′-GCAGACAGTGACCATCTACAGC-3′ 5′-GCCATCCTTTAGACACTTGAGC-3′ |
60 °C |
| human MIP-2 | 5′-CTTGCCAGCTCTCCTCCTC-3′ 5′-GCTTTCTGCCCATTCTTGAG-3′ |
64 °C |
| GAPDH | 5′-ACCACAGTCCATGCCATCAC-3′ 5′-CACCACCCTGTTGCTGTAGCC-3′ |
65 °C |
Proximal tubule cell culture and TLR9 agonist treatment
Immortalized human proximal tubular cells (HK-2; ATCC, Manassas, VA) were grown as described (45, 46). Mouse kidney proximal tubules were isolated and grown using Percoll density gradient separation as described previously (34). Confluent cells were treated with control oligonucleotides or with selective 1–5 μM TLR9 ligands [ODN2006 (human specific), ODNBW006 (human and mouse specific) or ODN1668 (mouse specific)] for 3 days as described (47). Proximal tubule cells were subjected to quantitative RTPCR for pro-inflammatory genes with primers listed in Table 2. We also performed caspase 3 and caspase 8 immunoblotting in HK-2 cells (43). Finally, HK-2 cell nuclear and cytosolic fractions were prepared and subjected to p65 and phospho-p65 subunit of NFκB with antibodies from Santa Cruz Biotechnologies (Santa Cruz, CA) as described (48).
Statistical analysis
Data were analyzed with Student’s t-test, one-way ANOVA plus Tukey’s post hoc multiple comparison test or Mann–Whitney nonparametric U test to analyze renal injury scores. All data are expressed throughout the text as means ± SEM.
Results
Genotyping and confirmation of renal proximal tubular TLR9 deletion
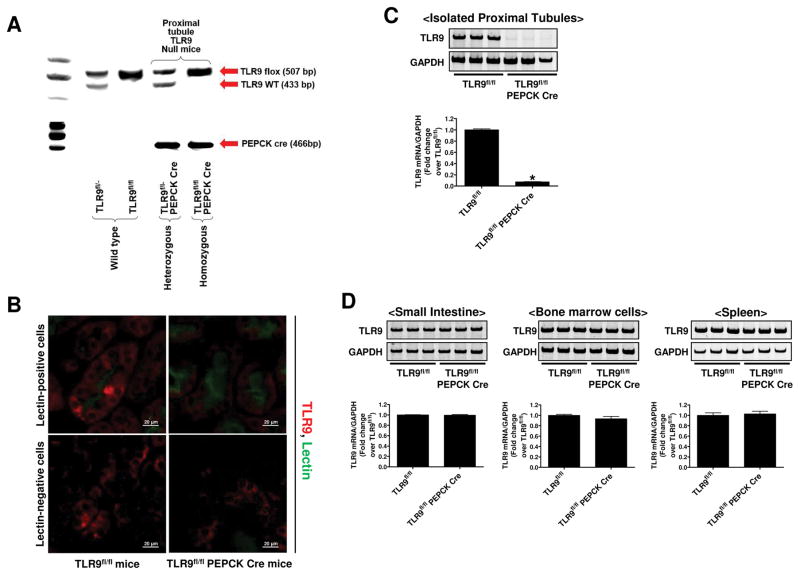
Tail PCR confirmed the genotypes of proximal tubule-specific TLR9 null mice (TLR9fl/fl PEPCK Cre) and the control wild type mice (TLR9fl/fl) generated (Figure 1A). PEPCK Cre primers generated a 466-bp fragment. TLR9 flox primers amplified a 507-bp fragment for the TLR9 floxed allele and a 433-bp fragment for the TLR9 wild type allele.
Figure 1. Genotyping and Confirmation of renal proximal tubule cell TLR9 deletion in proximal tubule cell TLR9 null mice.
A) Tail PCR confirming the genotypes of renal proximal tubule-specific TLR9 null mice (TLR9fl/fl PEPCK Cre) and the control transgenic mice (TLR9fl/fl). Bands were cut from multiple genotype gel images to create this figure to illustrate how we were able to differentiate TLR9 null mice and control mice. B) To confirm deletion of renal proximal tubular TLR9 in TLR9fl/fl PEPCK Cre mice, we simultaneously stained kidney sections for TLR9 immunofluorescence (red) as well as Phaseolus vulgaris leucoagglutinin lectin to identify the brush border of proximal tubules (green). C) and D) Representative gel images and band intensity quantifications of TLR9 mRNA normalized to GAPDH from RT-PCR reactions in isolated proximal tubule cells, small intestine, bone-marrow cells, and spleen of TLR9fl/fl and TLR9fl/fl PEPCK Cre mice. For statistical analysis, the student’s t-test was used to detect significant changes. *P<0.05 vs. TLR9fl/fl mice (N=3). Error bars represent 1 SEM.
We simultaneously stained kidney sections for TLR9 immunofluorescence (red) as well as Phaseolus vulgaris leucoagglutinin lectin to identify the brush border of proximal tubules (green) (Figure 1B, representative of 4 experiments). Merged images demonstrate that cytosolic TLR9 staining was visible in lectin-positive proximal tubule cells as well as lectin-negative renal cells in TLR9fl/fl mice (left panel). In contrast, TLR9 staining was nearly absent in lectin-positive proximal tubule cells but was visible in lectin-negative renal cells in TLR9fl/fl PEPCK Cre mice (right panel).
To further confirm renal proximal tubule cell specific deletion of TLR9 in TLR9fl/fl PEPCK Cre mice, we measured the TLR9 mRNA expression in isolated proximal tubules (Figure 1C). TLR9 mRNA expression in renal proximal tubules from TLR9fl/fl PEPCK Cre mice were decreased by >95% compared to TLR9fl/fl mice. However, TLR9 mRNA expressions in isolated bone marrow cells, intestine and spleen were equivalent between in TLR9fl/fl PEPCK Cre mice and in TLR9fl/fl mice (Figure 1D) again demonstrating renal proximal tubule specific deletion of TLR9 in TLR9fl/fl PEPCK Cre mice.
Kidney IR injury induces renal tubular TLR9 expression
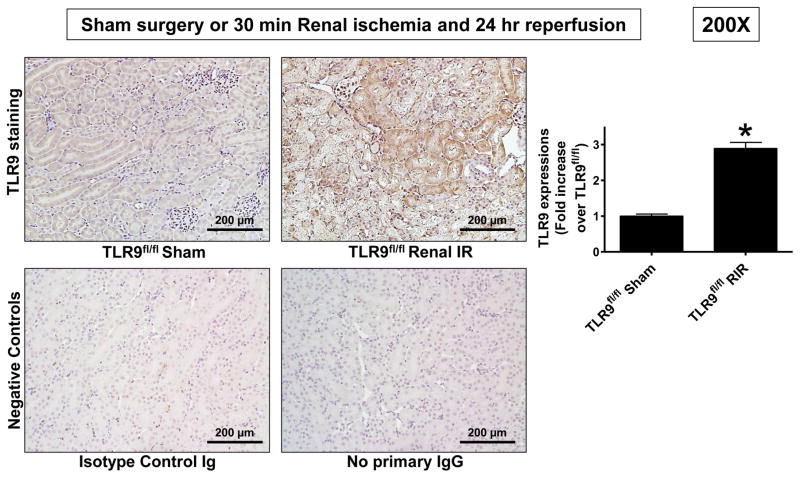
Figure 2 shows a representative TLR9 staining in TLR9fl/fl mice subjected to sham operation or to 30 min renal IR injury. Faint cytosolic TLR9 staining is visible in renal tubules from sham-operated mice. Kidneys from mice subjected to Renal IR injury show increased cytosolic TLR9 staining compared with sham-operated mice (200X, representative of 6 immunohistochemistry experiments).
Figure 2. Ischemic AKI induces renal tubular TLR9 expression.
Representative of 6 immunohistochemistry experiments for TLR9 protein staining in TLR9fl/fl mice subjected to sham operation or to 30 min renal IR injury. Two negative control slides show that staining is specific for TLR9. TLR9 expression was quantified using the imageJ software as described in Methods (N=6). For statistical analysis, the student’s t-test was used to detect significant changes. *P<0.05 vs. TLR9fl/fl mice subjected to sham surgery. Error bars represent 1 SEM.
Renal proximal tubular TLR9 plays a critical role in ischemic AKI
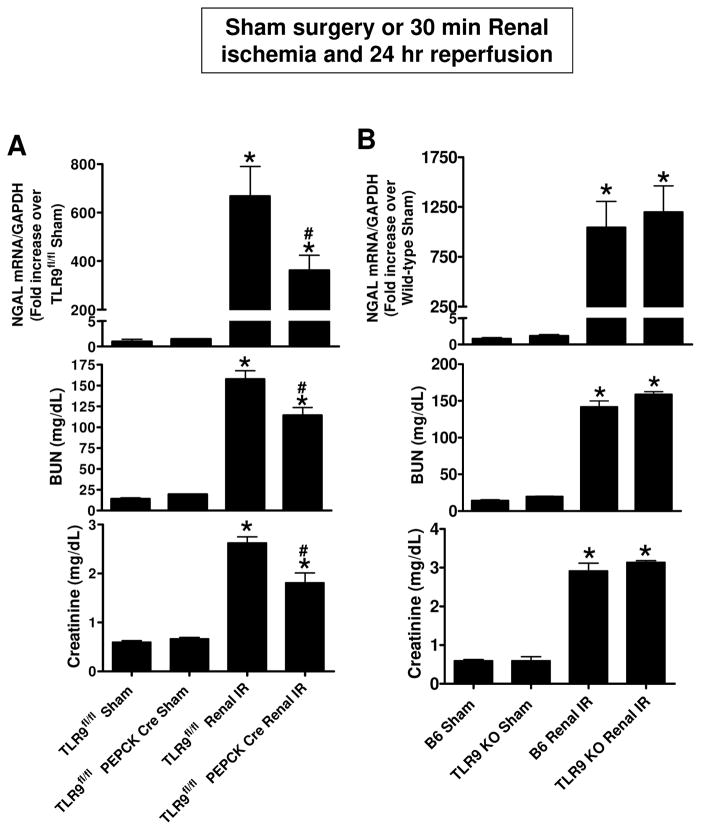
Plasma creatinine values were similar between TLR9fl/fl mice and renal proximal tubular TLR9 null mice subjected to sham-operation (Figure 3A). TLR9fl/fl mice subjected to renal IR had significantly increased plasma BUN and creatinine as well as kidney NGAL mRNA (N=6–8) compared to sham-operated mice (N=4–5). We show here that mice lacking renal proximal tubular TLR9 were protected against ischemic AKI compared to TLR9fl/fl mice as demonstrated by reduced plasma BUN and creatinine as well as kidney NGAL mRNA expression (N=6–8). In contrast, global TLR9KO mice are not protected against renal IR injury and had similar increases in plasma BUN and creatinine and kidney NGAL mRNA expression compared to B6 mice (N=5–7) (Figure 3B).
Figure 3. Renal proximal tubular TLR9 plays a critical role in ischemic AKI.
A) TLR9fl/fl PEPCK Cre mice and TLR9fl/fl mice were subjected to sham-surgery or to 30 min renal ischemia and 24 hr reperfusion (IR). Plasma BUN and creatinine as well as kidney NGAL mRNA (N=6–8) were measured. B) Global TLR9KO mice had similar increases in plasma BUN and creatinine and kidney NGAL mRNA expression when compared to B6 wild type mice (N=5–7). For statistical analysis, the one-way ANOVA plus Tukey’s post hoc multiple comparison test were used to detect significant changes. *P<0.05 vs. TLR9fl/fl mice subjected to sham surgery. #P<0.05 vs. TLR9fl/fl mice subjected to renal IR. Error bars represent 1 SEM.
Reduced renal tubular necrosis in proximal tubular TLR9 deficient mice after ischemic AKI
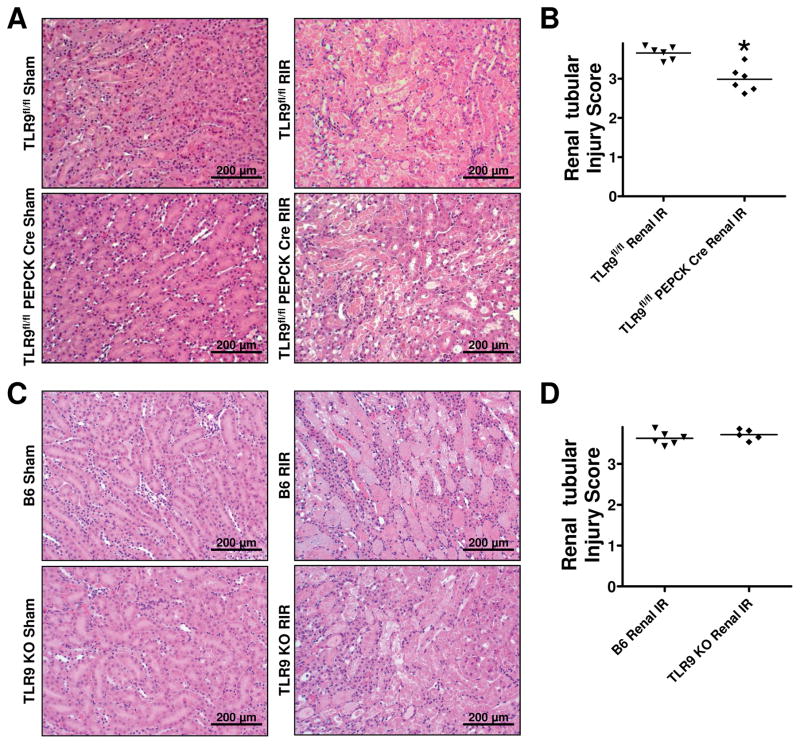
Figure 4A shows representative H&E images of renal proximal tubular TLR9 null mice and TLR9fl/fl mice subjected to sham surgery or 30 min renal IR and 24 hr reperfusion (magnification 200X, N=6). TLR9fl/fl mice subjected to renal IR showed severe tubular necrosis and proteinaceous casts as well as increased tubular dilatation and congestion. Renal proximal tubular TLR9 null mice had decreased renal tubular necrosis, congestion and cast formation compared to TLR9fl/fl mice subjected to renal IR. Kidneys from renal proximal tubular TLR9 null mice had slightly but significantly reduced renal tubular injury score compared to control TLR9fl/fl mice after IR (Figure 4B). Unlike reduced necrosis observed in renal proximal tubule TLR9 null mice, global TLR9KO mice and control B6 wild type mice showed similarly severe renal tubular necrotic injury after IR (Figure 4C and 4D).
Figure 4. Reduced renal tubular necrosis in proximal tubule TLR9 deficient mice after ischemic AKI.
A) Representative H&E images (from 5–6 experiments) of renal proximal tubular TLR9 null mice and TLR9fl/fl mice subjected to 30 min renal ischemia and 24 hrs reperfusion (magnification 200X). B) Renal injury scores assessing the degree of renal tubular necrosis (scale: 0–4,(41)) 24 hrs after renal IR (N=6). *P<0.05 vs. TLR9fl/fl mice subjected to renal IR injury. Representative H&E images (C) and renal injury scores (D) of global TLR9KO mice and B6 wild type mice subjected to sham-surgery or to 30 min renal ischemia and 24 hr reperfusion (magnification 200X, N=5–6). For statistical analysis, the Mann–Whitney nonparametric test was used to detect significant changes.
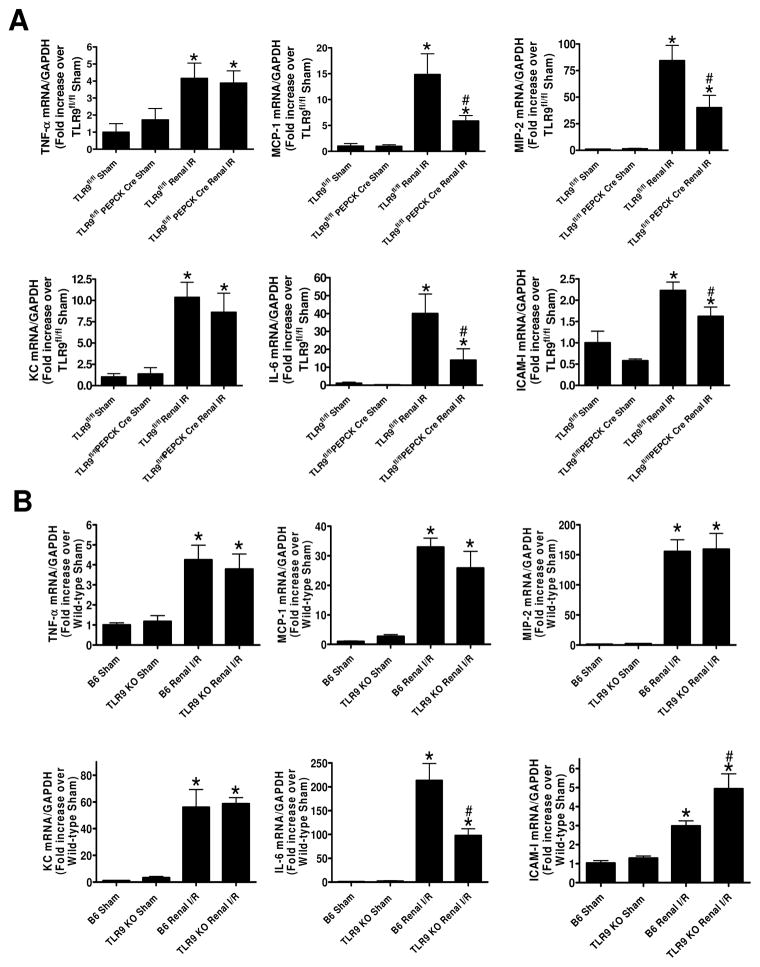
Renal proximal tubular TLR9 regulates pro-inflammatory chemokine and cytokine induction after ischemic AKI
Figure 5A shows fold increases in pro-inflammatory mRNAs normalized to GAPDH for each indicated mRNA (N=4–6). Ischemic AKI increased all of the pro-inflammatory genes measured in TLR9fl/fl mice. Consistent with the critical role of renal proximal tubular TLR9 for the induction of neutrophil and macrophage attracting chemokines, we show that MIP-2 and MCP-1 expression was significantly attenuated in renal proximal tubular TLR9 null mice. Moreover, IL-6 as well as ICAM-1 induction was attenuated by renal proximal tubular TLR9 deficiency. In contrast, we determined that global TLR9KO mice had similar degree of induction in KC, TNF-α, MIP-2 and MCP-1 after ischemic AKI when compared TLR9 competent mice (Figure 5B). Interestingly, global TLR9KO mice had significantly reduced kidney IL-6 mRNA expression but had higher kidney ICAM-1 mRNA expression. These data again suggest fundamental differences in renal inflammatory response between renal proximal tubular TLR9 null mice and global TLR9KO mice.
Figure 5. Renal proximal tubular TLR9 regulates kidney pro-inflammatory chemokine/cytokine induction and neutrophil infiltration after ischemic AKI.
With quantitative RT-PCR, we measured the expression of pro-inflammatory cytokine and chemokine mRNAs in the kidney [keratinocyte-derived cytokine (KC), monocyte chemoattractive protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and intercellular adhesion molecule-1 (ICAM-1)] 24 hr after sham-surgery or 30 min renal ischemia. Fold increases in pro-inflammatory mRNAs normalized to GAPDH from quantitative RT-PCR reactions for each indicated mRNA (N=4–6) are shown. For statistical analysis, the one-way ANOVA plus Tukey’s post hoc multiple comparison test were used to detect significant changes. *P < 0.05 vs. sham-operated mice. #P < 0.05 vs. TLR9fl/fl or B6 wild-type mice subjected to renal IR injury. Error bars represent 1 SEM.
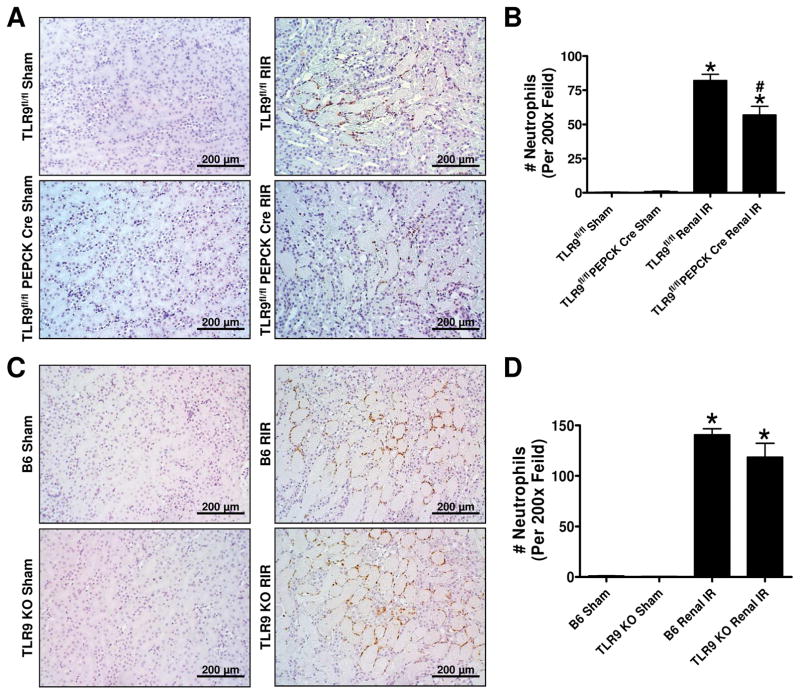
Renal proximal tubule TLR9 deficient mice have reduced kidney neutrophil infiltration after ischemic AKI
Figure 6A shows representative immunohistochemistry images and Figure 6B shows counts of infiltrating kidney neutrophils (N=6) in the kidneys of TLR9fl/fl mice and TLR9fl/fl PEPCK Cre subjected to sham surgery or renal IR (magnification 200X). Kidney neutrophil infiltration was significantly higher in the TLR9fl/fl wild type mice compared to TLR9fl/fl PEPCK Cre mice after renal IR. In contrast, kidney neutrophil infiltration was similar between B6 mice and global TLR9KO mice after ischemic AKI (Figure 6C, D).
Figure 6. Reduced kidney neutrophil infiltration in renal proximal tubule TLR9 deficient mice after ischemic AKI.
Representative images of immunohistochemistry for neutrophils (dark brown, A) and counts of infiltrating kidney neutrophils (B, N=6) in the kidneys of TLR9fl/fl mice and renal proximal tubule TLR9 null mice subjected to sham-surgery or to 30 min renal ischemia and 24 hr reperfusion (magnification 200X). Representative images of immunohistochemistry for neutrophils (C) and counts of infiltrating kidney neutrophils (D, N=5–6) in the kidneys of B6 wild type mice and global TLR9KO mice subjected to 30 min renal ischemia and 24 hr reperfusion (magnification 200X). For statistical analysis, the one-way ANOVA plus Tukey’s post hoc multiple comparison test were used to detect significant changes. *P<0.05 vs. TLR9fl/fl mice subjected to sham surgery. #P<0.05 vs. TLR9fl/fl mice subjected to renal IR. Error bars represent 1 SEM.
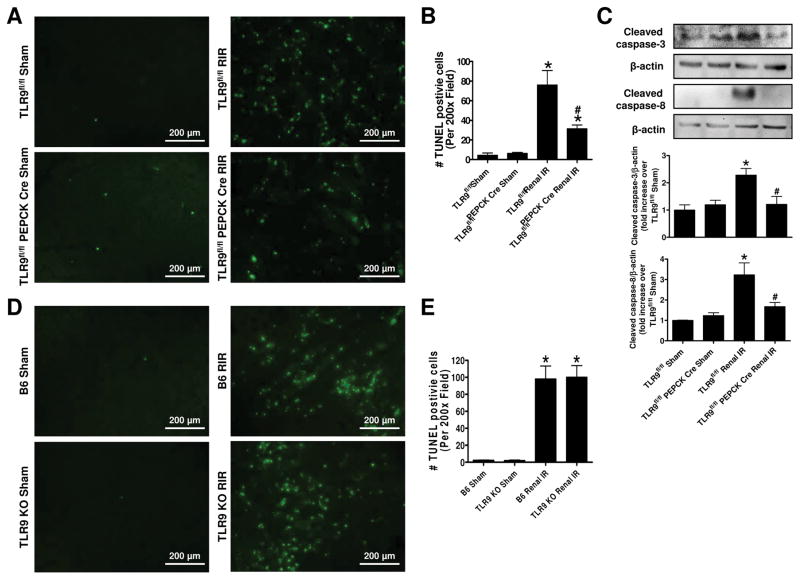
Reduced kidney apoptosis in renal proximal tubule TLR9 deficient mice after ischemic AKI
Figure 7A shows representative TUNEL staining images indicative of renal apoptosis and Figure 7B shows counts of TUNEL positive kidney cells (B, N=5) from TLR9fl/fl mice and TLR9fl/fl PEPCK Cre subjected to sham surgery or to renal IR (magnification 200X). Many TUNEL (fragmented DNA) positive cells were detected suggestive of renal tubular apoptosis in the kidneys from TLR9fl/fl mice subjected to renal IR injury. TUNEL positive kidney cell counts were significantly reduced in proximal tubular TLR9 null mice compared to TLR9fl/fl mice. We also detected caspase 3 and caspase 8 activation by measuring cleaved caspase 3 and caspase 8 in the kidney lysates from sham-operated mice and mice subjected to renal IR injury (Figure 7C). Caspase 3 and caspase 8 cleavage was increased in TLR9fl/fl mice subjected to renal IR that were significantly reduced in proximal tubular TLR9 null mice. In contrast, TUNEL positive kidney cells were equivalent between B6 mice and global TLR9KO mice after ischemic AKI (Figure 7D, E).
Figure 7. Reduced kidney apoptosis in renal proximal tubule TLR9 deficient mice after ischemic AKI.
Representative images of terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) staining indicative of renal tubular apoptosis (A) and counts of TUNEL positive kidney cells (B, N=5) from TLR9fl/fl wild type mice and renal proximal tubule TLR9 null mice subjected to sham-surgery or to 30 min renal ischemia and 24 hr reperfusion (magnification 200X). We also detected caspase 3 and caspase 8 activation by measuring cleaved caspase 3 and caspase 8 in the kidney lysates from sham-operated mice and mice subjected to renal IR injury (C). Representative images of TUNEL staining (D) and counts of TUNEL positive kidney cells (E, N=5) from B6 mice and global TLR9KO mice subjected to sham-surgery or to 30 min renal ischemia and 24 hr reperfusion (magnification 200X). For statistical analysis, the one-way ANOVA plus Tukey’s post hoc multiple comparison test were used to detect significant changes. *P<0.05 vs. TLR9fl/fl mice subjected to sham surgery. #P<0.05 vs. TLR9fl/fl mice subjected to renal IR. Error bars represent 1 SEM.
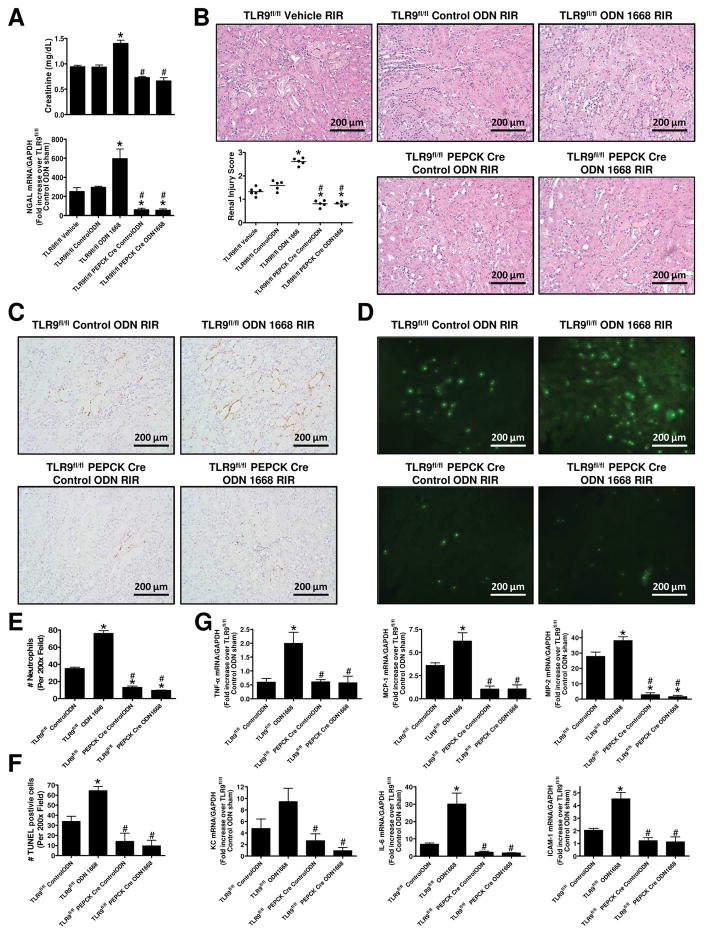
TLR9 activation exacerbates ischemic AKI and increases renal tubular necrosis, inflammation and apoptosis
There were no differences of plasma creatinine, kidney NGAL mRNA as well as renal tubular necrosis between vehicle (saline)-treated mice and control ODN-treated mice (Figure 8A, B). TLR9fl/fl mice subjected to 20 min. renal IR after ODN-1668 treatment had significantly increased plasma creatinine as well as kidney NGAL mRNA compared to control ODN-treated mice (N=5–6, Figure 8A). In contrast, not only mice lacking renal proximal tubular TLR9 had reduced renal injury after 20 min renal IR, ODN-1668 treatment failed to exacerbate ischemic AKI in these mice (N=5–6). Moreover, selective TLR9 activation with ODN-1668 increased renal tubular necrosis (Figure 8B), neutrophil infiltration (Figure 8C, E), pro-inflammatory cytokine mRNA induction (Figure 8G) and TUNEL positive cells (Figure 8D, F) in TLR9fl/fl mice compared to control ODN-treated TLR9fl/fl mice. However, ODN-1668 failed to increases these markers of renal injury and inflammation in renal proximal tubular TLR9 null mice. Furthermore, mice lacking renal proximal tubular TLR9 had reduced renal injury scores, neutrophil infiltration, pro-inflammatory cytokine induction and TUNEL staining compared to TLR9fl/fl mice after 20 min renal IR injury.
Figure 8. Renal proximal tubular TLR9 activation exacerbates ischemic AKI.
TLR9fl/fl mice or renal proximal tubular TLR9 null mice were pretreated with saline (vehicle), control oligonucleotide (control-ODN) or with 1 mg/kg ODN-1668 (a selective TLR9 agonist) and subjected to 20 min renal ischemia and 24 hr reperfusion (IR). A) TLR9fl/fl mice subjected to 20 min. renal IR after ODN-1668 treatment had significantly increased plasma creatinine as well as kidney NGAL mRNA compared to control ODN-treated mice (N=5–6). In contrast, not only mice lacking renal proximal tubular TLR9 had reduced renal injury after 20 min renal IR, ODN-1668 treatment failed to exacerbate ischemic AKI in these mice (N=5–6). B) Representative H&E images (magnification 200X) and scatter plot renal injury scores (N=5–6). Representative images of immunohistochemistry for neutrophils (C, 200X) and counts of infiltrating kidney neutrophils (E) (N=5–6). Representative images TUNEL staining (D, 200X) and counts of TUNEL positive kidney cells (F) (N=5–6). G) Quantitative RT-PCR measured the expression of pro-inflammatory cytokine and chemokine mRNAs in the kidney [KC, MCP-1, MIP-2, TNF-α, IL-6 and ICAM-1] Fold increases in pro-inflammatory mRNAs normalized to GAPDH from quantitative RT-PCR reactions of control ODN-treated sham-operated mice for each indicated mRNA (N=5–6) are shown. For statistical analysis, the one-way ANOVA plus Tukey’s post hoc multiple comparison test were used to detect significant changes. *P < 0.05 vs. control ODN-treated TLR9fl/fl mice subjected to renal IR injury. #P < 0.05 vs. ODN-1668-treated TLR9fl/fl mice subjected to renal IR injury. Error bars represent 1 SEM.
TLR9 agonists induce renal proximal tubular NFκB activation, caspase cleavage and pro-inflammatory mRNA induction
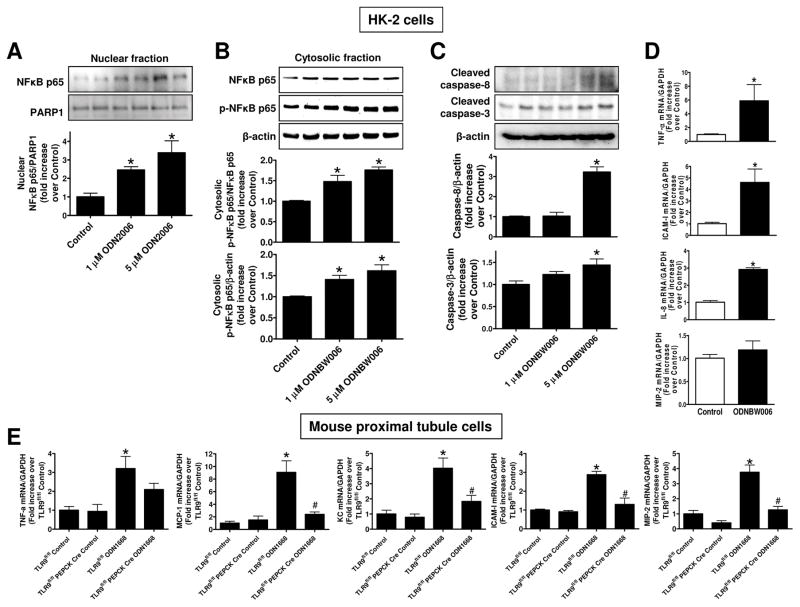
Figure 9A shows a representative immunoblotting for nuclear p65 NFκB subunit and band intensity quantifications normalized to PARP-1 in HK-2 cells (N=4). TLR9 activation with ODN2006 increased nuclear translocation of p65 NFκB subunit in HK-2 cells. Moreover, TLR9 activation with another TLR9 ligand (ODNBW006) in HK-2 cells also increased the phosphorylated p65 NFκB subunit in the cytosol as shown by the representative immunoblotting images for cytosolic p65 subunit (top) and band intensity quantifications normalized to p65 subunit or β-actin (N=4, bottom) (Figure 9B). Figure 9C shows a representative immunoblotting experiment for cleaved caspase 3 and caspase 8 (top) and band intensity quantifications normalized to β-actin (N=4, bottom) demonstrating increased TLR9 ligand-mediated cleavage of caspase 3 and caspase 8 in HK-2 cells.
Figure 9. TLR9 agonists induce NFκB activation, caspase cleavage and pro-inflammatory mRNA induction in renal proximal tubule cells.
A–D. HK-2 human renal proximal tubule cells were treated with 1–5 μM TLR9 agonist (ODN2006 or ODNBW006) or with respective control oligonucleotide (ODN2137 or ODN BW007) for 3 days. A. A representative immunoblotting experiment for nuclear p65 NFκB subunit (top) and band intensity quantifications normalized to PARP-1 (N=4, bottom). B. A representative immunoblotting experiment for cytosolic p65 subunit (top) and band intensity quantifications normalized to p65 subunit or β-actin (N=4, bottom) are shown. C. A representative immunoblotting experiment for cleaved caspase 3 and caspase 8 (top) and band intensity quantifications normalized to β-actin (N=4, bottom) are shown. D. With quantitative RTPCR, we measured the expression of pro-inflammatory IL-8, TNF-α, ICAM-1 and MIP-2 mRNA expression in HK-2 cells. Fold increases in pro-inflammatory mRNAs normalized to GAPDH from quantitative RTPCR reactions for each indicated mRNA (N=4) are shown. E. Finally, primary cultures of mouse renal proximal tubules from wild type TLR9fl/fl mice and renal proximal tubule TLR9 null mice were treated with a mouse TLR9 selective oligonucleotide ODN1688 for 3 days. With quantitative RTPCR, we measured the expression of pro-inflammatory IL-8, TNF-α, ICAM-1, MCP-1 and MIP-2 mRNA expression in primary cultures of mouse renal proximal tubules. Fold increases in pro-inflammatory mRNAs normalized to GAPDH from quantitative RTPCR reactions for each indicated mRNA (N=4) are shown. For statistical analysis, the one-way ANOVA plus Tukey’s post hoc multiple comparison test were used to detect significant changes. *P<0.05 vs control oligonucleotide-treated cells. #P<0.05 vs. TLR9fl/fl renal proximal tubule cells treated with ODN1668. Error bars represent 1 SEM.
Quantitative RTPCR revealed that a selective TLR9 ligand ODNBW006 treatment significantly induced the expression of IL-8, TNF-α and ICAM-1 mRNAs measured without changing the MIP-2 expression (Figure 9D, N=4) in HK-2 cells. In addition, we show that TLR9 agonist ODN1668 treatment significantly induced the expression of pro-inflammatory mRNAs (IL-8, TNF-α, ICAM-1, MCP-1 and MIP-2) in renal proximal tubules from TLR9fl/fl mice but not in renal proximal tubules isolated from renal proximal tubule TLR9 null mice (Figure 9E, N=4).
Discussion
TLR9 activation mediates IR injury in several organs including the heart, and liver but plays no role in intestinal IR injury (16, 18, 49). In cardiac and hepatic IR injury, TLR9 activation by endogenous DAMPs plays a critical role in cardiomyocyte and hepatocyte death (18, 21, 30, 50). In particular, mitochondrial DNA with high un-methylated CpG motif that can strongly stimulate TLR9 (30, 51). Previous studies show that circulating and locally released mitochondrial DNA can activate TLR9 to promote liver injury after trauma or IR (21, 30).
However, the role for TLR9 in ischemic AKI remains unclear. Suggesting a detrimental role of TLR9 in the kidney, mitochondrial DNA-induced TLR9 activation appears to play a critical role in septic AKI as TLR9 signaling inhibition or genetic TLR9 deletion attenuates CLP-induced sepsis in mice (17, 24). Moreover, siRNA-mediated TLR9 deletion also protects against CLP-sepsis (25). In addition, TLR9 activation by endogenous DNA induces kidney podocyte apoptosis (51). These findings in various kidney pathologies suggest kidney TLR9 may play a role in ischemic AKI. However, 2 previous studies using global TLR9 null mice failed to show a detrimental role for TLR9 in ischemic AKI (22, 23).
We demonstrate here that renal proximal tubule TLR9 plays a critical role in ischemic AKI as renal tubular TLR9 null mice had significantly reduced renal tubular necrosis and apoptosis after IR. Necrotic renal cells after IR release mitochondrial DNA that can target intracellular renal tubular TLR9 (24). In particular, we demonstrate that TLR9 signaling regulates renal proximal tubular apoptosis after kidney IR injury. Mice deficient in renal tubular TLR9 had reduced caspase 3 and 8 activation after renal IR compared to control TLR9fl/fl mice. Moreover, TLR9 selective ligand induced proximal tubule apoptosis in culture. Our data is consistent with the hypothesis that renal IR injury causes renal proximal tubular TLR9 activation to induce kidney apoptosis via caspase 3/8 activation.
Renal proximal tubule TLR9 null mice had significantly attenuated kidney IL-6, MCP-1, MIP-2 and ICAM-1 synthesis expression after ischemic AKI when compared to TLR9fl/fl mice (Figure 5). Consistent with reduced pro-inflammatory cytokines and chemokines, renal proximal tubule TLR9 null mice had reduced neutrophil infiltration after IR. Moreover, TLR9 selective ligand induced pro-inflammatory cytokines and chemokines in cultured human and mouse renal proximal tubule cells. Finally, TLR9 specific agonist ligand only induced inflammatory cytokines in TLR9fl/fl mice but not in TLR9 null renal proximal tubules. Taken together, our studies suggest that renal proximal tubules are the major source of pro-inflammatory cytokines and chemokines and renal tubular TLR9 is a key regulator for several of these critical inflammatory mediators.
Neutrophils infiltrating the kidney after renal IR are major contributor to additional renal injury after reperfusion and are able to recruit other inflammatory leukocytes including natural killer cells, monocytes and macrophages (52–54). Reduced kidney neutrophil infiltration most likely attenuated additional cytokine generation in renal proximal tubule TLR9 null mice that may have contributed to improved renal function after ischemic AKI. Consistent with this, renal proximal tubule cell TLR9 null mice also had significantly reduced MCP-1 expression in the kidney after IR. MCP-1 (CCL2) is the major chemokine that promote macrophage infiltration and migration after IR (55, 56).
Although our findings suggest that renal IR-induced TLR9 activation directly promotes inflammation and apoptosis in renal proximal tubular cells and exacerbates ischemic AKI, it is interesting that mice globally deficient in TLR9 were not protected against ischemic AKI as shown previously by other investigators (22, 23). These discrepancies in renal injury between tissue specific TLR9 null mice and global TLR9KO mice point out major differences between these mice and suggest caution in interpreting studies with global genetic deletions studies. These differences also suggest divergent effects of TLR9 activation depending on the cell and tissue types studied. Although renal proximal tubular TLR9 activation causes kidney injury by inducing apoptosis and inflammation after IR, other cell types in the kidney may benefit from TLR9 signaling. Indeed, a renal protective role for TLR9 in cisplatin-induced AKI model has been described as TLR9 deficient mice had exacerbated renal injury with higher BUN, tubular injury and inflammatory cytokine induction (57). In cisplatin-induced AKI as well as in murine lupus model, TLR9 may promote tissue protection by promoting accumulation of beneficial regulatory T-cells (57, 58). Directly relevant to our studies, global TLR9 deficiency leads to enhanced renal inflammation in several murine models of lupus (59, 60). Therefore, it is possible that global TLR9KO mice were not protected against ischemic AKI as they may lack the tissue protective regulatory T-cells modulated by TLR9 signaling. Furthermore, not all TLR9 signaling results in exacerbation of IR injury as TLR9 activation protects against cerebral as well as cardiac IR injury via activation of PI3K/Akt signaling (26, 27). Finally, TLR9 activation may mediate cardiac and neuronal protection by modulating energy metabolism (61). Therefore, our study as well as previous studies suggest that kidney TLR9 signaling results in complex and perhaps divergent effects depending on the cells targeted. In addition, our findings also suggest that these variations in TLR9 signaling suggest caution in interpreting studies with global TLR9 gene deletion.
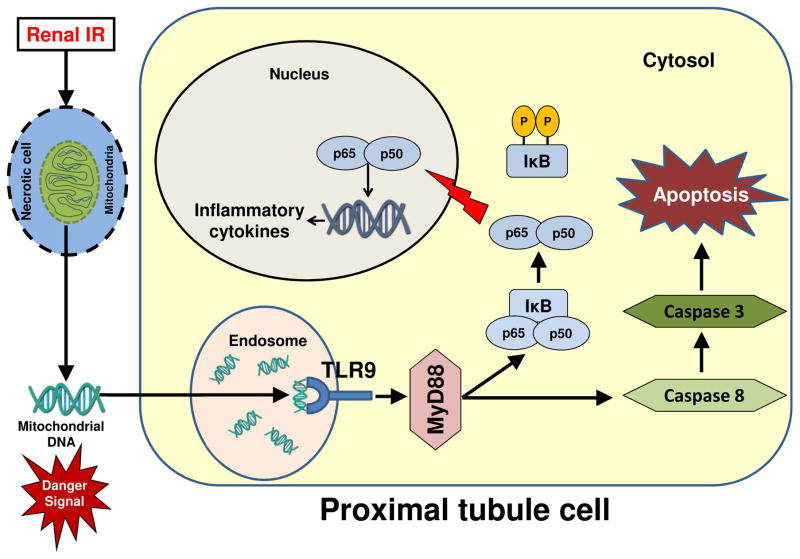
In summary, we demonstrate in this study that renal proximal tubular TLR9 plays a critical role in murine ischemic AKI by exacerbating necrosis, apoptosis and inflammation after IR. Our study suggest caution when interpreting data from global gene deletion studies as there were critical differences between proximal tubule TLR9 deficient mice and global TLR9 deficient mice subjected to ischemic AKI. Figure 10 shows a schematic of proposed mechanisms for renal proximal tubular TLR9-mediated exacerbation of ischemic AKI. After renal IR, damaged renal cells release endogenous TLR9 activators (presumably mitochondrial DNA products) leading to NFκB-mediated induction of pro-inflammatory chemokines and cytokines as well as Caspase 3/8-mediated induction of renal tubular apoptosis.
Figure 10. Schematic of proposed mechanisms for renal proximal tubular TLR9-mediated exacerbation of ischemic AKI.
After renal ischemia and reperfusion, damaged renal cells release endogenous TLR9 activators (presumably mitochondrial DNA products). Renal proximal tubular TLR9 activation would then lead to NFκB-mediated induction of pro-inflammatory chemokines and cytokines as well as Caspase 3/8-mediated induction of renal tubular apoptosis.
Acknowledgments
We thank Dr. Volker Haas (Vanderbilt University, Nashville, TN) for providing us the PEPCK Cre mice.
This work was supported by Department of Anesthesiology, Columbia University and in part by NIH grants DK-109544 and DK-115694.
Abbreviations
- AKI
acute kidney injury
- IR
ischemia reperfusion
- TLR
Toll-like receptor
- IL-6
Interleukin-6
- ICAM-1
intercellular adhesion molecule-1
- MCP-1
monocyte chemoattractive protein-1
- MIP-2
macrophage inflammatory protein-2
- TNF-α
tumor necrosis factor-α
- KC
Keratinocyte chemoattractant
- NGAL
Neutrophil gelatinase-associated lipocalin
- HK-2
Immortalized human proximal tubular cells
- ODN
oligonucleotide
Footnotes
Disclosures: No conflict of interest exists for each author.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Jones DR, Lee HT. Perioperative renal protection. Best Pract Res Clin Anaesthesiol. 2008;22:193–208. doi: 10.1016/j.bpa.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Aronson S, Blumenthal R. Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothorac Vasc Anesth. 1998;17:117–130. doi: 10.1016/s1053-0770(98)90106-9. [DOI] [PubMed] [Google Scholar]
- 4.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11:88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 5.Kinsey GR, Okusa MD. Expanding role of T cells in acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23:9–16. doi: 10.1097/01.mnh.0000436695.29173.de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusch A, Hoff U, Bubalo G, Zhu Y, Fechner M, Schmidt-Ullrich R, Marko L, Muller DN, Schmidt-Ott KM, Gurgen D, Blum M, Schunck WH, Dragun D. Novel signalling mechanisms and targets in renal ischaemia and reperfusion injury. Acta Physiol (Oxf) 2013;208:25–40. doi: 10.1111/apha.12089. [DOI] [PubMed] [Google Scholar]
- 7.Kork F, Balzer F, Spies CD, Wernecke KD, Ginde AA, Jankowski J, Eltzschig HK. Minor Postoperative Increases of Creatinine Are Associated with Higher Mortality and Longer Hospital Length of Stay in Surgical Patients. Anesthesiology. 2015;123:1301–1311. doi: 10.1097/ALN.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilmi IA, Damian D, Al-Khafaji A, Planinsic R, Boucek C, Sakai T, Chang CC, Kellum JA. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth. 2015;114:919–926. doi: 10.1093/bja/aeu556. [DOI] [PubMed] [Google Scholar]
- 9.Leventhal JS, Schroppel B. Toll-like receptors in transplantation: sensing and reacting to injury. Kidney Int. 2012;81:826–832. doi: 10.1038/ki.2011.498. [DOI] [PubMed] [Google Scholar]
- 10.Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J. The role of Toll-like receptors in renal diseases. Nat Rev Nephrol. 2010;6:224–235. doi: 10.1038/nrneph.2010.16. [DOI] [PubMed] [Google Scholar]
- 11.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 12.Robson MG. Toll-like receptors and renal disease. Nephron Exp Nephrol. 2009;113:e1–e7. doi: 10.1159/000228077. [DOI] [PubMed] [Google Scholar]
- 13.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, Lotze MT, Geller DA, Billiar TR. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 14.Rusai K, Sollinger D, Baumann M, Wagner B, Strobl M, Schmaderer C, Roos M, Kirschning C, Heemann U, Lutz J. Toll-like receptors 2 and 4 in renal ischemia/reperfusion injury. Pediatr Nephrol. 2010;25:853–860. doi: 10.1007/s00467-009-1422-4. [DOI] [PubMed] [Google Scholar]
- 15.Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der PT, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bamboat ZM, V, Balachandran P, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology. 2010;51:621–632. doi: 10.1002/hep.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda H, Leelahavanichkul A, Tsunoda S, Dear JW, Takahashi Y, Ito S, Hu X, Zhou H, Doi K, Childs R, Klinman DM, Yuen PS, Star RA. Chloroquine and inhibition of Toll-like receptor 9 protect from sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F1050–F1058. doi: 10.1152/ajprenal.00461.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, Liao X, Billiar T, Xu J, Esmon CT, Tsung A. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 20.Itagaki K, Kaczmarek E, Lee YT, Tang IT, Isal B, Adibnia Y, Sandler N, Grimm MJ, Segal BH, Otterbein LE, Hauser CJ. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS One. 2015;10:e0120549. doi: 10.1371/journal.pone.0120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, Wang S, Kim J, Billiar T, Wang Y, Tsung A. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–614. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Yun Z, Tan Z, Li S, Wang D, Ma K, Chi N, Liu J, Chen F, Gao G. The role of Toll-like receptor (TLR) 2 and 9 in renal ischemia and reperfusion injury. Urology. 2013;81:1379e1315–1320. doi: 10.1016/j.urology.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Bakker PJ, Scantlebery AM, Butter LM, Claessen N, Teske GJ, van der Poll T, Florquin S, Leemans JC. TLR9 Mediates Remote Liver Injury following Severe Renal Ischemia Reperfusion. PLoS One. 2015;10:e0137511. doi: 10.1371/journal.pone.0137511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuji N, Tsuji T, Ohashi N, Kato A, Fujigaki Y, Yasuda H. Role of Mitochondrial DNA in Septic AKI via Toll-Like Receptor 9. J Am Soc Nephrol. 2016;27:2009–2020. doi: 10.1681/ASN.2015040376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L, Li Y, Hu Z, Su J, Huo Y, Tan B, Wang X, Liu Y. Small interfering RNA targeting Toll-like receptor 9 protects mice against polymicrobial septic acute kidney injury. Nephron Exp Nephrol. 2012;122:51–61. doi: 10.1159/000346953. [DOI] [PubMed] [Google Scholar]
- 26.Lu C, Ha T, Wang X, Liu L, Zhang X, Kimbrough EO, Sha Z, Guan M, Schweitzer J, Kalbfleisch J, Williams D, Li C. The TLR9 ligand, CpG-ODN, induces protection against cerebral ischemia/reperfusion injury via activation of PI3K/Akt signaling. J Am Heart Assoc. 2014;3:e000629. doi: 10.1161/JAHA.113.000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Z, Ren D, Ha T, Liu L, Wang X, Kalbfleisch J, Gao X, Kao R, Williams D, Li C. CpG-ODN, the TLR9 agonist, attenuates myocardial ischemia/reperfusion injury: involving activation of PI3K/Akt signaling. Biochim Biophys Acta. 2013;1832:96–104. doi: 10.1016/j.bbadis.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisener-Dorman AF, Lawrence DA, Bolivar VJ. Cautionary insights on knockout mouse studies: the gene or not the gene? Brain Behav Immun. 2009;23:318–324. doi: 10.1016/j.bbi.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–864. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66:2576–2583. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan TN, Sinniah R. Study of renal tubular glycoconjugates in tubulointerstitial damage using conjugated lectins. J Pathol. 1993;170:187–196. doi: 10.1002/path.1711700215. [DOI] [PubMed] [Google Scholar]
- 33.Truong LD, V, Phung T, Yoshikawa Y, Mattioli CA. Glycoconjugates in normal human kidney. A histochemical study using 13 biotinylated lectins. Histochemistry. 1988;90:51–60. doi: 10.1007/BF00495707. [DOI] [PubMed] [Google Scholar]
- 34.Vinay P, Gougoux A, Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981;241:F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]
- 35.Park SW, Kim JY, Ham A, Brown KM, Kim M, D’Agati VD, Lee HT. A1 adenosine receptor allosteric enhancer PD-81723 protects against renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2012;303:F721–F732. doi: 10.1152/ajprenal.00157.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SW, Kim M, Brown KM, D’Agati VD, Lee HT. Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2012;23:266–280. doi: 10.1681/ASN.2011050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M, Park SW, Kim M, Chen SW, Gerthoffer WT, D’Agati VD, Lee HT. Selective Renal Over-Expression of Human Heat Shock Protein 27 Reduces Renal Ischemia-Reperfusion Injury in Mice. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HT, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D’Agati VD, Cox GN. Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2012;303:F1216–F1224. doi: 10.1152/ajprenal.00220.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruifrok AC, Johnston DA. Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol. 2001;23:291–299. [PubMed] [Google Scholar]
- 40.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 41.Khalid U, Pino-Chavez G, Nesargikar P, Jenkins RH, Bowen T, Fraser DJ, Chavez R. Kidney ischaemia reperfusion injury in the rat: the EGTI scoring system as a valid and reliable tool for histological assessment. Journal of Histology and Histopathology. 2016;3:1. [Google Scholar]
- 42.Park SW, Chen SW, Kim M, D’Agati VD, Lee HT. Human heat shock protein 27 overexpressing mice are protected against acute kidney injury after hepatic ischemia and reperfusion. Am J Physiol Renal Physiol. 2009;297:F885–F894. doi: 10.1152/ajprenal.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HT, Xu H, Siegel CD, Krichevsky IE. Local Anesthetics Induce Human Renal Cell Apoptosis. Am J Nephrol. 2003;23:129–139. doi: 10.1159/000069304. [DOI] [PubMed] [Google Scholar]
- 44.Park SW, Kim M, Kim M, D’Agati VD, Lee HT. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int. 2011;80:1315–1327. doi: 10.1038/ki.2011.281. [DOI] [PubMed] [Google Scholar]
- 45.Kim M, Kim M, Park SW, Pitson SM, Lee HT. Isoflurane protects human kidney proximal tubule cells against necrosis via sphingosine kinase and sphingosine-1-phosphate generation. Am J Nephrol. 2010;31:353–362. doi: 10.1159/000298339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song JH, Kim M, Park SW, Chen SW, Pitson SM, Lee HT. Isoflurane via TGF-beta1 release increases caveolae formation and organizes sphingosine kinase signaling in renal proximal tubules. Am J Physiol Renal Physiol. 2010;298:F1041–F1050. doi: 10.1152/ajprenal.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai F, Homan PJ, Agrawal H, Misharin AV, Abdala-Valencia H, Haines GK, 3rd, Dominguez S, Bloomfield CL, Saber R, Chang A, Mohan C, Hutcheson J, Davidson A, Budinger GRS, Bouillet P, Dorfleutner A, Stehlik C, Winter DR, Cuda CM, Perlman H. Bim suppresses the development of SLE by limiting myeloid inflammatory responses. J Exp Med. 2017;214:3753–3773. doi: 10.1084/jem.20170479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rabadi MM, Kim M, D’Agati VD, Lee HT. Peptidyl arginine deiminase-4 deficient mice are protected against kidney and liver injury after renal ischemia and reperfusion. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00254.2016. ajprenal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slone EA, Pope MR, Roth M, Welti R, Fleming SD. TLR9 is dispensable for intestinal ischemia/reperfusion-induced tissue damage. Am J Clin Exp Immunol. 2012;1:124–135. [PMC free article] [PubMed] [Google Scholar]
- 50.Bliksoen M, Mariero LH, Torp MK, Baysa A, Ytrehus K, Haugen F, Seljeflot I, Vaage J, Valen G, Stenslokken KO. Extracellular mtDNA activates NF-kappaB via toll-like receptor 9 and induces cell death in cardiomyocytes. Basic Res Cardiol. 2016;111:42. doi: 10.1007/s00395-016-0553-6. [DOI] [PubMed] [Google Scholar]
- 51.Bao W, Xia H, Liang Y, Ye Y, Lu Y, Xu X, Duan A, He J, Chen Z, Wu Y, Wang X, Zheng C, Liu Z, Shi S. Toll-like Receptor 9 Can be Activated by Endogenous Mitochondrial DNA to Induce Podocyte Apoptosis. Sci Rep. 2016;6:22579. doi: 10.1038/srep22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109:e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–747. [PubMed] [Google Scholar]
- 54.Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am J Kidney Dis. 1999;34:384–399. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- 55.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006;21:1231–1239. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 56.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H. CCR2 Signaling Contributes to Ischemia-Reperfusion Injury in Kidney. J Am Soc Nephrol. 2003;14:2503–2515. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- 57.Alikhan MA, Summers SA, Gan PY, Chan AJ, Khouri MB, Ooi JD, Ghali JR, Odobasic D, Hickey MJ, Kitching AR, Holdsworth SR. Endogenous Toll-Like Receptor 9 Regulates AKI by Promoting Regulatory T Cell Recruitment. J Am Soc Nephrol. 2016;27:706–714. doi: 10.1681/ASN.2014090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–342. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 59.Nickerson KM, Wang Y, Bastacky S, Shlomchik MJ. Toll-like receptor 9 suppresses lupus disease in Fas-sufficient MRL Mice. PLoS One. 2017;12:e0173471. doi: 10.1371/journal.pone.0173471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bossaller L, Christ A, Pelka K, Nundel K, Chiang PI, Pang C, Mishra N, Busto P, Bonegio RG, Schmidt RE, Latz E, Marshak-Rothstein A. TLR9 Deficiency Leads to Accelerated Renal Disease and Myeloid Lineage Abnormalities in Pristane-Induced Murine Lupus. J Immunol. 2016;197:1044–1053. doi: 10.4049/jimmunol.1501943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shintani Y, Kapoor A, Kaneko M, Smolenski RT, D’Acquisto F, Coppen SR, Harada-Shoji N, Lee HJ, Thiemermann C, Takashima S, Yashiro K, Suzuki K. TLR9 mediates cellular protection by modulating energy metabolism in cardiomyocytes and neurons. Proc Natl Acad Sci U S A. 2013;110:5109–5114. doi: 10.1073/pnas.1219243110. [DOI] [PMC free article] [PubMed] [Google Scholar]