Abstract
Background
Many individuals with chronic kidney disease (CKD) do not receive guideline-concordant care. We examined the impact of a team-based primary care CKD registry on clinical measures and processes of care among patients CKD cared for in a public safety-net healthcare delivery system.
Study Design
Pragmatic trial of a CKD registry versus a usual care registry for one year
Setting and Participants
Primary care providers (PCPs) and their patients with CKD in safety-net primary care setting in San Francisco.
Intervention
The CKD registry identified at point of care: all patients with CKD, those with BP >140/90 mmHg, those without ACEi/ARB prescription, and those without albuminuria quantification in the past year. It also provided quarterly feedback pertinent to these metrics to promote CKD patient “outreach”. The usual care registry provided point-of-care cancer screening and immunization data.
Outcomes
Changes in systolic BP at 12 months (primary outcome), proportion of patients with BP control, prescription of ACEi/ARB, quantification of albuminuria, severity of albuminuria and eGFR.
Results
The patient population (n=746) had a mean age of 56.7 +/− 12.1 (SD) years, was 53% female and was diverse (8% non-Hispanic White, 35.7% Black, 24.5% Hispanic, 24.4% Asian). Randomization to the CKD registry (30 PCPs, 285 patients) versus the usual care registry (49 PCPs, 461 patients) was associated with a 2-fold greater odds of ACEi/ARB prescription (adjusted OR, 2.25; 95%CI, 1.45-3.49) and albuminuria quantification (adjusted OR, 2.44; 95% CI, 1.38-4.29) during the one-year study period. Randomization to the CKD registry was not associated with changes in systolic BP, proportion of patients with uncontrolled BP, or degree of albuminuria or eGFR.
Limitations
Potential misclassification of CKD; missing baseline medication data; limited to study of a public safety-net healthcare system
Conclusion
A team-based safety-net primary care CKD registry did not improve BP parameters, but led to greater albuminuria quantification and more ACEi/ARB prescriptions after one year. Adoption of team-based CKD registries may represent an important step in translating evidence into practice for CKD management.
Keywords: chronic kidney disease (CKD), CKD registry, process of care, evidence-based care, guideline implementation, blood pressure control, albuminuria, angiotensin converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), hypertension, disease progression, best practices, CKD management, pragmatic trial
Over 20 million Americans suffer from chronic kidney disease (CKD).1 Compared to individuals with normal kidney function, those with CKD have greater odds of experiencing a premature cardiovascular event or death, independent of age, gender, and comorbid conditions.2 Lower income and racial/ethnic minority patients are more likely to have kidney failure, placing a unique burden on healthcare systems that disproportionately provide for their care.3 Even though randomized controlled trials have shown that controlling blood pressure (BP) control and reducing proteinuria with angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) can delay CKD decline, progression to end stage renal disease (ESRD), and decrease CKD-associated morbidity and mortality,4–7 many individuals with CKD are not receiving these evidence based treatments.8 The failure to implement these best practices may be related to providers’ poor CKD awareness9,10 and limited confidence among primary care providers (PCPs) in delivering CKD care11 in the context of inefficient health care systems that rely on overburdened providers to deliver chronic disease care to complex patients.
Disease registries are information platforms that can enhance chronic disease management.12 Often embedded within electronic health records, disease registries capture and track patient-level data, allowing health care teams to proactively manage patients via “in-reach” at point-of-care, or via “outreach” through patient contact outside of scheduled appointment times. Registries have been documented to improve the quality of chronic disease care,13 including among patients with diabetes14 and congestive heart failure.15 Prior studies of CKD registries in the United States with computer-assisted prompts/alerts have not improved process outcomes related to CKD management or clinical outcomes,16,17 though they have been successful in the United Kingdom.18
We hypothesized that the prior negative results in the U.S were not likely due to unique refractoriness of CKD to the registry approach, but more likely because prior U.S. CKD registries have focused on behavior change among individual physicians rather than on entire health care teams and systems of care. With input from primary care leaders and quality improvement champions in a safety net health system, we created an electronic CKD registry that identifies patients with CKD and provides data about CKD management to the entire health care team.19 We then tested this approach to improve kidney health in a pragmatic trial in safety-net primary care clinics with a high burden of hypertension and CKD.
Methods
Study population, setting and study design
This pragmatic trial (ClinicalTrials.gov study number NCTXXXXXX) took place in two primary care clinics in San Francisco’s public healthcare delivery system in 2013-2015. These clinics were selected because of the high prevalence of CKD among their patient populations and because one was an academic training clinic and the other was a community clinic without any trainees. No other clinics were approached for participation. All PCPs who worked in practice teams and provided longitudinal primary care to patients were eligible to participate in this study. PCPs who solely provided specialty care, for example HIV services or urgent care, were excluded. Practice teams that consisted of several physicians (+/− trainees), one nurse, nurse practitioners, medical assistants and behaviorists, were randomized 1:1 to one of two arms with a random number generator: access to an electronic CKD registry or a usual care registry for 12 months. This randomization scheme minimized contamination by medical assistants, who work with several providers within a given practice team, but led to differences in the number of PCPs randomized to each arm, due to different practice team sizes.
Approval to conduct this study was granted by the Human Research Protection Program & Institutional Review Board at the University of California, San Francisco, with waived patient consent. Medical directors of the two participating clinics provided verbal consent to participate in this study; health care providers in the clinics implied consent if they used the CKD registry. Healthcare teams randomized to intervention had the option to not use the information provided by the CKD registry to manage their patients. The trial was not blinded to participating teams and providers, though the analytic team was blinded to group assignment.
Intervention
The CKD registry was designed to alert practice teams of a patient’s CKD-relevant information and enhance guideline concordant care delivery for patients with CKD. The CKD registry defined patients as having CKD if they had two values of dipstick albuminuria >1+ or albuminuria > 30mg/g or two values of race-concordant eGFR 15-59 ml/min/1.37m2 calculated by the Modification of Diet in Renal Disease (MDRD) Study equation (standard of care in the health system), separated by at least three months. It excluded individuals with eGFR <15 ml/min/1.37m2 and patients with ESRD. The CKD registry provided primary care practice teams with point-of-care data about patient-specific CKD status (eGFR, CKD on problem list), recent ambulatory clinic BP readings, status of ACEi/ARB prescription, and quantification of albuminuria (complete vs. not complete). The CKD registry also provided data about diabetes care, immunization status, and data pertinent to age appropriate cancer screening, to align with usual care. Medical assistants were encouraged to use these data to identify patients with CKD who needed albuminuria quantification and all patients (including those with CKD) who were due for cancer screening or immunizations. Point-of-care decision support embedded within the CKD registry reminded PCPs about guideline concordant care for individuals with non-dialysis-requiring CKD: target BP <140/90 mmHg, prescription of ACEi/ARB, avoidance of non-steroidal anti-inflammatory medications, and prescription of statin medications (Figure S1). Quarterly feedback to practice teams and individual PCPs identified patients with CKD with BP >140/90 mmHg, those not prescribed an ACEi/ARB, and those with persistent severely increased albuminuria for panel management, to reach patients who did not regularly visit their PCP and would thus not benefit from the “in-reach” component of the CKD registry. A document with clinical guidance accompanied each quarterly report (Item S1). No additional resources were provided to the teams randomized to the intervention arm of this study.
Usual care consisted of an electronic registry that was in use before trial implementation. It provided practice teams with point-of-care data about diabetes care, age-appropriate cancer screening and immunizations, but no CKD-related data. Medical assistants were encouraged to use the usual care registry to identify patients who were due for cancer screening or immunizations. Quarterly feedback was not provided for practice teams randomized to receive usual care.
Outcomes
All outcome data were captured from the electronic health record. The primary outcome was change in ambulatory clinic systolic BP from baseline to 12 months. Medical assistants use standard oscillometric devices to check BP in all ambulatory clinics (including primary and specialty care) with a standardized protocol. If the first BP is elevated, a second BP is obtained. While both BP measures are included in the medical record, only the second BP at each ambulatory clinic visit was used in this analysis. Secondary outcomes included changes in: proportion of patients with BP control defined by BP <140/<90 mmHg, proportion of patients whose albuminuria was quantified among those had not received quantification at trial initiation, albuminuria severity, and proportion of patients prescribed an ACEi/ARB or had a documented reason for no prescription (i.e., allergy, prior development of hyperkalemia, or acute kidney injury). Change in eGFR was not a pre-specified outcome, however it was included in a post-hoc analysis.
Covariates
Primary care provider status (trainee, attending provider, nurse practitioner), number of clinical sessions per clinic per week, and years of experience were self-reported prior to trial initiation, regardless of whether they cared for patients with CKD. Patient demographic data (age, gender, race/ethnicity, language preference, insurance status) and laboratory data (serum creatinine, glycosylated hemoglobin) were obtained from the electronic health record.
Study Power
Prior data from the health care network suggested a mean systolic BP of 151 mmHg for eligible patients, with virtually no between-provider variance over one year (intraclass coefficient=0.0008). We therefore used simple t-test calculations to conduct our power analyses. Using a 0.05 level of significance and a power of 0.8, we needed at least 200 patients (100 per arm) to detect a 6-point difference in the 12-month change in systolic BP between study arms, assuming a standard deviation of systolic BP change of 15 mmHg. This magnitude of BP change has been associated with substantial decreases in cardiovascular health at the population level.21
Statistical analyses
We used multi-level linear and logistic mixed models to assess the impact of the CKD registry (versus usual care registry) on change in systolic BP, change in proportion of patients with BP control, change in albuminuria over time, albuminuria quantification at least once among patients that were missing it at baseline, and use of ACEi/ARB. In all of these models, time was treated as a categorical fixed effect, with the primary test being the interaction of time by intervention arm. Models were adjusted for patient age, gender, race/ethnicity, baseline CKD stage, clinic, and participation in the nested Kidney Awareness Registry and Education (KARE) trial (n=137) that examined the synergistic and individual impact of provider access to a CKD registry and patient participation in a self-management support program on blood pressure ascertained at study visits.20 Models included random effects for primary care team and PCP and random slopes and intercepts within patients. Random effects that were not statistically significant were dropped from the analysis.
For each outcome, the latest data from each 3-month interval were used in the analysis. Between 78-80% of patients had data for every quarter they were enrolled in the study. We could not ascertain medication data from the newly implemented electronic medical record at trial initiation; these data become available afterwards. Thus, the outcome of ACEi/ARB was defined by an active prescription > 50% of the time after randomization. ACEi/ARB prescription data was validated against PCP intent using the primary care progress note as the gold standard for a sample of patients from the academic clinic. In a post-hoc analysis, mixed models were used to examine change in eGFR by trial arm by time; approximately 70% of patients had eGFR data for every quarter in which they were enrolled. To account for temporal trends, the effect of each registry was estimated at baseline and at months 3, 6, 9, and 12 (trial conclusion) using marginal effects estimation22 in STATA (Statacorp, College Station, Texas, USA). This analysis holds other covariates (age, gender, race/ethnicity, baseline CKD stage, clinic, and participation in nested KARE trial) fixed at their observed values while varying time and intervention. This was accomplished using the fitted model to calculate the predicted outcome for each quarter and arm, as if the patient were in each of the intervention arms at each time.
Interaction analyses were performed to examine the impact of the CKD registry in two subgroups of patients determined a priori – those with baseline moderate CKD (CKD stage 3 & 4) and those with uncontrolled BP (>140/>90 mmHg) at baseline. A post-hoc analysis was performed to see whether the impact of the registry for each outcome differed by PCP level of training (attending provider, trainee physician and nurse practitioner.
Results
Baseline characteristics
The patient population (n=746) had a mean age of 56.7 +/− 12.1 (SD) years, was 53% female and was racially/ethnically diverse (8% non-Hispanic White, 35.7% Black, 24.5% Hispanic, 24.4% Asian). Nearly 30% of patients with CKD had a non-English language preference and all were publically insured or uninsured. Of the patient population, 41.6% had CKD stage 1 or 2; 38.6% had stage 3a and 15% had CKD stage 3b; 4.8% of the patient population had CKD stage 4. Overall, 38% of the study population had albuminuria quantified at baseline, with an average albumin-creatinine ratio of 421 mg/g. Nearly 20% of patients had a glycosylated hemoglobin >6.5% and 36% of patients had uncontrolled BP, with average baseline systolic and diastolic BPs of 133.6 mmHg and 79.6 mmHg, respectively. Characteristics of patients whose providers were randomized to the CKD registry were similar to those whose providers were randomized to the usual care registry, except with regard to age, race/ethnicity and language preference (Table 1).
Table 1.
Baseline characteristics of patients whose provider teams were randomized to the CKD registry or the usual care registry.
| Patient Characteristics | All (N =746( | Usual care Registry (n=461( | CKD Registry (n=285) | p - value |
|---|---|---|---|---|
| Age | 56.7 (12.1) | 57.6 (11.6) | 55.3 (12.7) | 0.01 |
| Female Sex* | 377 (52.7) | 230 (51.2) | 147 (55.1) | 0.3 |
| Race/ethnicity | <0.01 | |||
| White | 56 (7.5) | 47 (10.2) | 9 (3.2) | |
| Black | 267 (35.8) | 154 (33.4) | 113 (39.7) | |
| Hispanic | 183 (24.5) | 107 (23.2) | 76 (26.7) | |
| Asian | 182 (24.4) | 126 (27.3) | 56 (19.7) | |
| Other | 58 (7.8) | 27 (5.8) | 31 (10.9) | |
| Non-English language** | 165 (27.8) | 111 (31.2) | 54 (22.8) | 0.03 |
| Health insurance*** | 0.6 | |||
| None | 142 (23.5) | 90 (25.2) | 52 (21.1) | |
| Medi-Cal | 291 (48.3) | 174 (48.7) | 117 (47.6) | |
| Medi-Care | 128 (21.2) | 70 (19.6) | 58 (23.6) | |
| Other | 42 (7.0) | 23 (6.4) | 19 (7.7) | |
| CKD | 0.2 | |||
| Stages 1-2 | 310 (41.6) | 182 (39.5) | 128 (44.9) | |
| Stage 3a | 288 (38.6) | 181 (39.2) | 107 (37.5) | |
| Stage 3b | 112 (15.0) | 70 (15.2) | 42 (14.7) | |
| Stage 4 | 36 (4.8) | 28 (6.1) | 8 (2.8) | |
| eGFR, mL/min/1.73 m2 | 65.6 (30.5) | 64.7 (30.4) | 67.9 (30.8) | 0.4 |
| Albuminuria quantification**** | 284 (38.0) | 170 (36.9) | 114 (40.0) | 0.4 |
| Albuminuria at first quantification, mg/g | 421 (951) | 440 (1087) | 394 (703) | 0.6 |
| HbA1c > 6.5% | 143 (19.1) | 89 (19.3) | 54 (19.0) | 0.9 |
| BP > 140/90 mmHg, | 268 (35.9) | 162 (35.1) | 106 (37.2) | 0.6 |
| Systolic BP (mmHg) | 133.6 (20.9) | 133.9 (20.3) | 133.2 (21.9) | 0.7 |
| Diastolic BP (mmHg) | 79.6 (12.7) | 79.6 (11.6) | 79.6 (14.3) | 0.9 |
Values for continuous data given as mean +/− SD; for categorical data as count (column percentage).
denominator is 715
denominator is 593
denominator is 603
albuminuria denominator, n=646
CKD = Chronic Kidney Disease; HbA1c = Glycosylated Hemoglobin; BP = Blood Pressure
Among 96 PCPs who cared for patients with CKD at trial initiation, 79 (82%) self-reported demographic data. Among those providers, 91% practiced at the academic training clinic. Nearly 27% of providers were attending physicians, 17% were nurse practitioners and 56% were resident trainees, without any difference in distribution by study arm (p=0.2). Many trainees practiced in one large team that was randomized to the usual care registry. Providers randomized to the CKD registry arm appeared to have greater years of experience compared to those randomized to usual care registry, but this was not statistically significant (p=0.1) (Table 2).
Table 2.
Baseline characteristics of providers randomized to the CKD registry or the usual care registry.
| Provider characteristics | All (n=79) | Usual Care Registry | CKD Registry | p - value |
|---|---|---|---|---|
| n=49 | n=30 | |||
| Clinic type | 0.6 | |||
| Academic training clinic | 72 (91) | 44 (90) | 28 (93) | |
| Community clinic | 7 (9) | 5 (10) | 2 (7) | |
| Provider type | 0.2 | |||
| Trainee | 44 (56) | 31 (65) | 13 (43) | |
| Attending provider | 21 (27) | 9 (19) | 12 (40) | |
| Nurse practitioner | 13 (17) | 8 (17) | 6 (20) | |
| Clinical half-days per week | 4.1 (2) | 4.3 (2) | 3.7 (2) | 0.2 |
| Years of experience after degree | 0.1 | |||
| < 5 | 44 (56) | 31 (63) | 13 (43) | |
| 5–15 | 19 (24) | 12 (25) | 7 (23) | |
| >16 | 16 (20) | 6 (12) | 10 (33) |
Values for categorical data given as count (column percentage), for continuous data as mean +/− SD. CKD = Chronic Kidney Disease; SD = standard deviation
Primary outcome
There were 285 patients among 30 PCPs randomized to the CKD registry and 461 patients among 49 PCPs randomized to usual care registry. Randomization to the CKD registry vs usual care registry was not associated with a change in systolic BP over time (ptime x registry= 0.9). Among patients randomized to the CKD registry, marginal estimates of systolic BP at trial initiation and conclusion were 133 mmHg and 132.8 mmHg, respectively. Among patients randomized to the usual care registry, marginal estimates of systolic BP at trial initiation and conclusion were 133.7 mmHg and 132.7 mmHg, respectively (Figure 1). Interaction analyses demonstrated that results did not differ by PCP level of training (ptime x registry x PCP training=0.8), baseline CKD stage (ptime x registry x CKD stage =0.8) or baseline controlled vs. uncontrolled BP (ptime x registry x baseline BP = 0.7). Marginal estimates of systolic BP for pre-determined subgroups are depicted in Figure 1.
Figure 1. Marginal estimates and 95% confidence intervals of systolic BP over time, by registry arm.
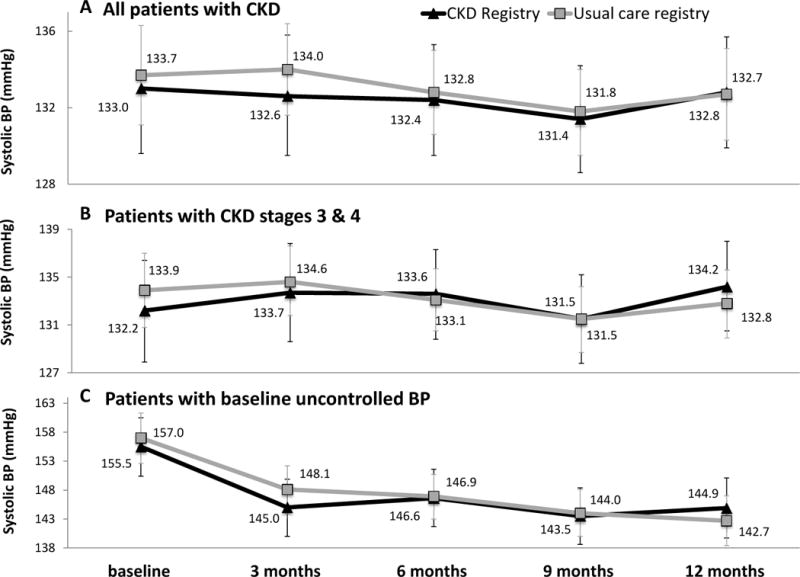
(A) all CKD patients (n=575;1230 observations; average 3.7 observations per patient), (B) patients with CKD stage 3 & 4 (n=397; 1534 observations; average 3.9 observations per patient), (C) patients with uncontrolled BP at baseline (n=207; 750 observations; average 3.6 observations per patient). Estimates are adjusted for age, gender, race/ethnicity, clinic, and participation in a health coaching study, as well as primary care team, primary care provider and patient clustering.
Secondary outcomes
Randomization to the CKD registry was not associated with a change in the proportion of patients with controlled BP <140/90 mmHg (ptime × registry=0.7). Results did not differ by PCP type (ptime × registry × PCP type = 0.8), CKD stage (ptime × registry × CKD stage =0.9) or baseline BP control (ptime × registry × baseline BP =0.9) (Figure 2). Randomization to the CKD registry was also not associated with change in eGFR overall (ptime × registry=0.2); results did not differ by baseline BP (ptime × registry × baseline BP=0.2) or CKD stage (ptime × registry × CKD stage =0.8). Change in albuminuria severity was also not different by registry group among the overall study population (ptime × registry=0.1), nor by PCP type (ptime × registry × PCP type = 0.7), baseline CKD severity (ptime × registry × CKD stage =0.7) or by baseline BP control (Ptime × registry × baseline BP=0.3) (Figure 3).
Figure 2. Marginal estimates and 95% confidence intervals of proportion of patients with BP < 140/90 mmHg over time by registry arm.
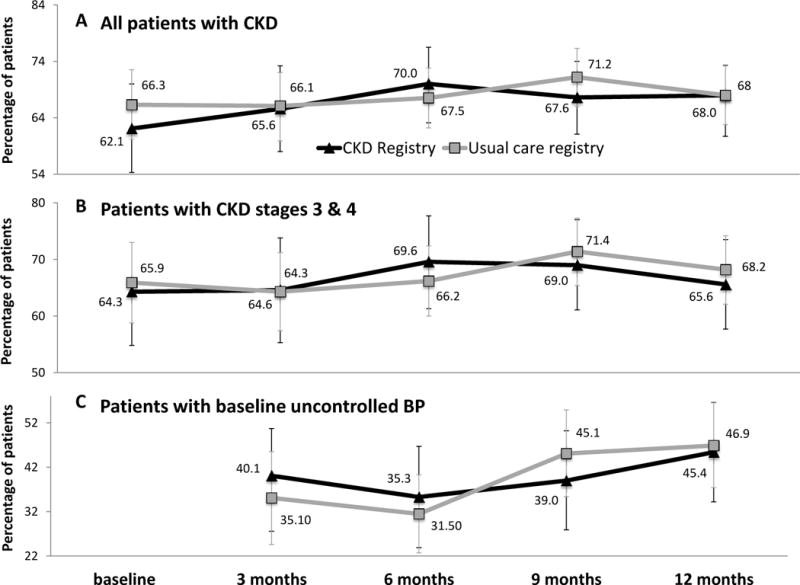
(A) all CKD patients (n=575; 1230 observations; average 3.7 observations per patient), (B) patients with CKD stage 3 & 4 (n=403; 1544 observations; average 3.8 observations per patient), (C) patients with uncontrolled BP at baseline (n=207; 630 observations; average 3.0 observations per patient). Estimates are adjusted for age, gender, race/ethnicity, clinic, and participation in a health coaching study, as well as primary care team, primary care provider and patient clustering.
Figure 3. Marginal estimates and 95% confidence intervals of change in albuminuria over time by registry arm.
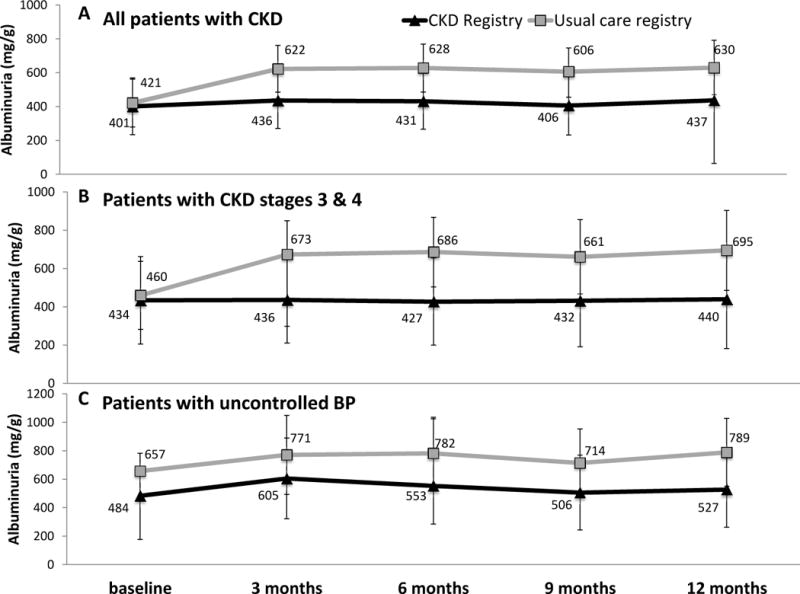
(A) all CKD patients (n=487; 1736 observations; average 3.6 observations per patient), (B) patients with CKD stage 3 & 4 (n=347; 1272 observations; average 3.7 observations per patient), (C) patients with uncontrolled BP at baseline (n=175; 622 observations; average 3.6 observations per patient). Estimates are adjusted for age, gender, race/ethnicity, clinic, and participation in a health coaching study, as well as primary care team, primary care provider and patient clustering.
Randomization to the CKD registry was associated with greater odds of ACEi/ARB prescription compared to the usual care registry among all CKD patients (adjusted OR, 2.25; 95% CI, 1.45-3.49). Marginal estimates of patients with an active ACEI/ARB prescription over 50% of the time was 82.2% in the registry group compared to 68.2% in the usual care group. Interaction analyses demonstrated that results differed by baseline CKD stage (pregistry × CKD stage =0.002) and by baseline BP (Pregistry × baseline BP =0.0004). Adjusted odds ratios for pre-determined subgroups are depicted in Figure 4a. Impact of the registry on ACEi/ARB prescription also differed by PCP type (pregistry × PCP training=0.002), with greatest impact among trainees. Odds of albuminuria quantification were higher among CKD patients whose providers were randomized to the CKD registry versus those randomized to the usual care registry (adjusted OR, 2.44; 95% CI, 1.38-4.29). Odds were higher among individuals with moderate (vs. mild) CKD (pregistry × CKD stage =0.01) and among those with uncontrolled (vs. controlled) BP at baseline (pregistry × baseline BP =0.01). Adjusted odds ratios for pre-determined subgroups are depicted in Figure 4b. Impact of the registry on quantification of albuminuria also differed by PCP training (pregistry × PCP training = 0.01), with odds of albuminuria quantification highest among attending providers and lowest among trainees.
Figure 4. Adjusted odds and 95% confidence intervals of ACEi/ARB prescription and albuminuria quantification among patients randomized to the CKD registry vs. usual care registry.
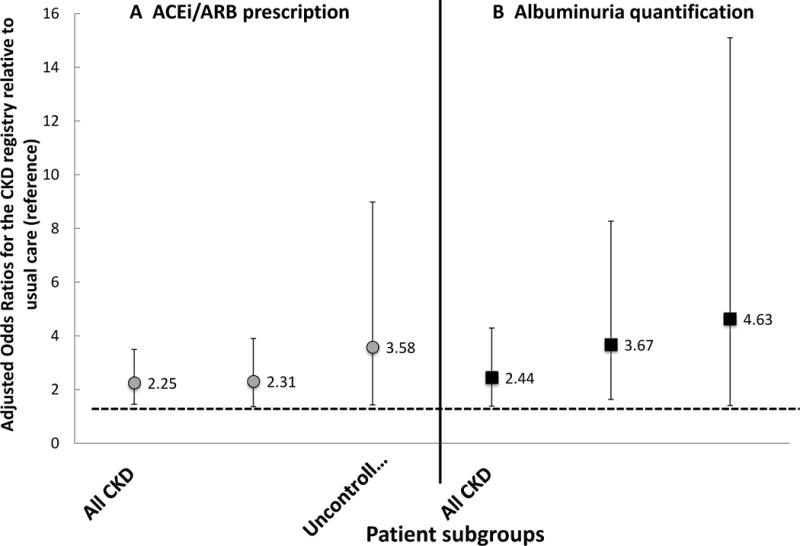
(A) ACEi/ARB prescription; (B) albuminuria quantification. Results are displayed for three patient groups: all patients with CKD (n=575), patients with CKD stage 3 & 4 (n=403), and patients with uncontrolled BP at baseline (n=207). Models are adjusted for age, gender, race/ethnicity, clinic, participation in a health coaching study, as well as primary care team, primary care provider and patient clustering. *denominator is 715
Discussion
In our study, a safety-net primary care CKD registry directed at the entire primary care team enhanced the delivery of guideline concordant CKD care, including ACEi/ARB use and albuminuria quantification, but did not improve BP control or impact change in eGFR after 12 months. This success builds upon prior interventions in the United States directed towards PCPs that have moderately improved CKD management. Studies have previously demonstrated that automated laboratory eGFR reporting has led to greater identification of CKD among PCPs and higher rates of nephrology referrals.23 Registries with electronic decision support for PCPs have been shown to increase serum laboratory testing for patients with kidney disease.24
The premise of the Chronic Care Model is that well informed patients and adequately prepared practice teams interact productively in a way that improves outcomes.25 The model supplies a framework for delivering high-quality chronic disease care that relies upon a team-based primary care approach. The CKD registry we studied aligns with the Chronic Care Model and differs from prior kidney registry efforts by empowering non-physician health care providers to enhance care delivery alongside PCPs during clinic visits with decision support and to perform outreach and panel management outside of clinic visits. This team-based population health approach has been successful at improving process outcomes among patients with multi-morbidity.26 This study extends this success to individuals with kidney disease.
ACEi/ARB prescription among patients with kidney disease is an essential component of CKD management. A recent meta-analysis of 119 randomized controlled trials examining the effects of ACEi or ARB therapy on health outcomes among 64,768 patients with all stages of CKD, demonstrated that ACEi or ARB reduced the odds of kidney failure by 30%-39% compared to placebo and by 25-35% compared to other anti-hypertensive medications. Their use was also associated with an 18-24% reduced odds of cardiovascular events compared to placebo, though these results were not necessarily independent of BP control or albuminuria level.27 From the public health perspective, data from the last decade of NHANES (National Health and Nutrition Examination Surveys) suggest that national prevalence of CKD has stabilized overall, including among those with CKD stages 3 & 4.28 These trends have been observed while the application of known CKD interventions, including BP control,29 glycemic control,30 and use of ACEi/ARB31 have increased. While the cross-sectional nature of NHANES data can only infer that application of CKD interventions have contributed to stabilization of CKD prevalence, these data underscore the importance of our registry’s impact on increased ACEi/ARB prescription among patients with CKD.
Importantly, randomization to the CKD registry was associated with greater quantification of albuminuria by health care teams. Presence of albuminuria is a strong risk factor for CKD progression,32 acute kidney injury33 and cardiovascular events,34 and contributes substantially to risk prediction models for the development of ESRD.35 Decreases in albuminuria over a two-year period have been associated with lower risk of incident ESRD and death among CKD patients with and without diabetes.36 Measuring albuminuria among those with low eGFR and pharmacologically minimizing that albuminuria is an essential component of optimal CKD care.37 Currently, albuminuria is grossly under-ascertained among those at risk for and with CKD, particularly among individuals without diabetes.38 This gap in evidence-based CKD care highlights the need for system-based interventions such as a team-based CKD registry.
Randomization to the CKD registry was not associated with improvement in systolic BP or greater BP control. These null results could be attributed to poor fidelity of the intervention (a common challenge for pragmatic clinical trials39), poor medication adherence among patients despite an increase in ACEi/ARB prescription in the intervention arm, or inadequate medication dosing of ACEi/ARB. It could also be due to fairly well-controlled BP at baseline with an average systolic BP of 134 mmHg. Results may also suggest that implementation of single elements of the Chronic Care Model (e.g., access to resources, provision of patient self-management support, redesign of delivery systems, team-based decision support) within the context of a robust primary care team, may not be sufficient to see improvements in clinical outcomes. Interventions that enhance clinical outcomes, such as BP control or change in eGFR, may need to incorporate several elements of the Chronic Care Model. This is consistent with data from Kaiser Permanente Northern California, an integrated health system that previously demonstrated marked improvements in BP control after implementing a large-scale hypertension program40 that consisted of a hypertension registry with “in-reach” and “outreach” components, as well delivery system re-design with medical assistant visits for BP measurements, and increased provider access to BP treatment algorithms. Similar data for the management of other chronic diseases have emerged from the North Carolina Improving Performance in Practice program, which found better cholesterol levels in patients with diabetes were associated in a graded fashion with the degree to which clinical practices adopted components of multi-level interventions.41 Improving BP among patients with CKD in safety-net settings may thus require interventions that synergistically target health systems and patients, as patients face additional challenges to optimal health care, including low health literacy and food insecurity.42
This study’s results must be taken in context of its limitations. All patients with two abnormal eGFR or albuminuria separated by three months were considered to have CKD. However, the registry could not exclude individuals with acute kidney injury, nor ascertain complications of increased ACEi/ARB prescription such as hyperkalemia, though the prevalence of these complications would not likely differ by trial arm. Also, this pragmatic trial was subject to the information technology constraints of the reporting tools inherent to the electronic health record used in the safety-net health delivery system. As such, medication data were not available at baseline, and only ACEi/ARB prescription became available shortly after study implementation. The cluster randomized approach to randomization led to differing numbers of PCPs and patients per arm, with more trainees randomized to the usual care arm. While this difference was not anticipated, the number of trainees was not statistically significantly different by study arm. Reassuringly, prevalence of uncontrolled diabetes, uncontrolled blood pressure, and severe CKD among patients were also not different by study arm. Poor fidelity of the intervention or contamination across study arms despite team (versus provider) randomization could have contributed to smaller differences in BP than anticipated between the study arms. These challenges, common to pragmatic trials, should be considered alongside the value afforded by this type of study design, including the participation of a diverse patient population, the direct application of the intervention to patient care, and external validity.39
In conclusion, a primary care registry directed towards the entire healthcare team with point-of-care alerts and outreach lists can improve essential CKD processes of care. In the context of health care reform and the evolution of patient-centered medical homes with team-based care chronic care delivery, this has wide public health implications. While this study needs to be replicated in a larger delivery system with long-term outcomes, adoption of team-based CKD registries may represent an important step of translating evidence into practice for CKD management, particularly in health delivery systems that care for patient populations with a high burden of kidney disease.
Supplementary Material
Figure S1. Example of point-of-care decision support for a patient with CKD.
Item S1. Example of a quarterly report provided to a health care team.
Acknowledgments
We thank the providers and patients of the San Francisco Health Network for their participation in this study.
Support: This work was supported by K23DK094850, R01DK104130 and R34DK093992 all from the National Diabetes and Digestive and Kidney Diseases. CYH is additionally supported by K24DK92291. DS is additionally supported by P60MD006902 from the National Institute of Minority Health and Health Disparities and P30DK092924 from the National Institute of Diabetes and Digestive and Kidney Diseases. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary Material
Supplementary Material Descriptive Text for Online Delivery
Supplementary Figure S1 (PDF). Example of point-of-care decision support for a patient with CKD.
Supplementary Item S1 (PDF). Example of a quarterly report provided to a health care team.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions: Research idea and study design: DST, NRP; data acquisition: DST, AV; data analysis/interpretation: DST, AV, CYH, DS, MH, CEM, NRP; statistical analysis: DST, CEM; supervision or mentorship: DS, CYH, NRP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3 suppl 1):A7–A8. doi: 10.1053/j.ajkd.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett BJ. Applying multiple interventions in chronic kidney disease. Semin Dial. 2003;16(2):157–164. doi: 10.1046/j.1525-139x.2003.16032.x. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142(5):342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJ, Steinberg EP. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12(8):1713–1720. doi: 10.1681/ASN.V1281713. [DOI] [PubMed] [Google Scholar]
- 7.Allen AS, Forman JP, Orav EJ, Bates DW, Denker BM, Sequist TD. Primary care management of chronic kidney disease. J Gen Intern Med. 2011;26(4):386–392. doi: 10.1007/s11606-010-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuot DS, Plantinga LC, Hsu CY, Powe NR. Is awareness of chronic kidney disease associated with evidence-based guideline-concordant outcomes? Am J Nephrol. 2012;35(2):191–197. doi: 10.1159/000335935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17(3):225–236. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuot DS, Plantinga LC, Hsu CY, et al. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6(8):1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahir MA, Dmitrieva O, de Lusignan S, et al. Confidence and quality in managing CKD compared with other cardiovascular diseases and diabetes mellitus: a linked study of questionnaire and routine primary care data. BMC Fam Pract. 2011;12:83. doi: 10.1186/1471-2296-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon J, Powers M. Chronic Disease Registries: A product review. California HealthCare Foundation; 2004. [Google Scholar]
- 13.Weingarten SR, Henning JM, Badamgarav E, et al. Interventions used in disease management programmes for patients with chronic illness-which ones work? Meta-analysis of published reports. BMJ. 2002;325(7370):925. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgiou A, Burns J, McKenzie S, Penn D, Flack J, Harris MF. Monitoring change in diabetes care using diabetes registers–experience from divisions of general practice. Aust Fam Physician. 2006;35(1–2):77–80. [PubMed] [Google Scholar]
- 15.Gheorghiade M, Albert NM, Curtis AB, et al. Medication dosing in outpatients with heart failure after implementation of a practice-based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail. 2012;18(1):9–17. doi: 10.1111/j.1751-7133.2011.00250.x. [DOI] [PubMed] [Google Scholar]
- 16.Drawz PE, Miller RT, Singh S, Watts B, Kern E. Impact of a chronic kidney disease registry and provider education on guideline adherence–a cluster randomized controlled trial. BMC Med Inform Decis Mak. 2012;12:62. doi: 10.1186/1472-6947-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Kader K, Fischer GS, Li J, Moore CG, Hess R, Unruh ML. Automated clinical reminders for primary care providers in the care of CKD: a small cluster-randomized controlled trial. Am J Kidney Dis. 2011;58(6):894–902. doi: 10.1053/j.ajkd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones C, Roderick P, Harris S, Rogerson M. An evaluation of a shared primary and secondary care nephrology service for managing patients with moderate to advanced CKD. Am J Kidney Dis. 2006;47(1):103–114. doi: 10.1053/j.ajkd.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 19.McBride D, Dohan D, Handley MA, Powe NR, Tuot DS. Developing a CKD registry in primary care: provider attitudes and input. Am J Kidney Dis. 2014;63(4):577–583. doi: 10.1053/j.ajkd.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuot DS, Velasquez A, McCulloch CE, et al. The Kidney Awareness Registry and Education (KARE) study: protocol of a randomized controlled trial to enhance provider and patient engagement with chronic kidney disease. BMC Nephrol. 2015;16:166. doi: 10.1186/s12882-015-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA. 2002;288(15):1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 22.Vittinghoff E. Regression methods in biostatistics : linear, logistic, survival, and repeated measures models. 2nd. New York: Springer; 2012. [Google Scholar]
- 23.Kagoma YK, Weir MA, Iansavichus AV, et al. Impact of estimated GFR reporting on patients, clinicians, and health-care systems: a systematic review. Am J Kidney Dis. 2011;57(4):592–601. doi: 10.1053/j.ajkd.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 24.Galbraith L, Jacobs C, Hemmelgarn BR, Donald M, Manns B, Jun M. Chronic disease management interventions for people with chronic kidney disease in primary care: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017 doi: 10.1093/ndt/gfw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving Chronic Illness Care: Translating Evidence Into Action. Health Affairs. 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 26.Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. VA Evidence-based Synthesis Program Evidence Briefs. Washington (DC): 2011. Evidence Brief: Effectiveness of Intensive Primary Care Programs. [PubMed] [Google Scholar]
- 27.Xie X, Liu Y, Perkovic V, et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am J Kidney Dis. 2016;67(5):728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Murphy D, McCulloch CE, Lin F, et al. Trends in Prevalence of Chronic Kidney Disease in the United States. Ann Intern Med. 2016;165(7):473–481. doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension. 2015;65(1):54–61. doi: 10.1161/HYPERTENSIONAHA.114.04012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med. 2014;160(8):517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health And Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126(17):2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 32.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grams ME, Sang Y, Ballew SH, et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Age, Race, and Sex With Acute Kidney Injury. Am J Kidney Dis. 2015;66(4):591–601. doi: 10.1053/j.ajkd.2015.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 36.Carrero JJ, Grams ME, Sang Y, et al. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 2017;91(1):244–251. doi: 10.1016/j.kint.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 38.Manns L, Scott-Douglas N, Tonelli M, et al. A Population-Based Analysis of Quality Indicators in CKD. Clin J Am Soc Nephrol. 2017;12(5):727–733. doi: 10.2215/CJN.08720816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Boer IH, Kovesdy CP, Navaneethan SD, et al. Pragmatic Clinical Trials in CKD: Opportunities and Challenges. J Am Soc Nephrol. 2016;27(10):2948–2954. doi: 10.1681/ASN.2015111264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310(7):699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halladay JR, DeWalt DA, Wise A, et al. More extensive implementation of the chronic care model is associated with better lipid control in diabetes. J Am Board Fam Med. 2014;27(1):34–41. doi: 10.3122/jabfm.2014.01.130070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuot DS, Grubbs V. Chronic kidney disease care in the US safety net. Adv Chronic Kidney Dis. 2015;22(1):66–73. doi: 10.1053/j.ackd.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Example of point-of-care decision support for a patient with CKD.
Item S1. Example of a quarterly report provided to a health care team.


