Abstract
Inspired by cisplatin’s deactivation by glutathione (GSH) in cancer, a GSH responsive nanogel loaded with doxorubicin (Dox) was prepared using hyaluronan as a matrix and cisplatin as a crosslinker. The elevated GSH depletes the cisplatin crosslinker in the nanogel, enhances Dox release and boosts cytotoxicity, thus providing a new GSH responsive fashion to reverse cisplatin resistance.
Cancer lesion has a significantly higher level of glutathione (GSH) in comparison to normal tissue.1–3 The high GSH level in tumor could result from a combination of active secreting of GSH by tumor stromal cells and effective cytosolic accumulation in tumor cells.4–6 In fact, the higher GSH level was commonly found in cisplatin resistant cancers than in the non-resistant ones.7, 8 GSH could influence drug resistance either through cellular signaling or direct drug binding.9 It was suggested that the platinum of cisplatin has a strong tendency towards binding with thiol-containing species, including GSH in cells, leading to the deactivation of cisplatin.10 Along with the acceleration of the efflux event, the intracellular concentration of the cisplatin-GSH adduct was further reduced, which collaboratively contributed to the drug’s resistance.11, 12
To overcome the cisplatin detoxification rendered by GSH, several nanoparticle strategies were proposed, such as restricting the GSH-cisplatin contact by shielding the cisplatin core with a hydrophilic shell, or reducing the GSH-cisplatin interaction by co-delivering a glutathione S-transferases inhibitor.13, 14 However, those strategies did not fully solve the problem. Once cisplatin is released from the nanocarrier, its exposure to GSH remains unavoidable. Conversely, GSH responsive delivery strategies were also proposed to improve drug delivery. Such delivery systems take advantage of the GSH’s reduction capability to release the conjugated or encapsulated anticancer drugs.15, 16
Besides its typical function as a chemotherapeutic drug, cisplatin is used as a cross-linker in constructing different nanoparticles.17–19 We have previously demonstrated that cisplatin was able to cross-link hyaluronan (HA), a polysaccharide presented in extracellular space, to co-deliver a series of drugs for anticancer application.19, 20 HA, a natural polymer, has been widely used as a surgical filler due to its biodegradability and minimal immunogenicity.21 In addition, HA is a ligand for CD44 receptor highly expressed on many cancer cells, thus conferring HA-based nanocarriers targeting capability towards CD44 positive tumor cells.21, 22 In view of the elevated GSH level in cisplatin resistant cells, we hypothesize that cisplatin could be used as a GSH-responsive cross-linker to combat its own drug resistance by including a second drug. Since the cisplatin cross-linker is essential to maintain the compactness of the HA nanogel, a GSH assisted depletion could accelerate the leakage/release of the co-loaded drug and lead to an enhanced cell killing.
Doxorubicin (Dox) was selected as a representative drug to demonstrate the concept, because its fluorescence property would ease nanogel characterization and subsequent cellular imaging. Dox is widely used as a first-line of treatment for multiple cancer types including breast, lung, etc.23 To prepare the cisplatin cross-linked HA nanogel co-loaded with Dox (HA/Cis/Dox), an aqueous solution of HA, cisplatin and doxorubicin was heated at 90 °C, cooled on ice and purified by dialysis in water. In this system, cisplatin acts as a crosslinker to induce the nanogel formation. However, the HA/Cis only nanogel, which has no Dox or other hydrophobic components, forms loosely packed structure with high polydispersity.20, 24 When Dox was introduced, the acquired HA/Cis/Dox nanogel was 45 ± 9.9 nm and seen as even spheres under the transmission electron microscopy (TEM) (Fig. 1a). Further dynamic light scattering (DLS) analysis suggested that the nanogel was negatively charged with a hydrodynamic diameter of 195.8 ± 10.5 nm in water (Table S1, ESI†), that is around 4 folds larger than it determined by TEM. This swelling fully supports the hydrogel nature of HA/Cis/Dox by absorbing water in solution.25 The incorporation efficiency of cisplatin and Dox was around 38.9% and 92.1%, corresponding to a final molar ratio of 1.8 to 1 in the nanogel (Table S1, ESI†). The Dox was aggregated in the nanogel as indicated by the red shifted absorption spectra and the quenched fluorescence in comparison to free Dox (Fig. 1b, c). The same changes to its optical properties was observed on our prior studies of similar nanogels.19, 24 Both cisplatin and Dox was slowly released in PBS at 37 °C, however Dox release was much slower; only around 10% of Dox was released after 3 days of incubation (Fig. S1, ESI†). The HA/Cis/Dox nanogel only slightly swelled in 3 days, evidenced by DLS measurement (Fig. 1d). This was presumably due to cisplatin enforced tight compacting of Dox.
Fig. 1.
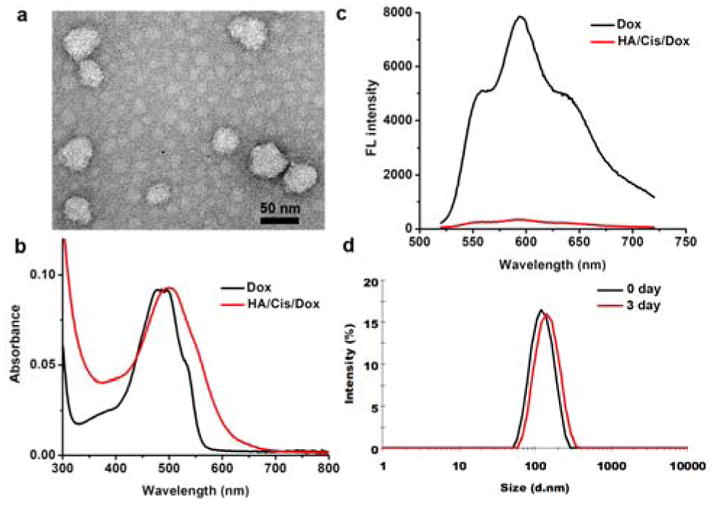
a) Morphology of HA/Cis/Dox nanogel. b) Absorption and c) fluorescence spectra of HA/Cis/Dox in comparison to free Dox. d) Size distribution of HA/Cis/Dox in PBS at 37 °C for 3 days determined by DLS.
To validate the GSH assisted depletion of cisplatin in the nanogel, the o-phenylenediamine (OPDA) method, which relies on the reaction between cisplatin and OPDA, was used (Fig. S2, ESI†).24, 26 The cisplatin-OPDA adducts give an absorbance peak at 705 nm, thus providing a facile and cost effective way to monitor the binding events of cisplatin. Incubating a cisplatin solution (10 μg/ml) with a GSH dose ranging from 0.005 to 5 mM, showed that the formation of cisplatin-OPDA adducts was gradually inhibited (Fig. S2c, ESI†), hinting an effective GSH binding to cisplatin. The same phenomenon was observed when incubating HA/Cis/Dox with 5 mM of GSH, a reported GSH concentration in tumor; the absorption peak corresponding to the cisplatin-OPDA adducts was completely eliminated (Fig. S2d, ESI†). The binding of platinum with a sulfur atom is known to be more efficient than that with either a nitrogen atom or carboxyl oxygen,27, 28 thus, the introduced GSH was able to inhibit the OPDA reaction and deplete cisplatin from nanogel.
The cisplatin depletion resulted in an accelerated release of Dox from the nanogel in a GSH dependent manner. As shown in Fig. 2a–b, 5 mM of GSH increased the release of Dox by 4 folds after 3 days of incubation. This provides an alternative GSH responsive strategy to the commonly used reducible connecting motifs such as disulfide bonding.29 Although the mean DLS sizes of nanogels didn’t change much in the GSH containing buffer, the increased polydispersion index (PDI) at 5 mM suggested a loosened structure (Fig. S3, ESI†) as seen in the TEM images (Fig. 2c). The ultrastructure observation by TEM verified the morphology change of HA/Cis/Dox in the presence of GSH (Fig. 2c). After 3 days of incubation, the nanogels partially collapsed and left no dense aggregation, which well corroborated with the increased Dox release in response to GSH.
Fig. 2.
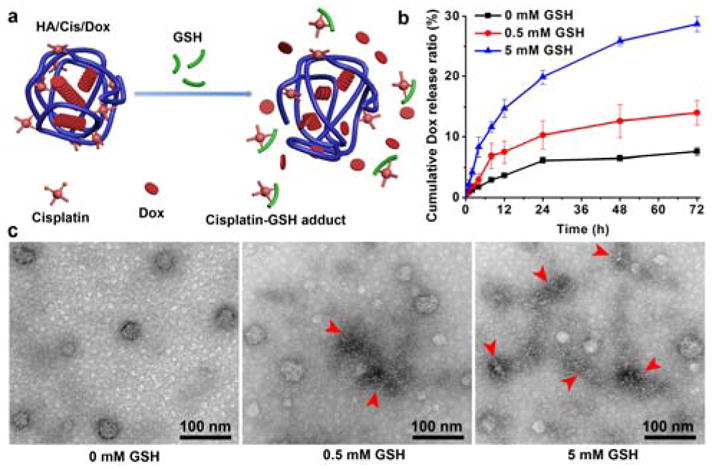
a) Schematic illustration of Cisplatin depletion by GSH in nangel. b–c) Dox release and morphology change of HA/Cis/Dox in presence of GSH, respectively. Arrowheads point the representative nanogels with altered morphology in GSH solution.
To verify the GSH responsive release, ovarian cancer A2780 cells and its cisplatin-resistant lineage A2780cis cells were selected for the study (Fig. S4a, ESI†). The initial dose escalation study indicated that A2780cis cells were about 10-fold less sensitive to cisplatin than its parent A2780 cells. The A2780cis cells were also found slightly resistant to doxorubicin, this probably due to its multi-drug resistance (Fig. S4b, ESI†). Studies have shown that the extracellular GSH provided by stromal cells is able to induce more GSH production in A2780cis cells, thus, promoting cisplatin resistance.4
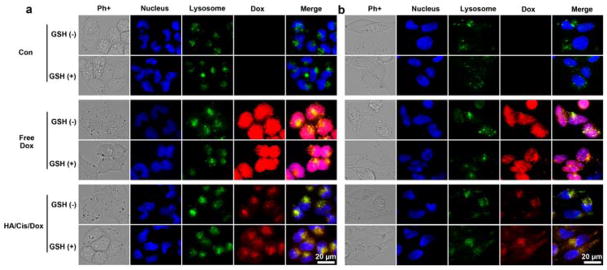
To imitate the tumor stromal microenvironment, the culture medium was supplemented with 1 mM of GSH.4, 5 After 8 hours of incubation with free Dox, Dox stained all nuclei in both A2780 and A2780cis cells regardless of the presence of GSH or not (Fig. 3). This was due to its free diffusion property and preferable binding with DNA. However, Dox encapsulated in the HA/Cis/Dox nanogel mostly resided within the lysosomes under normal culture conditions, which has a negligible GSH. Such lysosomal enrichment was often observed in nanoparticles, attributed to their sizes.19, 22, 30 Conversely, when GSH presented in the media, a high Dox signal was observed outside of the lysosome within the HA/Cis/Dox treated cells, while no clear difference was found in the free Dox treated cells. The increased Dox signals in cytoplasm indicated that, following the endocytosis of the nanogels, the encapsulated Dox was released by the intracellular GSH. This GSH dependent effect was confirmed by flow cytometry (FACS) (Fig. S5, ESI†). The right shifted peak indicated a higher Dox fluorescence intensity in the GSH treated cells, reflecting an increased Dox release post cisplatin depletion. More importantly, the Dox signal that appeared within the nucleus in the GSH-supplemented cells was significantly increased (Fig. 3).
Fig. 3.
Distribution of Dox in A2780 (a) and A2780cis (b) cells. Cells were treated as indicated for 8 hours with or without GSH (1mM). Dox concentration was set at 13.5 μM for free Dox and HA/Cis/Dox. The control experiments have no Dox.
To confirm the GSH responsive toxicity of the nanogel, both cell lines were incubated with free cisplatin, free Dox, free cisplatin plus Dox (Cis+Dox), or HA/Cis/Dox with or without GSH (1 mM) for 1 day, and then the MTS assay was performed to evaluate the cytotoxicity.
Generally, the presence of GSH reduced the cytotoxicity of cisplatin and Cis+Dox because of the GSH detoxification effect (Fig. 4). However, the GSH dramatically increased the cell killing effects of HA/Cis/Dox, which was even better than the free drug combination (Fig. 4). Apparently the A2780cis cells demonstrated resistance towards all treatments but the cisplatin tied HA/Cis/Dox nanogel. HA/Cis/Dox with GSH (1mM) was able to kill all cells, suggesting the potential of using HA/Cis/Dox to overcome cisplatin resistance. In the subsequent study, the [GSH] was reduced to 0.5 and 0.05 mM, a value that equivalent to those detected in fibroblast-conditioned medium.4, 31 The viability of A2780cis cells was then monitored 2 days after incubating with cisplatin and HA/Cis/Dox. Compared with free cisplatin, the 0.5 mM of GSH clearly induced an enhanced cytotoxicity for HA/Cis/Dox while 0.05 mM of GSH demonstrated a marginal enhancement only with cisplatin at 32 μM (Fig. S6, ESI†). Although the exact extracelluar concentration of GSH in tumor tissue is not known, our study demonstrated that the HA/Cis/Dox cytotoxicity could be enhanced by a broad range of [GSH].
Fig. 4.
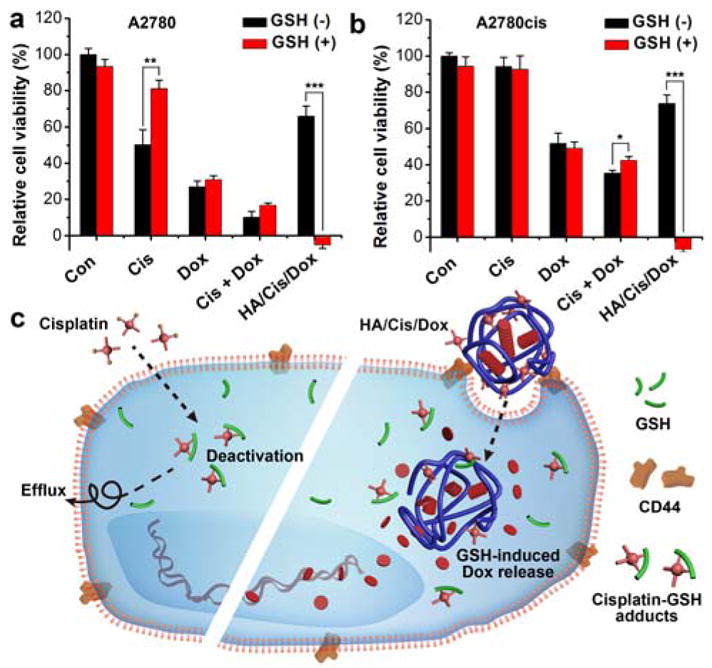
Cytotoxicity of HA/Cis/Dox on A2780 (a) and A2780cis (b) cells with or without GSH in medium (1 mM), respectively. Cells were treated for 1 day and the involved cisplatin and Dox were set at 24 μM and 13.5 μM, respectively. p < 0.05(*), p < 0.01(**) or p < 0.001(***) suggests significant difference determined by 2-sided Student’s t test. c) Plausible mechanism of HA/Cis/Dox’s GSH responsiveness in drug resistance.
It was noted that a higher intracellular Dox signal was observed in free Dox treated cells but its cytotoxicity was weaker than that exerted by HA/Cis/Dox in the presence of GSH (Fig. 3 and 4). This suggested other mechanisms provided by the HA/Cis/Dox nanogel, such as altered drug trafficking and depletion of GSH by cisplatin, might also contribute to this boost in cytotoxicity.32, 33 Future efforts will be needed to scrutinize the mechanism-of-action. Nevertheless, the clear GSH responsiveness and facile manipulation of this cisplatin cross-linked HA nanogel provided a promising strategy to fight cisplatin resistance.
Tumor associated GSH caused cisplatin detoxification is a serious issue with drug resistance. Cisplatin loses its toxicity to locally elevated GSH levels before reaching its intended target, the nucleus. Inspired by this unfavored reaction, a cisplatin crosslinked nanogel was developed to take advantage of this unusual environment. When cisplatin is depleted by GSH, the nanogel loses its integrity and releases the encapsulated drugs (Fig. 4c). This strategy relies on cisplatin deactivation, a process that is inevitable when cisplatin acts only as a drug. Given that the versatility of current cisplatin crosslinked nanogel to load different drugs, a flexible drug combination could be expected with a rational design and formulation optimization.
Supplementary Material
Acknowledgments
This work was supported in part by NIH GM094880.
Footnotes
Conflicts of interest
The authors declare no competing financial interest.
Notes and references
- 1.Estrela JM, Ortega A, Obrador E. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 2.Gamcsik MP, Kasibhatla MS, Teeter SD, Colvin OM. Biomarkers. 2012;17:671–691. doi: 10.3109/1354750X.2012.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB. Cancer Res. 2002;62:307–312. [PubMed] [Google Scholar]
- 4.Wang W, Kryczek I, Dostal L, Lin H, Tan L, Zhao L, Lu F, Wei S, Maj T, Peng D, He G, Vatan L, Szeliga W, Kuick R, Kotarski J, Tarkowski R, Dou Y, Rattan R, Munkarah A, Liu JR, Zou W. Cell. 2016;165:1092–1105. doi: 10.1016/j.cell.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Trachootham D, Liu J, Chen G, Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W, Keating MJ, Huang P. Nat Cell Biol. 2012;14:276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akkari L, Joyce JA. Cell Res. 2016;26:867–868. doi: 10.1038/cr.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee RFS, Riedel T, Escrig S, Maclachlan C, Knott GW, Davey CA, Johnsson K, Meibom A, Dyson PJ. Metallomics. 2017;9:1413–1420. doi: 10.1039/c7mt00153c. [DOI] [PubMed] [Google Scholar]
- 8.Min YZ, Mao CQ, Chen SM, Ma GL, Wang J, Liu YZ. Angew Chem Int Edit. 2012;51:6742–6747. doi: 10.1002/anie.201201562. [DOI] [PubMed] [Google Scholar]
- 9.Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C. Oxid Med Cell Longevity. 2013:972913. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marques MPM, Gianolio D, Cibin G, Tomkinson J, Parker SF, Valero R, Lopes RP, de Carvalho LAEB. Phys Chem Chem Phys. 2015;17:5155–5171. doi: 10.1039/c4cp05183a. [DOI] [PubMed] [Google Scholar]
- 11.Kelland L. Nature reviews Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 12.Amable L. Pharmacol Res. 2016;106:27–36. doi: 10.1016/j.phrs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Surnar B, Sharma K, Jayakannan M. Nanoscale. 2015;7:17964–17979. doi: 10.1039/c5nr04963f. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Li C, Jin S, Liu J, Xue X, Eltahan AS, Sun J, Tan J, Dong J, Liang XJ. Biomaterials. 2017;144:119–129. doi: 10.1016/j.biomaterials.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. J Controlled Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Huo M, Yuan J, Tao L, Wei Y. Polym Chem. 2014;5:1519–1528. [Google Scholar]
- 17.Li M, Tang Z, Lv S, Song W, Hong H, Jing X, Zhang Y, Chen X. Biomaterials. 2014;35:3851–3864. doi: 10.1016/j.biomaterials.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Deshpande NU, Jayakannan M. Biomacromolecules. 2017;18:113–126. doi: 10.1021/acs.biomac.6b01411. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Tung CH. ACS Appl Mater Interfaces. 2017;9:8547–8555. doi: 10.1021/acsami.6b16500. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Tung CH. ACS Appl Mater Interfaces. 2018;10:4343–4348. doi: 10.1021/acsami.7b16575. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Jeong H, Han S, Beack S, Hwang BW, Shin M, Oh SS, Hahn SK. Biomaterials. 2017;123:155–171. doi: 10.1016/j.biomaterials.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Li SD, Howell SB. Mol pharmaceutics. 2010;7:280–290. doi: 10.1021/mp900242f. [DOI] [PubMed] [Google Scholar]
- 23.Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Pharmacogenet Genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Zhang Z, Tung CH. Chem Commun. 2017;53:779–782. doi: 10.1039/c6cc08230k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasqui D, De Cagna M, Barbucci R. Polymers. 2012;4:1517–1534. [Google Scholar]
- 26.Golla ED, Ayres GH. Talanta. 1973;20:199–210. doi: 10.1016/0039-9140(73)80267-x. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann T, Chval Z, Burda JV. J Phys Chem B. 2009;113:3139–3150. doi: 10.1021/jp807645x. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann T, Zeizinger M, Burda JV. J Inorg Biochem. 2005;99:2184–2196. doi: 10.1016/j.jinorgbio.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Fukino T, Yamagishi H, Aida T. Adv Mater. 2017:29. doi: 10.1002/adma.201603888. [DOI] [PubMed] [Google Scholar]
- 30.Chou LY, Ming K, Chan WC. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 31.Cheteh EH, Augsten M, Rundqvist H, Bianchi J, Sarne V, Egevad L, Bykov VJ, Ostman A, Wiman KG. Cell Death Dis. 2017;8:e2848. doi: 10.1038/cddis.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng X, Morgenstern R, Nystrom AM. Biomaterials. 2014;35:1227–1239. doi: 10.1016/j.biomaterials.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Zhang ZH, Li YJ, Wan JX, Long PH, Guo J, Chen GS, Wang CC. Polym Chem. 2017;8:2410–2422. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.