Abstract
20-HETE is a cytochrome P450-derived metabolite of arachidonic acid that has both pro- and anti-hypertensive actions that result from modulation of vascular and kidney function. In the vasculature, 20-HETE sensitizes vascular smooth muscle cells to constrictor stimuli and increases myogenic tone. By promoting smooth muscle cell migration and proliferation, as well as by acting on the vascular endothelium to cause endothelial dysfunction, angiotensin converting enzyme (ACE) expression, and inflammation, 20-HETE contributes to adverse vascular remodeling and increased blood pressure. A G protein-coupled receptor was recently identified as the effector for the vascular actions of 20-HETE. In addition, evidence suggests that 20-HETE contributes to hypertension via positive regulation of the renin-angiotensin-aldosterone system, as well as by causing renal fibrosis. On the other hand, 20-HETE exerts anti-hypertensive actions by inhibiting sodium reabsorption by the kidney in both the proximal tubule and thick ascending limb of Henle. This review discusses the pro- and anti-hypertensive roles of 20-HETE in the pathogenesis of hypertension-associated renal disease, the association of gene polymorphisms of cytochrome P450 enzymes with the development of hypertension and renal end organ damage in humans, and 20-HETE related pharmaceutical agents.
Keywords: Cytochrome P450, 20-HETE, hypertension, hypertensive nephropathy, vascular function, sodium transport, genetic polymorphisms
1. Introduction
It has long been recognized that arachidonic acid (AA) is metabolized by cyclooxygenase (COX) and lipoxygenase (LOX) to produce 5-, 12-, and 15-hydroxyeicosatetraenoic acids, leukotrienes, prostacyclin, and prostaglandins. These metabolites modulate renal function, vascular tone, and inflammatory responses (Fan et al., 2016; Fan et al., 2015b; Roman, 2002). However, a third pathway for the metabolism of AA exists in some tissues like kidney and liver, where AA is also metabolized by cytochrome P450 (CYP) enzymes into epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids (HETEs) (Capdevila et al., 1981; McGiff and Quilley, 1999). Notably, 20-hydroxy-5, 8, 11, 14-eicosatetraenoic acid (20-HETE) is the metabolite of ω-hydroxylation of AA formed by enzymes of the CYP4A and CYP4F families (Fan et al., 2016; Fan et al., 2015b; Roman, 2002). Once produced, 20-HETE can be catalyzed by alcohol dehydrogenase (ADH) to the carboxylic acid, which is further metabolized by β-oxidation (Collins et al., 2005; Kaduce et al., 2004). In addition, 20-HETE is also metabolized by epoxygenases, COX, and LOX (Hill et al., 1992; Rosolowsky et al., 1996). After its conjugation with UDP-glucuronosyltransferases, 20-HETE can be excreted in the urine (Jarrar et al., 2014).
The isoforms of CYP enzymes that are responsible for the production of 20-HETE are different among species. CYP4A11, −4F2, and −4F3 are the isoforms that contribute to the production of 20-HETE in humans (Gainer et al., 2005; Hirani et al., 2008; Lasker et al., 2000; Powell et al., 1998). Among these isoforms, CYP4F2 is primarily responsible for the formation of 20-HETE in the kidney (Powell et al., 1998), and CYP4F3 is mainly expressed in polymorphonuclear leukocytes (PMNs) (Rosolowsky et al., 1996). CYP4A1, −4A2, −4A3, −4A8, −4F1, and −4F4 are the 20-HETE producing isoforms in rats (Kawashima et al., 1997; Kikuta et al., 1999; Nguyen et al., 1999; Williams et al., 2012; Xu et al., 2004). Among them, CYP4A1 exhibits the greatest catalytic activity (Xu et al., 2004). In mice, CYP4A10, −4A12a, −4A12b, and −4A14 are constitutively expressed, but only CYP4A12a is able to metabolize AA to 20-HETE (Dordea et al., 2016; Holla et al., 2001; Muller et al., 2007; Wu et al., 2013). However, unlike CYP4A10 and CYP4A14, CYP4A12 expression is weakly induced by fibrates and is also expressed differently in the tissues of male versus female mice (Holla et al., 2001). It should be noted that CYP4A genes are found in a single cluster on the same chromosome suggesting gene duplication. There are four genes of the 4A family in mice, rats, rabbits, and dogs. Man is the exception with only 2 isoforms and, unlike other species, has two related CYP4F genes on different chromosomes that produce 20-HETE. This could have occurred during a crossover event in evolution. Within the kidney, 20-HETE is expressed in pre-glomerular arterioles, glomeruli, proximal convoluted tubules, and pars recta. Of note, 20-HETE is the primary metabolite of AA in the thick ascending loop of Henle (TALH) in the nephron.
Recently, the first 20-HETE receptor was identified (Garcia et al., 2017). It was reported that 20-HETE affects vascular function by binding to the Gq protein-coupled receptor GPR75, which previously was identified as a receptor for the chemokine CCL5 (RANTES) (Ignatov et al., 2006; Liu et al., 2013). In endothelial cells, 20-HETE acts to impair vasodilation and increase vasoconstrictive signaling. Binding of 20-HETE to GPR75 causes dissociation of the Gαq/11 subunit, produces inositol trisphosphate (IP3), increases intracellular Ca2+, and activates MAPK. These signaling events ultimately lead to endothelial dysfunction as marked by deceased nitric oxide (NO) and increased reactive oxygen species due to uncoupling and/or loss of endothelial nitric oxide synthase (eNOS) (Fig. 1). 20-HETE also activates NF-κB signaling, which results in increased angiotensin-converting enzyme (ACE) expression that favors formation of hypertensive angiotensin II (Ang II). As discussed elsewhere (Fan and Roman, 2017), 20-HETE may also cause endothelial dysfunction and ACE expression via transactivation of the epidermal growth factor receptor (EGFR) (not shown).
Figure 1. Overview of signaling events in endothelial and vascular smooth muscle cells contributing to hypertension.
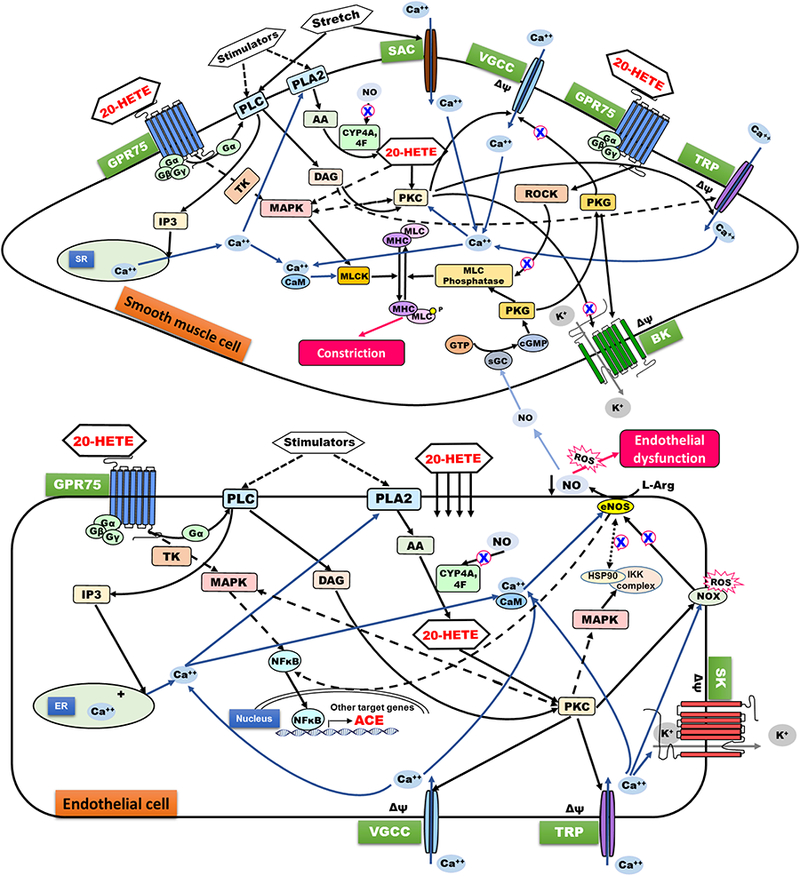
Stretch or vasoconstrictors increase intracellular Ca2+ and activate PLA2 to generate 20-HETE, which can act either intracellularly to stimulate PKC or extracellularly via the Gαq/11 protein-coupled receptor GPR75 (or other yet-to-be identified GPCR). Overall, 20-HETE antagonizes the actions of the vasodilator NO, which can down-regulate levels of 20-HETE-forming CYPs. In VSMC (top figure), 20-HETE and GPR75 couple to increase intracellular Ca2+ and contraction by 1) activation of MLCK (downstream of Ca2+/calmodulin or MAPK), (2) ROCK-mediated inhibition of MLC phosphatase, as well as by PKC-mediated (3a) activation of VGCC, (3b) phosphorylation of contractile proteins, and (3c) inhibition of BK. In the endothelium (bottom figure), 20-HETE-mediated activation of GPR75 promotes loss of NO and endothelial dysfunction by uncoupling eNOS, activating NOX, and disrupting eNOS-HSP90 association, thus promoting reactive oxygen species formation. 20-HETE also induces expression of angiotensin-converting enzyme (ACE), thereby increasing levels of the vasoconstrictor Ang II. 20-HETE promotes endothelial inflammation by increasing expression of adhesion molecules, cytokines, and chemokines for leukocytes. ACE, angiotensin converting enzyme; BK, calcium-activated potassium channel; CaM, calmodulin; DAG, diacylglycerol; eNOS, endothelial nitric oxide synthase; ER, endoplasmic reticulum; GPCR, G-protein coupled receptor; HSP90, heat shock protein 90; IP3, inositol trisphosphate; L-Arg, L-arginine; MAPK, mitogen-activated protein kinases; MHC, myosin heavy chain; MLC, myosin light chain; MLCK, myosin light-chain kinase; NFκB, nuclear factor κ-light-chain-enhancer of activated B cells; PKC, protein kinase C; PKG, protein kinase G; PLA2, phospholipase A2; PLC, phospholipase C; ROCK, Rho-activated kinase; SAC, stretch-activated calcium channel; SK, small conductance calcium-activated potassium channels; sGC, soluble guanylyl cyclase; SR, sarcoplasmic reticulum; TK, tyrosine kinases; TRP, transient receptor potential channels; VGCC, voltage-gated calcium channel. See text and (Fan and Roman, 2017) for additional details.
In vascular smooth muscle cells (VSMCs), GPR75 activation is linked to protein kinase C (PKC)-mediated phosphorylation of the β subunit of the large conductance calcium-and voltage-activated potassium (BK) channels, which inhibits BK channel activity and causes vasoconstriction (Garcia et al., 2017) (Fig. 1). GPR75 may explain the actions of 20-HETE in VSMCs and endothelial cells; however, this remains to be verified in proximal tubular and TALH cells, and to be determined whether GPR75 is the only 20-HETE receptor or whether others will be identified (Fan and Roman, 2017).
As discussed in our review and summarized in Table 1, 20-HETE has both pro- and anti-hypertensive actions. The former are attributed to its aforementioned actions on the endothelial cells and VSMC of both the renal and peripheral vasculature. The anti-hypertensive and extravascular actions of 20-HETE result from natriuretic and diuretic actions arising from inhibition of sodium reabsorption in both the proximal tubule (PT) and TALH. Confoundingly, the intra-renal, anti-hypertensive actions of 20-HETE are opposed by the renin-angiotensin-aldosterone-system (RAAS), which may be activated by 20-HETE.
Table 1 -.
Cell-specific actions of 20-HETE that affect blood pressure
| Cell Type | ↑ Blood Pressure | ↓ Blood Pressure |
|---|---|---|
| EC | ↓ NO, inflammation, ↑ACE | |
| VSMC | ↑ contractility leading to ↓ GFR & ↑ PVR | |
| PT | ↑Na2+ & H2O reabsorption from ↑renal Ang II | ↓ Na2+ & H2O reabsorption |
| DT | ↑Na2+ reabsorption from ↑renal Ang II | |
| TALH | ↑Na2+ & H2O reabsorption from ↑renal Ang II | ↓ Na2+ & H2O reabsorption |
DT, distal tubule; EC, endothelial cell; PVR, peripheral vascular resistance; PT, proximal tubule; TALH, thick ascending limb of Henle.
2. Role of 20-HETE in promoting hypertension
20-HETE acts as a potent vasoconstrictor of VSMC by multiple means (Fig. 1). It blocks BK channel activity, leading to a fall in membrane potential, which enhances calcium entry via voltage-gated L-type Ca2+ channels and transient receptor potential cation channel 6 (TRPC6), resulting in vasoconstriction. (Fan et al., 2013b; Gebremedhin et al., 1998; Roman, 2002; Williams et al., 2010). 20-HETE activates mitogen-activated protein kinases (MAPKs) (Garcia et al., 2016), PKC (Sun et al., 1999), Rho-kinase/ROCK, and tyrosine-kinases (Parmentier et al., 2001b; Sun et al., 1999), which lead to VSMC contraction either via increased intracellular Ca2+ or by enhanced phosphorylation of contractile elements (Fig. 1) (Fan et al., 2013b; Gebremedhin et al., 1998; Roman, 2002; Williams et al., 2010).
Elevations in transmural pressure promote 20-HETE production and inhibition of 20-HETE synthesis impairs myogenic reactivity in renal arteries (Gebremedhin et al., 2000). 20-HETE enhances vascular response to stretch (Gao et al., 2008; Goodman et al., 2003; Nakayama et al., 2003), Ang II (Fan et al., 2013b; Lima et al., 2013), endothelin 1 (Berg, 2016; Oyekan et al., 1999), vasopressin (Omata et al., 1992a), serotonin (Cambj-Sapunar et al., 2003; Roman et al., 2006), and contributes to oxidative stress (Dunn et al., 2008; Hou et al., 2010; Singh et al., 2007), endothelial dysfunction (Dunn et al., 2008), and inflammation (Hoopes et al., 2015; Miyata and Roman, 2005), all of which alter renal hemodynamics (enhance afferent arteriole autoregulation and decrease renal blood flow) and increase peripheral vascular resistance, thereby promoting development of hypertension.
The first implication of the relationship between 20-HETE and hypertension was reported in 1989 (Sacerdoti et al., 1989). The investigators found that selective depletion of renal cytochrome P450 and AA metabolites prevented elevated blood pressure in the Spontaneously Hypertensive Rat (SHR). Direct evidence was then provided in 1991 that SHR elevated in the kidney in SHR (Ishizuka et al., 2004; Kroetz et al., 1997; Omata et al., 1992a; Omata et al., 1992b; Schwartzman et al., 1996). Therefore, it seems that the hypertensive phenotype in the SHR is due to elevated renal 20-HETE formation and, thus, it would be expected that inhibition of renal production of 20-HETE would attenuate the degree of hypertension in this model. Indeed, induction of heme oxygenase (HO) reduced (Goodman et al., 2003) or blocked formation of 20-HETE and slowed development of hypertension in male (Gebremedhin et al., 1993; Imig et al., 1993; Kroetz et al., 1997; Omata et al., 1992a; Schwartzman et al., 1996; Zhang et al., 2005) and post-menopausal female SHR (Yanes et al., 2011). Administration of dihydrotestosterone (DHT) in both male and female Sprague Dawley rats upregulated 20-HETE production in renal parenchymal and vascular tissues by elevating expression of CYP4A8 through the androgen receptor, and promoted development of hypertension (Singh et al., 2007; Singh and Schwartzman, 2008; Wu and Schwartzman, 2011).
The relationship of 20-HETE and hypertension was also investigated in hypertensive mouse models. Upregulation of CYP4A12 by exogenous DHT, or as is seen in nitric oxide (NO) receptor deficient sGCαl (−/−) mice, produced 20-HETE-dependent hypertension and vascular dysfunction (Dordea et al., 2016; Muller et al., 2007; Wu et al., 2013). CYP4A14 is highly expressed in female mice (Heng et al., 1997; Holla et al., 2001; Muller et al., 2007), but knockout of CYP4A14 (Fidelis et al., 2010; Holla et al., 2001; Muller et al., 2007) induced hypertension in male mice only. This was associated with an increase in plasma testosterone levels and renal CYP4A12 expression and 20-HETE formation, as well as reduced renal production of NO (Fidelis et al., 2010). These findings suggest that CYP4A14 may produce a metabolite that inhibits testosterone production. Hypertension in this model was reversed by castration (Holla et al., 2001) and the elevation in renal perfusion pressure to phenylephrine was blunted by an inhibitor of 20-HETE synthesis (Fidelis et al., 2010). More recent studies indicate that knock-in of human CYP4A11 (Savas et al., 2016) and CYP4F2 (Cai, 2009; Fava et al., 2009; Liu et al., 2009) in mice enhanced renal production of 20-HETE and promoted development of hypertension. Levels of 20-HETE were also elevated in various tissues including kidneys and endothelial cells in endothelial-specific human CYP4F2 transgenic mice (Cheng et al., 2014); however, blood pressure was not altered in this model. These studies indicate that increased formation of 20-HETE is associated with elevations in blood pressure in male, but not female mice, since it is linked to elevations in testosterone-induced CYP4A12 expression. The relationship between these findings and the influence of 20-HETE on the development of hypertension in man remain to be determined.
3. Impact of 20-HETE on hypertensive vascular remodeling and nephropathy
20-HETE plays a pro-fibrotic role in hypertensive nephropathy. In streptozotocin (STZ) treated diabetic CYP4A14 KO mice, the increased renal 20-HETE production (due to increased plasma testosterone levels and consequent induction of CYP4A12 gene expression) was associated with hypertension (Gangadhariah et al., 2015; Holla et al., 2001) and exacerbated renal injury. Increased urinary protein excretion, expansion of mesangial and glomerular basement membranes, and deposition of glomerular matrix were observed (Gangadhariah et al., 2015; Holla et al., 2001). These results are consistent with previous studies that enhanced CYP4A/20-HETE levels accompanied elevated reactive oxygen species production in cultured mouse podocytes (Eid et al., 2009) and rat proximal tubular epithelial cells (Eid et al., 2013). Increased expression of 20-HETE in STZ-treated mice and rats was also associated with increased reactive oxygen species generation, NADPH oxidase activity (Eid et al., 2009), transforming growth factor-β1 (TGF-β1) and fibronectin expression, as well as glomerular matrix formation, podocyte apoptosis, and urinary protein excretion (Eid et al., 2009; Elmarakby et al., 2013).
Vascular remodeling is one of the key pathophysiological processes in hypertension and is related to an increase in the media-to-lumen ratio of small arteries and arterioles, leading to vascular hyperactivity to constrictor stimuli and enhanced peripheral resistance (Renna et al., 2013). The renal expression of 20-HETE is increased in SHR, and 20-HETE is involved in the augmented renal vascular reactivity to Ang II, which results in profound vascular remodeling (Gebremedhin et al., 1993). 20-HETE also contributes to vascular hypertrophy in a CYP4A12 transgenic mouse independent of the increase in mean arterial pressure (Wu et al., 2013). These studies demonstrate that 20-HETE plays a role in vascular remodeling, a process that involves activation of multiple vascular components such as endothelium and VSMCs, and deposition of extracellular matrix (ECM) and basement membrane (McGrath et al., 2005).
Besides promoting proliferation of VSMCs (Fan et al., 2016; Orozco et al., 2013), which has been implicated in restenosis, 20-HETE also stimulates mitogenesis of endothelial (Chen et al., 2012; Cheng et al., 2014; Guo et al., 2007) and renal epithelial cells (Akbulut et al., 2009). 20-HETE-induced endothelial cell proliferation may contribute to atherosclerotic neovascularization/angiogenesis and related plaque instability and rupture (Chen et al., 2012; Moreno et al., 2006; Sun, 2014), events that are associated with and exacerbated by hypertension (Picariello et al., 2011). Renal epithelial cell proliferation has significant implications for polycystic kidney disease (PKD) (Park et al., 2009), which commonly is associated with hypertension, although the mechanism is unclear. 20-HETE is stimulated by pro-angiogenic factors such as hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) to promote cell migration and proliferation (Chen et al., 2014; Muthalif et al., 1998; Parmentier et al., 2001a; Stec et al., 2007a). Moreover, 20-HETE induces VEGF expression that drives endothelial cell proliferation in a signaling cascade involving apocynin-insensitive reactive oxygen species production and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) activation (Chen et al., 2014; Guo et al., 2007). In kidney epithelial cells, 20-HETE was found to activate the mitogenic Raf/MEK/ERK and protein kinase B (PKB/Akt) signaling pathways via c-Src-mediated transactivation of EGFR (Akbulut et al., 2009). Whether 20-HETE also acts on renal epithelial cells through the same GPR75 receptor as on endothelial and VSMCs remains to be determined (Fan and Roman, 2017; Garcia et al., 2017). Overall, these studies suggest that 20-HETE plays a role in vascular or kidney remodeling, which might be associated with its effect to promote angiogenesis and endothelial or renal epithelial cell proliferation.
20-HETE has also been known to play a role in promoting vascular inflammation. Arteries that were treated with a biosynthesis inhibitor of 20-HETE, HET0016 resulted in attenuation of reactive oxygen species and vascular nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, and reduction of vascular inflammation, which was associated with decreases in pro-inflammatory cytokine expression, such as tumor necrosis factor-alpha (TNFα), interleukin-1 beta (IL-1β), and IL-6 (Toth et al., 2013). 20-HETE has also been found to induce expression of multiple adhesion molecules such as monocyte chemotactic protein 1 (MCP-1), intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion protein 1 (VCAM-1) in the vasculature (Hoopes et al., 2015). The adhesion molecules increase vascular inflammation by recruiting monocytes and macrophages to the vascular wall.
4. Interplay between 20-HETE and the renin-angiotensin-aldosterone system (RAAS) in hypertension and hypertensive nephropathy
The renin-angiotensin-aldosterone system, which acts either in a systemic endocrine or in a local paracrine/autocrine fashion, plays an important role in cardiovascular homeostasis (Harrison-Bernard, 2009). Ang II is the central bioactive component of the RAAS and exerts a crucial role in regulating vascular myogenic tone in normal physiology, as well as in the pathogenesis of hypertension and other cardiovascular disorders (Mehta and Griendling, 2007; Paul et al., 2006). Reduced arterial blood pressure, a decrease in sodium load, or activation of the sympathetic nervous system (SNS) all stimulate renin secretion (Paul et al., 2006). Renin cleaves angiotensinogen to produce Ang I, which is further converted to Ang II by ACE (Roman et al., 2016). Tissue-specific renin- or ACE-independent routes for Ang II formation have also been identified (Forrester et al., 2018). In addition, evidence has suggested that the kidney expresses all necessary components for Ang II formation and thus possesses a local renin-angiotensin-system, although recent reports question the intra-renal origins of angiotensinogen and other system components (Roman et al., 2016).
Emerging evidence suggests a complex interplay between 20-HETE and the RAAS. A positive feedback between 20-HETE and Ang II exists and contributes to vasoconstriction and hypertension (Alonso-Galicia et al., 2002; Chu et al., 2000; Croft et al., 2000; Fan et al., 2013b; Joly et al., 2006; Park et al., 2001). Ang II increases renal production of 20-HETE (Alonso-Galicia et al., 2002), while the RAAS is suppressed in a salt-sensitive hypertensive rat with decreased expression of glomerular CYP4A1 (Ito and Roman, 1999). Chronic deoxycorticosterone acetate (DOCA)-salt treatment suppresses the systemic renin-angiotensin system, and 20-HETE expression was reduced in a DOCA-salt hypertensive mouse model (Honeck et al., 2000); however, 20-HETE levels were elevated in the DOCA-salt hypertensive rat (Oyekan et al., 1999), perhaps implicating a direct role for the mineralocorticoid receptor in this model.
Recent studies demonstrated that 20-HETE activates the RAAS in part by inducing vascular expression of ACE downstream of NF-κB activation (Cheng et al., 2012; Garcia et al., 2016; Sodhi et al., 2010); however, 20-HETE-mediated microvascular remodeling in hypertension did not fully rely on ACE activity in the vascular endothelium (Cheng et al., 2012; Garcia et al., 2015). Moreover, increased 20-HETE may not necessarily be associated with enhanced RAAS activity. For instance, fenofibrate treatment induced intrarenal 20-HETE production, thereby attenuating hypertension in an Ang II-dependent mouse model via enhanced sodium excretion (see below, Role of 20-HETE in preventing hypertension and hypertensive nephropathy) (Vera et al., 2005). In contrast, fenofibrate increased renal production of 20-HETE and plasma renin activity in both Stroke-Prone Spontaneously Hypertensive Rats (SHRSP) and salt-sensitive hypertensive Dahl S rats (Shatara et al., 2000). In a human-derived 20-HETE-producing CYP4A11 transgenic mouse, 20-HETE enhanced the production of renal angiotensinogen and activation of Ang II type 1 (AT1) receptor and this enhancement paralleled an increase in plasma potassium level and in activities of the sodium chloride co-transporter (NCC) and serum/glucocorticoid regulated kinase 1 (SGK1), even though plasma aldosterone, Ang II, and renin activities remained unchanged (Savas et al., 2016). This investigation suggests that 20-HETE may contribute to hypertension and its complications via upregulation of sodium transport in the distal nephron secondary to increased activity of RAAS.
Hypertension in the SHR is dependent on both the RAAS and 20-HETE and is associated with elevated sympathetic tone. Little is known as to whether 20-HETE interacts with Ang II to increase sympathetic activity or potentiates the effects of norepinephrine at the level of smooth muscle. More studies are necessary to elucidate whether the link between 20-HETE and RAAS is mediated solely via changes in vascular reactivity, sodium retention activation of the SNS, or via combinational effects.
5. Hypertensive actions of 20-HETE summarized
20-HETE promotes hypertension by several means involving both the peripheral vasculature and kidney. It serves as an autocrine second messenger and enhances the vascular response to constrictor stimuli in peripheral VSMCs, as well as renal afferent arteries. It also promotes endothelial dysfunction, and induces vascular inflammation, and RAAS activation. Furthermore, 20-HETE promotes renal oxidative stress and fibrosis, directly or via apoptosis of kidney cells. These factors, in our opinion, mainly account for the role of 20-HETE in promoting hypertension and contributing to hypertension associated renal end organ damage.
6. Role of 20-HETE in preventing hypertension and hypertensive nephropathy
On the other hand, 20-HETE prevents hypertension by inhibiting sodium reabsorption and promoting natriuresis (Fig. 2). It inhibits Na+-K+-ATPase activity and internalizes sodium-hydrogen exchanger 3 (NHE3) in the PT, thereby diminishing sodium transport in renal tubules (Capdevila et al., 2003; Fan et al., 2016; Fan et al., 2015b; Roman, 2002). In the TALH, 20-HETE inhibits Na+ reabsorption and induces natriuresis by several means: 20-HETE a) inhibits the basolateral Na+/K+-ATPase and luminal Na+-K+−2Cl− cotransporter; and b) inhibits both the luminal 70 pS K+ channel responsible for K+ back-leak that sustains cotransporter activity and the basolateral 50 pS K+ channel that affects cotransporter activity by controlling the driving force for Cl− cellular exit (Fan et al., 2016; Fan et al., 2015b; Gu and Wang, 2002; Roman, 2002; Yu et al., 2007)
Figure 2. The effect of 20-HETE on different segments of the nephron to enhance natriuresis.
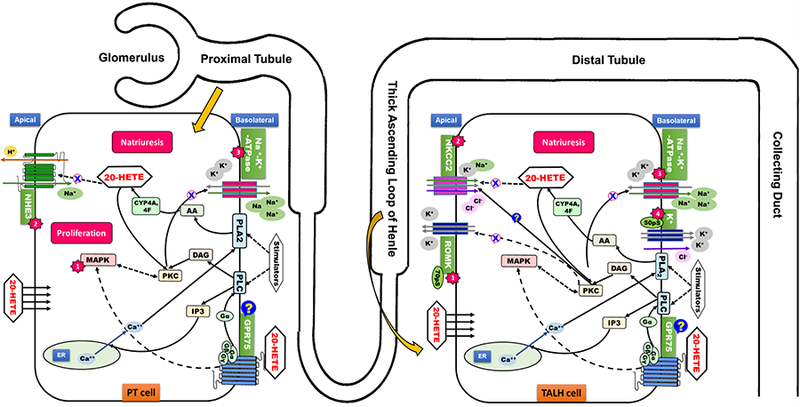
20-HETE is actively produced from PLA2-mediated formation of AA in proximal tubular cells and TALH cells by hormonal and stress stimulators downstream of PLC-mediated increases in intracellular Ca2+. 20-HETE may act intracellularly or via GPR75. In proximal tubular cells, 20-HETE promotes cellular proliferation by activation of PKC/MAPK mediated signaling pathway (1). 20-HETE also internalizes NHE3 via an undefined process (2) and inhibits activity of Na+-K+-ATPase by PKC-mediated phosphorylation (3), thereby diminishing sodium transport from the apical (luminal) to basolateral side of proximal tubule cells. In TALH cells, 20-HETE inhibits the apical NKCC2 cotransporter (2) and basolateral Na+-K+-ATPase (3) to prevent sodium reabsorption from the renal tubular lumen into the basolateral space. 20-HETE also inhibits the 70pS ROMK located on the apical side (1) and the 50pS ROMK located on the basolateral side (4), which normally contribute to NKCC2 cotransporter activity by allowing for K+ back-leak into tubular lumen and driving Cl− transcellular flux, respectively. All of the actions on proximal tubule cells and TALH cells by 20-HETE contribute to natriuresis and the prevention of water-sodium retention. NHE3, sodium-hydrogen antiporter 3; NKCC2, Na+-K+−2Cl− cotransporter; ROMK2, renal outer medullary potassium channel 2; PLA2, phospholipase A2; PLC, phospholipase C; PKC, protein kinase C; TALH, thick ascending loop of Henle. See text and Figure Legend 1 legend for additional details.
Induction of renal formation of 20-HETE with fibrates attenuates, rather than promotes, high blood pressure in SHRSP (Shatara et al., 2000) or SHR (Hou et al., 2010). The divergent results can be understood by the observation that fenofibrate increases renal 20-HETE and natriuresis, but does not alter vascular 20-HETE, because blood vessels do not express peroxisome proliferator-activated receptor alpha (PPAR-α) (Vera et al., 2005). This finding suggests that the 20-HETE produced in renal tubules may contribute to the blood pressure-lowering effects of fibrates treatment in Ang II-dependent hypertension without affecting or overweighting its effects on vascular tone. This viewpoint is supported by more recent studies in the Dahl S rats.
The Dahl S rat is a low renin, salt-sensitive model of hypertension due to enhanced reabsorption of sodium in the PT and TALH. In the Dahl S rat, reduced levels of CYP4A and 20-HETE are associated with rapid development of hypertension with salt diet, impaired pressure natriuresis relationship, and abnormal sodium transport in the kidney (Williams et al., 2008; Williams et al., 2007b). These rats also have impaired renal microvascular function (Fan et al., 2013a; Ge et al., 2014). These findings are similar to another study in SHR, whereby the induction of 20-HETE with fibrates attenuated hypertension and reduced proteinuria (Shatara et al., 2000). Transfer of chromosome 5 which contains 4 isoforms of CYP4A enzymes from normotensive Lewis or Brown Norway (BN) strains or transfer of a single BN CYP4A1 gene to the Dahl S rat prevented development of hypertension, improved natriuresis, and rescued the impaired myogenic response of both renal and cerebral arterioles (Fan et al., 2013a; Fan et al., 2014; Ge et al., 2014; Murphy et al., 2013; Williams et al., 2012; Williams et al., 2008).
Dahl S rats exhibit a deficiency in the formation of 20-HETE (Fan et al., 2015a; Williams et al., 2012; Williams et al., 2008), an elevation in glomerular capillary pressure, and an increase in permeability of the glomerulus to albumin, which are associated with enhanced renal TGF-β1 expression and development of hypertension-induced chronic kidney disease (Fan et al., 2015a; Williams et al., 2012; Williams et al., 2008). These rats also have impaired renal myogenic and TGF responses, increased renal interstitial pressure in response to elevation of renal perfusion pressure, and a reset of pressure-natriuresis (Fan et al., 2013a; Ge et al., 2014; Ren et al., 2014). The renal protective effect of 20-HETE was confirmed in transgenic Dahl S rats in which the CYP4A gene(s) was knocked in or overexpressed (Murphy et al., 2012; Murphy et al., 2013; Williams et al., 2012; Williams et al., 2008). These rats exhibited diminished albumin permeability, rescued glomerular filtration rate (GFR), and attenuated glomerular capillary leakage (Dahly-Vernon et al., 2005; McCarthy et al., 2005; Williams et al., 2007b). More evidence was recently provided that 20-HETE, a physiological substrate of ADH in podocytes, protected the glomerular permeability barrier in ethanol treated mice in which CYP4A12a was upregulated by blocking cytoskeletal derangement and production of superoxide (McCarthy et al., 2015).
The available evidence suggests that elevated 20-HETE induces hypertension, but prevents renal injury due to elevation of renal vascular tone in models associated with involvement of RAAS. Results with a 20-HETE inhibitor in Lyon hypertensive rats further support this conclusion (Lantelme et al., 1997; Messer-Letienne et al., 1999; Williams et al., 2007a). Deficiencies in 20-HETE in the kidney increase tubular sodium reabsorption and promote salt-sensitive hypertension and renal injury due to decreased renal vascular reactivity.
7. Human genetic studies
In humans, CYP4F2 is the most potent isoform, followed by CYP 4A11, among the predominant 20-HETE producing enzymes: CYP4A11, −4A22, −4F2, and −4F3 (Gainer et al., 2005; Hirani et al., 2008; Lasker et al., 2000; Powell et al., 1998). A number of single nucleotide polymorphisms (SNPs) in the CYP4F2 (V433M, G421C, GA/AA) and CYP4A11 (T8590C) genes have been reported to play a role in the development of hypertension in several cohorts (Gainer et al., 2008; Laffer et al., 2008; Liu et al., 2008; Liu et al., 2006; Mayer et al., 2005; Ward et al., 2008; Williams et al., 2011). Among these SNPs, the V433M variant (Stec et al., 2007b) in CYP4F2 and the T8590C variant (Gainer et al., 2005) in CYP4A11 decrease the activities of these enzymes, and the later SNP is also associated with the pathogenesis of salt-sensitive hypertension (Williams et al., 2007b; Yanes et al., 2011). Other reports showed that the renal excretion of 20-HETE-glucuronide is elevated in hypertensive patients with CYP4F2 SNPs (Liu et al., 2008; Ward et al., 2008). These authors suggested that 20-HETE might promote hypertension by enhancing renal vasoconstriction, but this hypothesis remains to be validated because renal blood flow (RBF) or GFR was not measured in these hypertensive patients. Moreover, recent studies have indicated that urinary glucuronide conjugate-20-HETE is not of renal origin, since the amount of the conjugated form of 20-HETE is higher in the plasma than in urine when the fractional excretion is <1% (Dreisbach et al., 2014).
Overall, these studies indicate that 20-HETE plays an important role in the development of hypertension in humans. However, more studies are needed to determine to what extent changes in 20-HETE production contribute to the antihypertensive effects of diuretics, ACE inhibitors, AT1 receptor antagonists, and other antihypertensive agents.
8. The development of 20-HETE related pharmaceutical agents
Along the way in studying the role of 20-HETE in various pathological processes, a variety of 20-HETE-related pharmaceutical agents have been synthesized (Williams et al., 2010; Yu et al., 2004) (Fig. 3). These include CYP inhibitors and inducers, and 20-HETE agonists and antagonists. In the early period, several specific inhibitors of CYP4A enzymes were synthesized and used for evaluating the role of CYP metabolites of AA, such as 1-aminobenzotriazole (ABT) and 17-octadecynoic acid (17-ODYA) (Knickle and Bend, 1992; Mathews et al., 1985; Zou et al., 1994). However, they were not satisfactory because they are not specific and could also block the formation of EETs (Knickle and Bend, 1992; Maier et al., 2000; Mathews et al., 1985; Zou et al., 1994), a group of AA metabolites with effects on vascular function generally opposite to 20-HETE. The second generation of inhibitors to specifically block formation of 20-HETE, 12, 12-dibromododec-11-enamide (DBDD) and N-methylsulfonyl-12, 12-dibromododec-11-enamide (DDMS), were synthesized by Dr. Falck (Alonso-Galicia et al., 1997; Wang et al., 1998). These compounds proved to completely inhibit formation of 20-HETE at a concentration of 10 μM, whereas the activity of EETs was only reduced by 10–20% under the same condition (Alonso-Galicia et al., 1997). The main limitation of these agents is their fatty acid property with a rather high albumin-binding rate in the plasma, which restricts distribution of these compounds to targeted tissues.
Figure 3. Development of 20-HETE-related pharmaceutical agents.
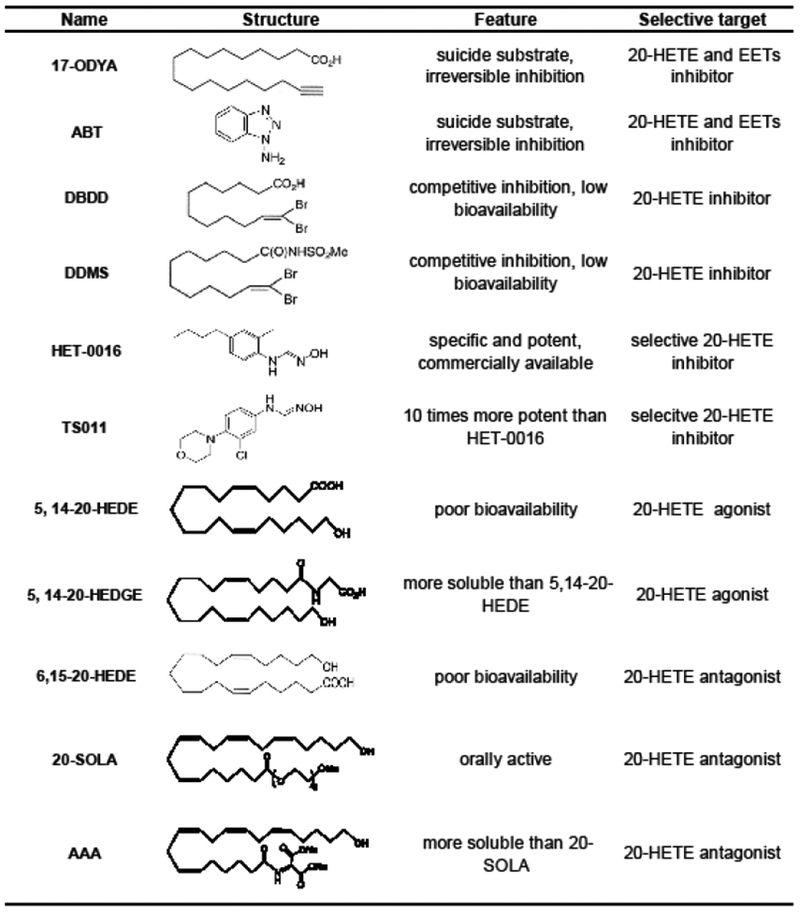
20-HETE-related pharmaceutical agents are summarized, including CYP inhibitors and inducers, and 20-HETE agonists and antagonists. Chronologically, these compounds became more potent, specific, and soluble with development. Abbreviations: 17-ODYA: 17-octadecynoic acid; ABT: 1-aminobenzotriazole; DBDD: 12, 12-dibromododec-11-enamide; DDMS: N-methylsulfonyl-12, 12-dibromododec-11-enamide; HET-0016: N-hydroxy-N’-(4-n-butyl-2-methylphenyl) formamidine; TS011: N-(3-Chloro-4-morpholin-4-yl) Phenyl-N’-hydroxyimido formamide; 5, 14–20-HEDE or WIT-003: 20-hydroxyeicosa-5(Z), 14(Z)-dienoic acid; 5, 14–20-HEDGE: N-[20-hydroxyeicosa-5(Z), 14(Z)-dienoyl] glycine; 6,15–20-HEDE or WIT-002: 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid; 20-SOLA: 2, 5, 8, 11, 14, 17-hexaoxanonadecan-19-yl 20-hydroxyeicosa-6(Z), 15(Z)-dienoate; AAA: sodium (S)-2-((6Z,15Z)-20-hydroxyicosa-6,15-dienamido)- succinate.
A new inhibitor of CYP4A enzymes, N-hydroxy-N’-(4-n-butyl-2-methylphenyl) formamidine (HET-0016) was created (Miyata et al., 2001). This compound could inhibit formation of 20-HETE in a potent and selective way for its IC50 is only 8.9 nM and it has no effect on other enzymes that are responsible for AA metabolism such as COX, epoxygenase, LOX, or other CYP isoforms that are not involved in producing 20-HETE (Miyata et al., 2001). Later, a more potent and selective HET-0016 analog, TS011 was developed (Williams et al., 2010). In general, HET-0016 is commercially available and is the most widely used inhibitor of 20-HETE synthesis.
A number of analogues of 20-HETE were created by Dr. Falck. Drs. Falck and Roman found that these compounds could act either as agonists or antagonists of 20-HETE by interacting with a putative 20-HETE receptor in vascular smooth muscle (Alonso-Galicia et al., 1998; Roman, 2003; Yu et al., 2004). The extensively used agonists of 20-HETE are 20-hydroxyeicosa-5(Z), 14(Z)-dienoic acid (5, 14–20-HEDE or WIT-003), and N-[20-hydroxyeicosa-5(Z), 14(Z)-dienoyl] glycine (5, 14–20-HEDGE) (Akbulut et al., 2009; Regner et al., 2009; Renic et al., 2012), while the most effective analogue to block the vasoconstrictor response to 20-HETE appears to be 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (6,15–20-HEDE or WIT-002) (Alonso-Galicia et al., 1999; Frisbee et al., 2001; Gebremedhin et al., 2000). However, the use of these compounds as drugs are limited because of protein binding, short half-life, and poor solubility.
Very recently, a novel water-soluble 20-HETE antagonist, 2, 5, 8, 11, 14, 17-hexaoxanonadecan-19-yl 20-hydroxyeicosa-6(Z), 15(Z)-dienoate (20-SOLA) was synthesized. Administration of 20-SOLA was found to reduce blood pressure as a result of increased natriuresis in hypertensive CYP4A14 KO mice (Pandey et al., 2017). This model of androgen-driven hypertension is associated with both 20-HETE-induced vasoconstriction and renal hypoperfusion, as well as excessive sodium and volume reabsorption due to Ang II-induced upregulation of NHE3 in the proximal tubule and NCC in the distal convoluted tubule. Increased renal Ang II is likely attributed to 20-HETE-driven vascular ACE or renal angiotensinogen expression. Thus, the anti-hypertensive actions of the 20-SOLA in this hypertensive model may have resulted from increased glomerular filtration and attenuation of the RAAS. Finally, Dr. Eric F. Johnson reported a more soluble 20-HETE antagonist than 6, 15–20-HEDE, sodium (S)-2-((6Z, 15Z)-20-hydroxyicosa-6, 15-dienamido)-succinate (AAA), which contributed to the reduced blood pressure in human CYP 4A11 transgenic mice (Savas et al., 2016). These compounds appear to be the most likely candidates for drug development for treatment of myocardial infarction, stroke, chronic kidney disease, neovascularization and other 20-HETE-associated cardiovascular complications.
9. Perspectives
In summary, 20-HETE plays an important role in the control of blood pressure. The regulation depends on the balance of 20-HETE’s pro-hypertensive effects on the vasculature and anti-hypertensive effects in the kidney. Identification of genetic variants in the 20-HETE-producing enzymes or development of drugs targeting the production of 20-HETE could provide useful information for the early diagnosis, prevention, and treatment of hypertension and hypertension-related tissue injuries. Additional research into the effect of high salt diet on vascular and renal 20-HETE and CYP isoform expression levels is needed to resolve this issue (Frisbee et al., 2000; Roman, 2002; Walkowska et al., 2015), as well as the role of the gut microbiota. A recent study showing rhythmic expression of EETs in the rat brain and vasculature would suggest that vascular and renal levels of 20-HETE may be under circadian control as well (Carver et al., 2014). Finally, therapeutic approaches based on genetic manipulations, such cell-targeted miRNA delivery, to separate the renal from vascular effects of 20-HETE or its synthesis, are needed and may prove beneficial for treating hypertension.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Richard. J. Roman (University of Mississippi Medical Center) and Dr. John R. Falck (University of Texas, Southwestern Medical Center) for their invaluable inputs. This study was supported by grants AG050049, P20GM104357, HL36279, DK104184 and HL138685, from the National Institutes of Health; 16GRNT31200036 from the American Heart Association; 201606100208 from China Scholarship Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
None.
REFERENCES
- Akbulut T, Regner KR, Roman RJ, Avner ED, Falck JR, Park F, 2009. 20-HETE activates the Raf/MEK/ERK pathway in renal epithelial cells through an EGFR- and c-Src-dependent mechanism. Am J Physiol Renal Physiol 297, F662–670. doi: 10.1152/ajprenal.00146.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ, 1997. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension 29, 320–325. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ, 1999. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol 277, F790–796. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, Maier KG, Greene AS, Cowley AW Jr., Roman RJ, 2002. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 283, R60–68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, Sun C, Harder D, Roman R, 1998. Blockade of 20-HETE formation mimics the vasodilator response to nitric oxide in renal arterioles, Hypertension. LIPPINCOTT WILLIAMS & WILKINS 227; EAST WASHINGTON SQ, PHILADELPHIA, PA 19106 USA, pp. 616–616. [Google Scholar]
- Berg RM, 2016. Myogenic and metabolic feedback in cerebral autoregulation: Putative involvement of arachidonic acid-dependent pathways. Med Hypotheses 92, 12–17. doi: 10.1016/j.mehy.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Cai Y, 2009. Arachidonic acid cytochrome P450 4F2 in hypertension: what can we learn from a transgenic mouse model?? Kidney Int 75, 1253–1254. doi: 10.1038/ki.2009.82. [DOI] [PubMed] [Google Scholar]
- Cambj-Sapunar L, Yu M, Harder DR, Roman RJ, 2003. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke 34, 1269–1275. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW, 1981. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci U S A 78, 5362–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila JH, Nakagawa K, Holla V, 2003. The CYP P450 arachidonate monooxygenases: enzymatic relays for the control of kidney function and blood pressure. Adv Exp Med Biol 525, 39–46. [DOI] [PubMed] [Google Scholar]
- Carver KA, Lourim D, Tryba AK, Harder DR, 2014. Rhythmic expression of cytochrome P450 epoxygenases CYP4×1 and CYP2c11 in the rat brain and vasculature. Am J Physiol Cell Physiol 307, C989–998. doi: 10.1152/ajpcell.00401.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ackerman R, Guo AM, 2012. 20-HETE in neovascularization. Prostaglandins Other Lipid Mediat 98, 63–68. doi: 10.1016/j.prostaglandins.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Chen L, Ackerman R, Saleh M, Gotlinger KH, Kessler M, Mendelowitz LG, Falck JR, Arbab AS, Scicli AG, Schwartzman ML, Yang J, Guo AM, 2014. 20-HETE regulates the angiogenic functions of human endothelial progenitor cells and contributes to angiogenesis in vivo. J Pharmacol Exp Ther 348, 442–451. doi: 10.1124/jpet.113.210120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Edin ML, Hoopes SL, Li H, Bradbury JA, Graves JP, DeGraff LM, Lih FB, Garcia V, Shaik JS, Tomer KB, Flake GP, Falck JR, Lee CR, Poloyac SM, Schwartzman ML, Zeldin DC, 2014. Vascular characterization of mice with endothelial expression of cytochrome P450 4F2. FASEB J 28, 2915–2931. doi: 10.1096/fj.13-241927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Garcia V, Ding Y, Wu CC, Thakar K, Falck JR, Ramu E, Schwartzman ML, 2012. Induction of angiotensin-converting enzyme and activation of the renin-angiotensin system contribute to 20-hydroxyeicosatetraenoic acid-mediated endothelial dysfunction. Arterioscler Thromb Vasc Biol 32, 1917–1924. doi: 10.1161/ATVBAHA.112.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ZM, Croft KD, Kingsbury DA, Falck JR, Reddy KM, Beilin LJ, 2000. Cytochrome P450 metabolites of arachidonic acid may be important mediators in angiotensin II-induced vasoconstriction in the rat mesentery in vivo. Clin Sci (Lond) 98, 277–282. [PubMed] [Google Scholar]
- Collins XH, Harmon SD, Kaduce TL, Berst KB, Fang X, Moore SA, Raju TV, Falck JR, Weintraub NL, Duester G, Plapp BV, Spector AA, 2005. Omega-oxidation of 20-hydroxyeicosatetraenoic acid (20-HETE) in cerebral microvascular smooth muscle and endothelium by alcohol dehydrogenase 4. J Biol Chem 280, 33157–33164. doi: 10.1074/jbc.M504055200. [DOI] [PubMed] [Google Scholar]
- Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA, 2000. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol 279, F544–551. Doi: [DOI] [PubMed] [Google Scholar]
- Dahly-Vernon AJ, Sharma M, McCarthy ET, Savin VJ, Ledbetter SR, Roman RJ, 2005. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension 45, 643–648. doi: 10.1161/01.HYP.0000153791.89776.43. [DOI] [PubMed] [Google Scholar]
- Dordea AC, Vandenwijngaert S, Garcia V, Tainsh RE, Nathan DI, Allen K, Raher MJ, Tainsh LT, Zhang F, Lieb WS, Mikelman S, Kirby A, Stevens C, Thoonen R, Hindle AG, Sips PY, Falck JR, Daly MJ, Brouckaert P, Bloch KD, Bloch DB, Malhotra R, Schwartzman ML, Buys ES, 2016. Androgen-sensitive hypertension associated with soluble guanylate cyclase-alpha1 deficiency is mediated by 20-HETE. Am J Physiol Heart Circ Physiol 310, H1790–1800. doi: 10.1152/ajpheart.00877.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach AW, Smith SV, Kyle PB, Ramaiah M, Amenuke M, Garrett MR, Lirette ST, Griswold ME, Roman RJ, 2014. Urinary CYP eicosanoid excretion correlates with glomerular filtration in African-Americans with chronic kidney disease. Prostaglandins Other Lipid Mediat 113–115, 45–51. doi: 10.1016/j.prostaglandins.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ, 2008. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295, H2455–2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE, 2009. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 58, 1201–1211. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid S, Maalouf R, Jaffa AA, Nassif J, Hamdy A, Rashid A, Ziyadeh FN, Eid AA, 2013. 20-HETE and EETs in diabetic nephropathy: a novel mechanistic pathway. PLoS One 8, e70029. doi: 10.1371/journal.pone.0070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmarakby AA, Faulkner J, Pye C, Rouch K, Alhashim A, Maddipati KR, Baban B, 2013. Role of haem oxygenase in the renoprotective effects of soluble epoxide hydrolase inhibition in diabetic spontaneously hypertensive rats. Clin Sci (Lond) 125, 349–359. doi: 10.1042/CS20130003. [DOI] [PubMed] [Google Scholar]
- Fan F, Chen CC, Zhang J, Schreck CM, Roman EA, Williams JM, Hirata T, Sharma M, Beard DA, Savin VJ, Roman RJ, 2015. a. Fluorescence dilution technique for measurement of albumin reflection coefficient in isolated glomeruli. Am J Physiol Renal Physiol 309, F1049–1059. doi: 10.1152/ajprenal.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, Booz GW, Roman RJ, 2016. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci (Landmark Ed) 21, 1427–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Ge Y, Murphy S, Geurts A, Jacob H, Roman R, 2013. a. CYP4A1 transgenic rats generated using Sleeping Beauty transposon system restores the impaired myogenic responses in the afferent arteriole of Dahl S rats. (Abstract) Hypertens 62, A39. [Google Scholar]
- Fan F, Muroya Y, Roman RJ, 2015. b. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens 24, 37–46. doi: 10.1097/MNH.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Pabbidi MR, Geurts AM, Jacob HJ, Roman RJ, 2014. Upregulation of CYP4A1 gene expression restores the impaired myogenic response and autoregulation of cerebral blood flow in Dahl salt sensitive rats (Abstract). HBPR 64. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Roman RJ, 2017. GPR75 Identified as the First 20-HETE Receptor: A Chemokine Receptor Adopted by a New Family. Circ Res 120, 1696–1698. doi: 10.1161/circresaha.117.311022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Sun CW, Maier KG, Williams JM, Pabbidi MR, Didion SP, Falck JR, Zhuo J, Roman RJ, 2013. b. 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS One 8, e82482. doi: 10.1371/journal.pone.0082482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava C, Montagnana M, Melander O, 2009. Overexpression of cytochrome P450 4F2 in mice increases 20-hydroxyeicosatetraenoic acid production and arterial blood pressure. Kidney Int 76, 913; author reply 913–914. doi: 10.1038/ki.2009.281. [DOI] [PubMed] [Google Scholar]
- Fidelis P, Wilson L, Thomas K, Villalobos M, Oyekan AO, 2010. Renal function and vasomotor activity in mice lacking the Cyp4a14 gene. Exp Biol Med (Maywood) 235, 1365–1374. doi: 10.1258/ebm.2010.009233. [DOI] [PubMed] [Google Scholar]
- Forrester S, Booz G, Sigmund C, Coffman T, Kawai T, Rizzo V, Scalia R, Eguchi S, 2018. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol Rev (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC, Falck JR, Lombard JH, 2000. Contribution of cytochrome P-450 omega-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol 278, H1517–1526. doi: 10.1152/ajpheart.2000.278.5.H1517. [DOI] [PubMed] [Google Scholar]
- Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH, 2001. Altered mechanisms underlying hypoxic dilation of skeletal muscle resistance arteries of hypertensive versus normotensive Dahl rats. Microcirculation 8, 115–127. [PubMed] [Google Scholar]
- Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, Cupples LA, Guo CY, Demissie S, O’Donnell CJ, Brown NJ, Waterman MR, Capdevila JH, 2005. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation 111, 63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- Gainer JV, Lipkowitz MS, Yu C, Waterman MR, Dawson EP, Capdevila JH, Brown NJ, Group AS, 2008. Association of a CYP4A11 variant and blood pressure in black men. J Am Soc Nephrol 19, 1606–1612. doi: 10.1681/ASN.2008010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadhariah MH, Luther JM, Garcia V, Paueksakon P, Zhang MZ, Hayward SW, Love HD, Falck JR, Manthati VL, Imig JD, Schwartzman ML, Zent R, Capdevila JH, Pozzi A, 2015. Hypertension is a major contributor to 20-hydroxyeicosatetraenoic acid-mediated kidney injury in diabetic nephropathy. J Am Soc Nephrol 26, 597–610. doi: 10.1681/ASN.2013090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Koba S, Sinoway L, Li J, 2008. 20-HETE increases renal sympathetic nerve activity via activation of chemically and mechanically sensitive muscle afferents. J Physiol 586, 2581–2591. doi: 10.1113/jphysiol.2008.150730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML, 2017. 20-HETE Signals Through G Protein-Coupled Receptor GPR75 (Gq) to Affect Vascular Function and Trigger Hypertension. Circ Res. doi: 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, Shkolnik B, Milhau L, Falck JR, Schwartzman ML, 2016. 20-HETE Activates the Transcription of Angiotensin-Converting Enzyme via Nuclear Factor-kappaB Translocation and Promoter Binding. J Pharmacol Exp Ther 356, 525–533. doi: 10.1124/jpet.115.229377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, Shkolnik B, Pandey V, Capdevila J, Falck J, Schwartzman M, 2015. 20-SOLA, a Novel Water Soluble 20-HETE Antagonist, Reduces Blood Pressure Through Regulation of Vascular ACE Expression via an IKK Dependent Pathway. FASEB Journal 29, 647–649.. [Google Scholar]
- Ge Y, Murphy SR, Fan F, Williams JM, Falck JR, Liu R, Roman RJ, 2014. Role of 20-HETE in the impaired myogenic and TGF responses of the Af-Art of Dahl salt-sensitive rats. Am J Physiol Renal Physiol 307, F509–515. doi: 10.1152/ajprenal.00273.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR, 2000. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87, 60–65. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR, 1998. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol 507 (Pt 3), 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Ma YH, Imig JD, Harder DR, Roman RJ, 1993. Role of cytochrome P-450 in elevating renal vascular tone in spontaneously hypertensive rats. J Vasc Res 30, 53–60. [DOI] [PubMed] [Google Scholar]
- Goodman AI, Quan S, Yang L, Synghal A, Abraham NG, 2003. Functional expression of human heme oxygenase-1 gene in renal structure of spontaneously hypertensive rats. Exp Biol Med (Maywood) 228, 454–458. [DOI] [PubMed] [Google Scholar]
- Gu RM, Wang WH, 2002. Arachidonic acid inhibits K channels in basolateral membrane of the thick ascending limb. Am J Physiol Renal Physiol 283, F407–414. doi: 10.1152/ajprenal.00002.2002. [DOI] [PubMed] [Google Scholar]
- Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG, 2007. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther 321, 18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- Harrison-Bernard LM, 2009. The renal renin-angiotensin system. Adv Physiol Educ 33, 270–274. doi: 10.1152/advan.00049.2009. [DOI] [PubMed] [Google Scholar]
- Heng YM, Kuo CS, Jones PS, Savory R, Schulz RM, Tomlinson SR, Gray TJ, Bell DR, 1997. A novel murine P-450 gene, Cyp4a14, is part of a cluster of Cyp4a and Cyp4b, but not of CYP4F, genes in mouse and humans. Biochem J 325 (Pt 3), 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E, Fitzpatrick F, Murphy RC, 1992. Biological activity and metabolism of 20-hydroxyeicosatetraenoic acid in the human platelet. Br J Pharmacol 106, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirani V, Yarovoy A, Kozeska A, Magnusson RP, Lasker JM, 2008. Expression of CYP4F2 in human liver and kidney: assessment using targeted peptide antibodies. Arch Biochem Biophys 478, 59–68. doi: 10.1016/j.abb.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla VR, Adas F, Imig JD, Zhao X, Price E Jr., Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH, 2001. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A 98, 5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeck H, Gross V, Erdmann B, Kargel E, Neunaber R, Milia AF, Schneider W, Luft FC, Schunck WH, 2000. Cytochrome P450-dependent renal arachidonic acid metabolism in desoxycorticosterone acetate-salt hypertensive mice. Hypertension 36, 610–616. [DOI] [PubMed] [Google Scholar]
- Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC, 2015. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat 120, 9–16. doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Shen YH, Li C, Wang F, Zhang C, Bu P, Zhang Y, 2010. PPARalpha agonist fenofibrate protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress and MAPK activity. Biochem Biophys Res Commun 394, 653–659. doi: 10.1016/j.bbrc.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Ignatov A, Robert J, Gregory-Evans C, Schaller HC, 2006. RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75. Br J Pharmacol 149, 490–497. doi: 10.1038/sj.bjp.0706909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig JD, Falck JR, Gebremedhin D, Harder DR, Roman RJ, 1993. Elevated renovascular tone in young spontaneously hypertensive rats. Role of cytochrome P-450. Hypertension 22, 357–364. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Ito O, Omata K, Ito S, 2004. [Role of androgens in the renal production of 20-hydroxyeicosatetraenoic acid in spontaneously hypertensive rats]. Nihon Jinzo Gakkai Shi 46, 685–692. [PubMed] [Google Scholar]
- Ito O, Roman RJ, 1999. Regulation of P-450 4A activity in the glomerulus of the rat. Am J Physiol 276, R1749–1757. [DOI] [PubMed] [Google Scholar]
- Iwai N, Inagami T, 1991. Isolation of preferentially expressed genes in the kidneys of hypertensive rats. Hypertension 17, 161–169. [DOI] [PubMed] [Google Scholar]
- Jarrar YB, Cha EY, Seo KA, Ghim JL, Kim HJ, Kim DH, Lee SJ, Shin JG, 2014. Determination of major UDP-glucuronosyltransferase enzymes and their genotypes responsible for 20-HETE glucuronidation. J Lipid Res 55, 2334–2342. doi: 10.1194/jlr.M051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly E, Seqqat R, Flamion B, Caron N, Michel A, Imig JD, Kramp R, 2006. Increased renal vascular reactivity to ANG II after unilateral nephrectomy in the rat involves 20-HETE. Am J Physiol Regul Integr Comp Physiol 291, R977–986. doi: 00401.2005 [pii] 10.1152/ajpregu.00401.2005. [DOI] [PubMed] [Google Scholar]
- Kaduce TL, Fang X, Harmon SD, Oltman CL, Dellsperger KC, Teesch LM, Gopal VR, Falck JR, Campbell WB, Weintraub NL, Spector AA, 2004. 20-hydroxyeicosatetraenoic acid (20-HETE) metabolism in coronary endothelial cells. J Biol Chem 279, 2648–2656. doi: 10.1074/jbc.M306849200. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Kusunose E, Thompson CM, Strobel HW, 1997. Protein expression, characterization, and regulation of CYP4F4 and CYP4F5 cloned from rat brain. Arch Biochem Biophys 347, 148–154. doi: 10.1006/abbi.1997.0342. [DOI] [PubMed] [Google Scholar]
- Kikuta Y, Kusunose E, Ito M, Kusunose M, 1999. Purification and characterization of recombinant rat hepatic CYP4F1. Arch Biochem Biophys 369, 193–196. doi: 10.1006/abbi.1999.1271. [DOI] [PubMed] [Google Scholar]
- Knickle LC, Bend JR, 1992. Dose-dependent, mechanism-based inactivation of cytochrome P450 monooxygenases in vivo by 1-aminobenzotriazole in liver, lung, and kidney of untreated, phenobarbital-treated, and beta-naphthoflavone-treated guinea pigs. Can J Physiol Pharmacol 70, 1610–1617. [DOI] [PubMed] [Google Scholar]
- Kroetz DL, Huse LM, Thuresson A, Grillo MP, 1997. Developmentally regulated expression of the CYP4A genes in the spontaneously hypertensive rat kidney. Mol Pharmacol 52, 362–372. [DOI] [PubMed] [Google Scholar]
- Laffer CL, Gainer JV, Waterman MR, Capdevila JH, Laniado-Schwartzman M, Nasjletti A, Brown NJ, Elijovich F, 2008. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension 51, 767–772. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantelme P, Lo M, Luttenauer L, Sassard J, 1997. Pivotal role of the renin-angiotensin system in Lyon hypertensive rats. Am J Physiol 273, R1793–1799. [DOI] [PubMed] [Google Scholar]
- Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK, 2000. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem 275, 4118–4126. [DOI] [PubMed] [Google Scholar]
- Lima R, Yanes LL, Davis DD, Reckelhoff JF, 2013. Roles played by 20-HETE, angiotensin II and endothelin in mediating the hypertension in aging female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 304, R248–251. doi: 10.1152/ajpregu.00380.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Hassan Z, Amisten S, King AJ, Bowe JE, Huang GC, Jones PM, Persaud SJ, 2013. The novel chemokine receptor, G-protein-coupled receptor 75, is expressed by islets and is coupled to stimulation of insulin secretion and improved glucose homeostasis. Diabetologia 56, 2467–2476. doi: 10.1007/s00125-013-3022-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhao Y, Nie D, Shi J, Fu L, Li Y, Yu D, Lu J, 2008. Association of a functional cytochrome P450 4F2 haplotype with urinary 20-HETE and hypertension. J Am Soc Nephrol 19, 714–721. doi: 10.1681/ASN.2007060713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhao YY, Gong W, Shi JP, Fu LY, Wang J, Li GY, Lu JY, 2006. [Correlation analysis and identification of G421C in regulatory region of CYP4F2 gene with essential hypertension]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 28, 143–147. [PubMed] [Google Scholar]
- Liu X, Zhao Y, Wang L, Yang X, Zheng Z, Zhang Y, Chen F, Liu H, 2009. Overexpression of cytochrome P450 4F2 in mice increases 20-hydroxyeicosatetraenoic acid production and arterial blood pressure. Kidney Int 75, 1288–1296. doi: 10.1038/ki.2009.67. [DOI] [PubMed] [Google Scholar]
- Maier KG, Henderson L, Narayanan J, Alonso-Galicia M, Falck JR, Roman RJ, 2000. Fluorescent HPLC assay for 20-HETE and other P-450 metabolites of arachidonic acid. Am J Physiol Heart Circ Physiol 279, H863–871. [DOI] [PubMed] [Google Scholar]
- Mathews JM, Dostal LA, Bend JR, 1985. Inactivation of rabbit pulmonary cytochrome P-450 in microsomes and isolated perfused lungs by the suicide substrate 1-aminobenzotriazole. J Pharmacol Exp Ther 235, 186–190. [PubMed] [Google Scholar]
- Mayer B, Lieb W, Gotz A, Konig IR, Aherrahrou Z, Thiemig A, Holmer S, Hengstenberg C, Doering A, Loewel H, Hense HW, Schunkert H, Erdmann J, 2005. Association of the T8590C polymorphism of CYP4A11 with hypertension in the MONICA Augsburg echocardiographic substudy. Hypertension 46, 766–771. doi: 10.1161/01.HYP.0000182658.04299.15. [DOI] [PubMed] [Google Scholar]
- McCarthy ET, Sharma R, Sharma M, 2005. Protective effect of 20-hydroxyeicosatetraenoic acid (20-HETE) on glomerular protein permeability barrier. Kidney Int 67, 152–156. doi: 10.1111/j.1523-1755.2005.00065.x. [DOI] [PubMed] [Google Scholar]
- McCarthy ET, Zhou J, Eckert R, Genochio D, Sharma R, Oni O, De A, Srivastava T, Sharma R, Savin VJ, Sharma M, 2015. Ethanol at low concentrations protects glomerular podocytes through alcohol dehydrogenase and 20-HETE. Prostaglandins Other Lipid Mediat 116–117, 88–98. doi: 10.1016/j.prostaglandins.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGiff JC, Quilley J, 1999. 20-HETE and the kidney: resolution of old problems and new beginnings. Am J Physiol 277, R607–623. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Deighan C, Briones AM, Shafaroudi MM, McBride M, Adler J, Arribas SM, Vila E, Daly CJ, 2005. New aspects of vascular remodelling: the involvement of all vascular cell types. Exp Physiol 90, 469–475. doi: 10.1113/expphysiol.2005.030130. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK, 2007. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292, C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Messer-Letienne I, Bernard N, Roman RJ, Sassard J, Benzoni D, 1999. 20-Hydroxyeicosatetraenoic acid and renal function in Lyon hypertensive rats. Eur J Pharmacol 378, 291–297. [DOI] [PubMed] [Google Scholar]
- Miyata N, Roman RJ, 2005. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res 41, 175–193. [DOI] [PubMed] [Google Scholar]
- Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K, 2001. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol 133, 325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno PR, Purushothaman KR, Sirol M, Levy AP, Fuster V, 2006. Neovascularization in human atherosclerosis. Circulation 113, 2245–2252. doi: 10.1161/CIRCULATIONAHA.105.578955. [DOI] [PubMed] [Google Scholar]
- Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH, 2007. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J 403, 109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Fan F, Baker R, Guerts A, Jacob H, Roman R, 2012. Upregulation of renal medullary 20-HETE production opposes the development of hypertension in Sleeping Beauty Transposon CYP4A1 transgenic Dahl S rats. The FASEB Journal 26, 1103–1113.. [Google Scholar]
- Murphy S, Fan F, Baker R, Roman R, 2013. Increases in renal medullary 20-HETE formation oppose the development of hypertension and improves pressure natriuresis in CYP4A1 transgenic Dahl S rats. The FASEB Journal 27, 1115.1113–1115.1113. doi: [Google Scholar]
- Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU, 1998. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci U S A 95, 12701–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Obara K, Tanabe Y, Ishikawa T, 2003. 20-Hydroxyeicosatetraenoic acid potentiates contractile activation of canine basilar artery in response to stretch via protein kinase Calpha-mediated inhibition of calcium-activated potassium channel. Adv Exp Med Biol 538, 411–416; discussion 416. [DOI] [PubMed] [Google Scholar]
- Nguyen X, Wang MH, Reddy KM, Falck JR, Schwartzman ML, 1999. Kinetic profile of the rat CYP4A isoforms: arachidonic acid metabolism and isoform-specific inhibitors. Am J Physiol 276, R1691–1700. [DOI] [PubMed] [Google Scholar]
- Omata K, Abraham NG, Escalante B, Schwartzman ML, 1992. a. Age-related changes in renal cytochrome P-450 arachidonic acid metabolism in spontaneously hypertensive rats. Am J Physiol 262, F8–16. [DOI] [PubMed] [Google Scholar]
- Omata K, Abraham NG, Schwartzman ML, 1992. b. Renal cytochrome P-450-arachidonic acid metabolism: localization and hormonal regulation in SHR. Am J Physiol 262, F591–599. [DOI] [PubMed] [Google Scholar]
- Orozco LD, Liu H, Perkins E, Johnson DA, Chen BB, Fan F, Baker RC, Roman RJ, 2013. 20-Hydroxyeicosatetraenoic acid inhibition attenuates balloon injury-induced neointima formation and vascular remodeling in rat carotid arteries. J Pharmacol Exp Ther 346, 67–74. doi: 10.1124/jpet.113.203844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyekan AO, McAward K, Conetta J, Rosenfeld L, McGiff JC, 1999. Endothelin-1 and CYP450 arachidonate metabolites interact to promote tissue injury in DOCA-salt hypertension. Am J Physiol 276, R766–775. [DOI] [PubMed] [Google Scholar]
- Pandey V, Garcia V, Gilani A, Mishra P, Zhang FF, Paudyal MP, Falck JR, Nasjletti A, Wang WH, Schwartzman ML, 2017. The Blood Pressure-Lowering Effect of 20-HETE Blockade in Cyp4a14(−/−) Mice Is Associated with Natriuresis. J Pharmacol Exp Ther 363, 412–418. doi: 10.1124/jpet.117.243618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park F, Sweeney WE Jr., Jia G, Akbulut T, Mueller B, Falck JR, Birudaraju S, Roman RJ, Avner ED, 2009. Chronic blockade of 20-HETE synthesis reduces polycystic kidney disease in an orthologous rat model of ARPKD. Am J Physiol Renal Physiol 296, F575–582. doi: 10.1152/ajprenal.90705.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KM, Chen A, Bonventre JV, 2001. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem 276, 11870–11876. doi: 10.1074/jbc.M007518200. [DOI] [PubMed] [Google Scholar]
- Parmentier JH, Muthalif MM, Nishimoto AT, Malik KU, 2001. a. 20-Hydroxyeicosatetraenoic acid mediates angiotensin ii-induced phospholipase d activation in vascular smooth muscle cells. Hypertension 37, 623–629. [DOI] [PubMed] [Google Scholar]
- Parmentier JH, Muthalif MM, Saeed AE, Malik KU, 2001. b. Phospholipase D activation by norepinephrine is mediated by 12(s)-, 15(s)-, and 20-hydroxyeicosatetraenoic acids generated by stimulation of cytosolic phospholipase a2. tyrosine phosphorylation of phospholipase d2 in response to norepinephrine. J Biol Chem 276, 15704–15711. doi: 10.1074/jbc.M011473200 M011473200 [pii]. [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R, 2006. Physiology of local renin-angiotensin systems. Physiol Rev 86, 747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Picariello C, Lazzeri C, Attana P, Chiostri M, Gensini GF, Valente S, 2011. The impact of hypertension on patients with acute coronary syndromes. Int J Hypertens 2011, 563657. doi: 10.4061/2011/563657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell PK, Wolf I, Jin R, Lasker JM, 1998. Metabolism of arachidonic acid to 20-hydroxy-5,8,11, 14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. J Pharmacol Exp Ther 285, 1327–1336. [PubMed] [Google Scholar]
- Regner KR, Zuk A, Van Why SK, Shames BD, Ryan RP, Falck JR, Manthati VL, McMullen ME, Ledbetter SR, Roman RJ, 2009. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int 75, 511–517. doi: 10.1038/ki.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, D’Ambrosio MA, Garvin JL, Peterson EL, Carretero OA, 2014. Mechanism of impaired afferent arteriole myogenic response in Dahl salt-sensitive rats: role of 20-HETE. Am J Physiol Renal Physiol 307, F533–538. doi: 10.1152/ajprenal.00283.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renic M, Kumar SN, Gebremedhin D, Florence MA, Gerges NZ, Falck JR, Harder DR, Roman RJ, 2012. Protective effect of 20-HETE inhibition in a model of oxygen-glucose deprivation in hippocampal slice cultures. Am J Physiol Heart Circ Physiol 302, H1285–1293. doi: 10.1152/ajpheart.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renna NF, de Las Heras N, Miatello RM, 2013. Pathophysiology of vascular remodeling in hypertension. Int J Hypertens 2013, 808353. doi: 10.1155/2013/808353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ, 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82, 131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Roman RJ, Falck JR, Alonso-Galicia M, Jacobs ER, Harder DR, 2003. 20-HETE antagonists and agonists. US: Patent 6,605,641 B2.
- Roman RJ, Fan F, Zhuo JL, 2016. Intrarenal Renin-Angiotensin System: Locally Synthesized or Taken up Via Endocytosis? Hypertension 67, 831–833. doi: 10.1161/HYPERTENSIONAHA.116.07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman RJ, Renic M, Dunn KM, Takeuchi K, Hacein-Bey L, 2006. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurol res 28, 738–749. [DOI] [PubMed] [Google Scholar]
- Rosolowsky M, Falck JR, Campbell WB, 1996. Metabolism of arachidonic acid by canine polymorphonuclear leukocytes synthesis of lipoxygenase and omega-oxidized metabolites. Biochim Biophys Acta 1300, 143–150. [DOI] [PubMed] [Google Scholar]
- Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML, 1989. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science 243, 388–390. [DOI] [PubMed] [Google Scholar]
- Savas U, Wei S, Hsu MH, Falck JR, Guengerich FP, Capdevila JH, Johnson EF, 2016. 20-Hydroxyeicosatetraenoic Acid (HETE)-dependent Hypertension in Human Cytochrome P450 (CYP) 4A11 Transgenic Mice: NORMALIZATION OF BLOOD PRESSURE BY SODIUM RESTRICTION, HYDROCHLOROTHIAZIDE, OR BLOCKADE OF THE TYPE 1 ANGIOTENSIN II RECEPTOR. J Biol Chem 291, 16904–16919. doi: 10.1074/jbc.M116.732297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman ML, da Silva JL, Lin F, Nishimura M, Abraham NG, 1996. Cytochrome P450 4A expression and arachidonic acid omega-hydroxylation in the kidney of the spontaneously hypertensive rat. Nephron 73, 652–663. [DOI] [PubMed] [Google Scholar]
- Shatara RK, Quest DW, Wilson TW, 2000. Fenofibrate lowers blood pressure in two genetic models of hypertension. Can J Physiol Pharmacol 78, 367–371. [PubMed] [Google Scholar]
- Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML, 2007. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension 50, 123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- Singh H, Schwartzman ML, 2008. Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep 60, 29–37. [PubMed] [Google Scholar]
- Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML, 2010CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension 56, 871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec DE, Gannon KP, Beaird JS, Drummond HA, 2007. a. 20-Hydroxyeicosatetraenoic acid (20-HETE) stimulates migration of vascular smooth muscle cells. Cell Physiol Biochem 19, 121–128. doi: 10.1159/000099200. [DOI] [PubMed] [Google Scholar]
- Stec DE, Roman RJ, Flasch A, Rieder MJ, 2007. b. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics 30, 74–81. doi: 10.1152/physiolgenomics.00003.2007. [DOI] [PubMed] [Google Scholar]
- Sun CW, Falck JR, Harder DR, Roman RJ, 1999. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension 33, 414–418. [DOI] [PubMed] [Google Scholar]
- Sun Z, 2014. Atherosclerosis and atheroma plaque rupture: normal anatomy of vasa vasorum and their role associated with atherosclerosis. ScientificWorldJournal 2014, 285058. doi: 10.1155/2014/285058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Csiszar A, Sosnowska D, Tucsek Z, Cseplo P, Springo Z, Tarantini S, Sonntag WE, Ungvari Z, Koller A, 2013. Treatment with the cytochrome P450 omega-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats. Br J Pharmacol 168, 1878–1888. doi: 10.1111/bph.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera T, Taylor M, Bohman Q, Flasch A, Roman RJ, Stec DE, 2005. Fenofibrate prevents the development of angiotensin II-dependent hypertension in mice. Hypertension 45, 730–735. [DOI] [PubMed] [Google Scholar]
- Walkowska A, Kuczeriszka M, Sadowski J, Olszynski KH, Dobrowolski L, Cervenka L, Hammock BD, Kompanowska-Jezierska E, 2015. High salt intake increases blood pressure in normal rats: putative role of 20-HETE and no evidence on changes in renal vascular reactivity. Kidney Blood Press Res 40, 323–334. doi: 10.1159/000368508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML, 1998. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther 284, 966–973. [PubMed] [Google Scholar]
- Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, Croft KD, 2008. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension 51, 1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]
- Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, Roman RJ, 2012. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 302, R1209–1218. doi: 10.1152/ajpregu.00604.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Murphy S, Burke M, Roman RJ, 2010. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 56, 336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Sarkis A, Hoagland KM, Fredrich K, Ryan RP, Moreno C, Lopez B, Lazar J, Fenoy FJ, Sharma M, Garrett MR, Jacob HJ, Roman RJ, 2008. Transfer of the CYP4A region of chromosome 5 from Lewis to Dahl S rats attenuates renal injury. Am J Physiol Renal Physiol 295, F1764–1777. doi: 10.1152/ajprenal.90525.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Sarkis A, Lopez B, Ryan RP, Flasch AK, Roman RJ, 2007. a. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension 49, 687–694. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- Williams JM, Sharma M, Anjaiahh S, Falck JR, Roman RJ, 2007b. Role of endogenous CYP450 metabolites of arachidonic acid in maintaining the glomerular protein permeability barrier. Am J Physiol Renal Physiol 293, F501–505. doi: 00131.2007 [pii] 10.1152/ajprenal.00131.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JS, Hopkins PN, Jeunemaitre X, Brown NJ, 2011. CYP4A11 T8590C polymorphism, salt-sensitive hypertension, and renal blood flow. J Hypertens 29, 1913–1918. doi: 10.1097/HJH.0b013e32834aa786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, Zhang F, Gotlinger K, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML, 2013. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12–20-HETE synthase. J Am Soc Nephrol 24, 1288–1296. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Schwartzman ML, 2011. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat 96, 45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Falck JR, Ortiz de Montellano PR, Kroetz DL, 2004. Catalytic activity and isoform-specific inhibition of rat cytochrome p450 4F enzymes. J Pharmacol Exp Ther 308, 887–895. doi: 10.1124/jpet.103.059626. [DOI] [PubMed] [Google Scholar]
- Yanes LL, Lima R, Moulana M, Romero DG, Yuan K, Ryan MJ, Baker R, Zhang H, Fan F, Davis DD, 2011. Postmenopausal hypertension: role of 20-HETE. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 300, R1543–R1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N, Ishimoto T, Reddy LM, Falck JR, Gebremedhin D, Harder DR, Roman RJ, 2004. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol 486, 297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Yu M, Lopez B, Dos Santos EA, Falck JR, Roman RJ, 2007. Effects of 20-HETE on Na+ transport and Na+-K+-ATPase activity in the thick ascending loop of Henle. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 292, R2400–R2405. [DOI] [PubMed] [Google Scholar]
- Zhang F, Chen CL, Qian JQ, Yan JT, Cianflone K, Xiao X, Wang DW, 2005. Long-term modifications of blood pressure in normotensive and spontaneously hypertensive rats by gene delivery of rAAV-mediated cytochrome P450 arachidonic acid hydroxylase. Cell Res 15, 717–724. doi: 10.1038/sj.cr.7290341. [DOI] [PubMed] [Google Scholar]
- Zou AP, Ma YH, Sui ZH, Ortiz de Montellano PR, Clark JE, Masters BS, Roman RJ, 1994. Effects of 17-octadecynoic acid, a suicide-substrate inhibitor of cytochrome P450 fatty acid omega-hydroxylase, on renal function in rats. J Pharmacol Exp Ther 268, 474–481. [PubMed] [Google Scholar]


