Abstract
Background
Moderate coffee consumption has been suggested to be associated with lower risk of chronic conditions such as diabetes, a major precursor to chronic kidney disease (CKD). However, the association between coffee and CKD has not been fully established.
Study Design
Prospective cohort study.
Setting and Participants
14,209 participants aged 45–64 years from the Atherosclerosis Risk in Communities (ARIC) Study.
Predictors
Coffee consumption (cups/day) was assessed at visit 1 (1987–89) and visit 3 (1993–95) using food frequency questionnaires.
Outcomes
Incident CKD defined as eGFR <60 mL/min/1.73 m2 accompanied by ≥25% eGFR decline, CKD-related hospitalization or death, or end-stage renal disease.
Results
There were 3,845 cases of incident CKD over a median of 24 years of follow-up. Men, whites, current smokers, and participants without comorbidities were more likely to consume higher amounts of coffee per day. After adjustment for demographic, clinical, and dietary factors, higher categories of coffee consumption were associated with lower risk of incident CKD compared to those who never consume coffee [hazard ratio (HR) for <1 cup/day, 0.90 (95% CI, 0.82–0.99); for 1 to <2 cups/day, 0.90 (95% CI, 0.82–0.99); for 2 to <3 cups/day, 0.87 (95% CI, 0.77–0.97); and for ≥3 cups/day, 0.84 (95% CI, 0.75–0.94)]. In continuous analysis, for each additional cup of coffee consumed per day, the risk of incident CKD was lower by 3% (HR, 0.97; 95% CI, 0.95–0.99; p<0.001).
Limitations
Self-reported coffee consumption and observational design.
Conclusions
Participants who drank higher amounts of coffee had a lower risk of incident CKD after adjusting for covariates. Coffee consumers may not be at adverse risk for kidney disease.
Index words: coffee, beverages, diet, chronic kidney disease (CKD), incident CKD, renal failure, modifiable risk factor
Introduction
Coffee is one of the most frequently consumed beverages in the U.S., with about 75% of the U.S. population aged 20 and older reported to be coffee drinkers (1). According to the 2015–2020 Dietary Guidelines for Americans, moderate coffee consumption (3–5 cups per day or 400 mg/d of caffeine) is not associated with long-term health risks such as cancer or cardiovascular disease and therefore can be incorporated into healthy eating styles (2). Coffee consumption has been shown to be protective against multiple chronic conditions such as diabetes (3, 4), coronary heart disease (5), cancer (6, 7), and all-cause mortality (8–11).
Sparse literature exists as to whether coffee consumption is associated with chronic kidney disease (CKD), a disease with growing prevalence and costs (12). A recent meta-analysis identified 4 cohort studies that found no significant association between coffee and CKD, but the pooled results suggested a potential inverse association among women (13). These four prior studies were conducted in Italy, Japan, and Korea; few prior studies have examined a longitudinal association between coffee and CKD in the U.S.
Our study seeks to examine the risk of incident CKD across different levels of coffee consumption in the U.S., using data from the community-based Atherosclerosis Risk in Communities (ARIC) study.
Methods
Study Population
The ARIC study is a community-based cohort of 15,792 middle-aged (45–64 years), predominantly black and white men and women (14). Study participants were recruited and enrolled in 1987–1989 from 4 U.S. communities: Forsyth County, North Carolina; Jackson Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Participants attended follow up visits in 1990–1992 (visit 2), 1993–1995 (visit 3), 1996–1998 (visit 4), and 2011–2013 (visit 5). An ethics committee at each site approved the study protocol, and study participants provided informed consent.
Due to small numbers, we excluded participants who were not black or white (n=48), blacks from Washington County, Maryland (n=33), and blacks from Minneapolis, Minnesota (n=22). We additionally excluded those who had prevalent CKD [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2] or missing serum creatinine (n=353), were not fasting or were missing glucose measurement (n=527), or missing BMI (n=10). We further excluded those who were missing data needed to calculate a DASH (Dietary Approaches to Stop Hypertension) diet score (n=435), missing coffee consumption data (n=28), missing alcohol status (n=34), or had implausible levels of total energy intake (<500 or >3,500 kcal/d for women; <700 or >4,500 kcal/d for men) (n=93). A total of 14,209 ARIC study participants were included in our analysis.
Assessment of Coffee Consumption
Coffee consumption was assessed via a 66-item semi-quantitative food frequency questionnaire (FFQ), which was administered in person by a trained interviewer at visit 1 (1987–89) and visit 3 (1993–95). Participants were asked to report how frequently they consumed an 8-ounce cup of regular (non-decaffeinated) coffee on average over the past year. Frequency options included: almost never, 1–3 cups per month, 1 cup per week, 2–4 cups per week, 5–6 cups per week, 1 cup per day, 2–3 cups per day, 4–6 cups per day, and >6 cups per day. From visit 1 to visit 3, 44% of participants did not change their response in the FFQ about coffee consumption and 25% changed their response by 1 category. These categorical responses were converted into cups per week as a continuous variable (e.g. for category 2–4 cups per week: 3 cups per week) (15). We used a cumulative approach to incorporate data from both visits 1 and 3 (15). For participants who remained event-free at visit 3, we used the mean coffee consumption of visits 1 and 3 for a more precise estimate. For participants who developed incident CKD between visit 1 and visit 3 or did not have visit 3 coffee data, we used their coffee consumption from visit 1 in order to capture the amount of coffee they drank prior to their CKD event. After taking into account cumulative consumption from visit 1 and 3 coffee intake, the continuous variable was converted into cups per week and re-categorized into 5 new groups: never, <1 cup/day, ≥1 to <2 cup/day, ≥2 to <3 cups/day, and ≥3 cups/day. Participants who responded “almost never” for both FFQs were categorized into the group labeled “never.”
Outcome Assessment
Incident CKD was defined as at least one of the following criteria: 1) eGFR <60 mL/min/1.73 m2 accompanied by ≥25% eGFR decline at any subsequent study visit relative to baseline, 2) International Classification of Diseases (ICD)-9/10 code for a hospitalization related to CKD stage 3+, 3) ICD-9/10 code for a death related to CKD stage 3+ identified through linkage to the National Death Index, 4) end-stage renal disease (ESRD) identified by linkage to the US Renal Data System (USRDS) registry (16). For ICD-9/10 codes, any diagnosis (primary, secondary, etc.) was included. eGFR was calculated with the 2009 CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation using serum creatinine measured using the modified kinetic Jaffé method (17–19). The competing events for this outcome included death due to malignant neoplasms in the lung, chronic obstructive pulmonary disease, acute myocardial infarction, and atherosclerotic heart disease.
As a sensitivity analysis for this composite definition of incident CKD, we defined incident CKD using visit-based measures only (eGFR<60 mL/min/1.73 m2 and ≥30% eGFR decline). The competing events were the same as for the composite definition of incident CKD.
We also assessed the development of advanced kidney disease using a secondary outcome of ESRD. Cases were identified by the USRDS registry, representing cases of kidney transplant and dialysis. Kidney failure was used as a sensitivity analysis for the ESRD definition (20), defined as: 1) ESRD, 2) ICD-9-CM and ICD-10-CM codes from hospitalizations and deaths that represented kidney failure, transplantation, and dialysis, 3) a study visit eGFR<15 mL/min/1.73 m2 (19). Any mention of the ICD-9/10 codes anywhere on the forms were included.
Assessment of Covariates
In our analysis, we adjusted for demographic, study design, lifestyle, and clinical factors that might influence coffee consumption and kidney outcomes. Information on age, sex, race, study center, education level, smoking status, physical activity, and alcohol status were assessed at baseline using an interviewer-administered questionnaire (14). Race and study center were combined into one variable given the non-uniform distribution of racial groups across study centers. Physical activity index score was calculated based on intensity and time dedicated to sport and non-sport exercise during leisure time. Possible values for this score ranged from 1 (lowest physical activity) to 5 (highest physical activity) (14). Alcohol status was categorized into never, former, current (moderate), and current (heavy). Moderate alcohol drinkers consisted of women who drank <1 drink/day and men who drank <2 drinks/day. Heavy alcohol drinkers consisted of women who drank ≥1 drink/day and men who drank ≥2 drinks/day. To capture diet quality, we used a DASH diet score based on: low intake of red and processed meat, sweetened beverages, and sodium as well as high intake of fruits, vegetables, whole grains, nuts and legumes, and low-fat dairy (21). Each component was scored from 1 to 5 based on ranked distribution in quintiles.
Clinical factors included systolic and diastolic blood pressure, fasting glucose, eGFR, diabetes, hypertension, and use of antihypertensive medication. Diabetes was defined at baseline as fasting blood glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, self-reported history of physician-diagnosed diabetes, or use of diabetes medication in the preceding 2 weeks. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication in the past 2 weeks.
Cumulative energy intake was determined by calculating the mean energy intake of visits 1 and 3 for participants who developed the outcome after visit 3 and for those who did not develop the outcome but had information for both visits. We used only visit 1 energy intake for participants who had an incident CKD event between visits 1 and 3 or were missing visit 3 data.
Statistical Analysis
We reported demographic risk factors and clinical risk factors according to different categories of coffee consumption. We estimated hazard ratios (HR) and associated 95% confidence intervals (CI) for incident CKD and secondary outcomes with different categories of coffee intake using cause-specific hazards models (22). Time to event accumulated from visit 1 (1987–1989) until end of follow-up (December 31, 2013). We evaluated three models: Model 1 included age, sex, race-center, education, and total energy intake; Model 2 additionally included physical activity, smoking, alcohol status, and DASH diet score; and Model 3 included all variables in model 2 plus systolic blood pressure, diabetes status, antihypertensive medication use, and baseline eGFR. We calculated p-values for trend across categories using the median value of each category of coffee consumption.
We also collapsed the coffee consumption categories to perform an analysis comparing participants who reported never drinking coffee to participants who reported drinking any coffee. To express the risk of CKD per additional cup of coffee, we analyzed coffee consumption using a continuous variable (cups/day).
We tested interaction terms between potential effect modifiers (sex, smoking, diabetes, race, physical activity, and DASH diet) and coffee consumption on incident CKD using the likelihood ratio test. We assessed the proportionality assumption in cause-specific models on the basis of Schoenfeld residuals.
All p-values were 2-tailed, and statistical significance was set a priori at P<0.05. All analyses were performed using Stata software (version 12.0; StataCorp, College Station, TX, USA).
Results
Baseline Characteristics of Participants
Of the 14,209 participants included in our study, 19% of the population almost never drank coffee, 21% drank <1 cup/d, 25% drank ≥1 to <2 cups/d, 15% drank ≥2 to <3 cups/d, and 19% drank ≥3 cups/d. Men, whites, and current smokers were more likely to consume higher amounts of coffee (Table 1). About 36% of the never drinkers were black compared to 7% of the ≥3 cups/d group. Participants with lower BMI, lower blood pressure, and without diabetes or hypertension also consumed more coffee. Among participants who drank at least 3 cups of coffee/day, baseline eGFR was slightly lower by about 3 mL/min/1.73 m2 compared to participants who never drank coffee. Serum magnesium slightly increased across coffee consumption categories.
Table 1.
Baseline Characteristics According to Daily Coffee Consumption
| Coffee Consumption Levela | ||||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | Never (n=2,758) | <1 cup/d (n=3,024) | ≥1 to <2 cups/d (n=3,578) | ≥2 to <3 cups/d (n=2,102) | ≥3 cups/d (n=2,747) | pd |
| Age, years | 53.9 ± 6 | 54.3 ± 6 | 54.6 ± 6 | 54.1 ± 6 | 53.5 ± 6 | 0.1 |
| Female, % | 63.6 | 56.9 | 56.2 | 50.9 | 49.1 | <0.001 |
| Race, % | <0.001 | |||||
| White | 63.6 | 69.5 | 70.4 | 84.0 | 92.9 | |
| Black | 36.4 | 30.5 | 29.7 | 16.0 | 7.1 | |
| Education level, % | <0.001 | |||||
| Less than high school | 24.9 | 22.1 | 26.3 | 21.0 | 18.2 | |
| High school or equivalent | 40.2 | 40.6 | 39.9 | 41.7 | 44.7 | |
| College or above | 34.9 | 37.3 | 33.9 | 37.4 | 37.2 | |
| Center, % | <0.001 | |||||
| Forsyth County, North Carolina | 22.1 | 25.9 | 28.0 | 29.9 | 23.9 | |
| Jackson, Mississippi | 31.8 | 26.3 | 27.4 | 14.4 | 6.2 | |
| Minneapolis, Minnesota | 19.7 | 23.9 | 19.4 | 27.7 | 43.0 | |
| Washington County, Maryland | 26.4 | 23.9 | 25.2 | 28.1 | 27.0 | |
| Smoking status, % | <0.001 | |||||
| Never smoker | 55.3 | 50.9 | 42.1 | 35.3 | 23.4 | |
| Former smoker | 26.0 | 30.9 | 34.7 | 35.8 | 34.8 | |
| Current smoker | 18.7 | 18.2 | 23.2 | 28.9 | 41.8 | |
| Physical activity index scoreb (1–5) | 2.4 ± 0.8 | 2.4 ± 0.8 | 2.4 ± 0.8 | 2.5 ± 0.8 | 2.5 ± 0.8 | 0.3 |
| BMI, kg/m2 | 28.2 ± 6 | 28.0 ± 5 | 27.8 ± 5 | 27.1 ± 5 | 26.7 ± 5 | <0.001 |
| Systolic BP, mmHg | 123.4 ± 20 | 121.5 ± 18 | 122.3 ± 19 | 119.4 ± 18 | 116.8 ± 17 | <0.001 |
| Diastolic BP, mmHg | 75.0 ± 12 | 74.4 ± 11 | 74.0 ± 11 | 72.7 ± 11 | 71.3 ± 11 | <0.001 |
| Diabetes, % | 13.4 | 11.4 | 11.0 | 8.9 | 6.1 | <0.001 |
| Hypertension, % | 40.2 | 37.3 | 36.6 | 30.2 | 21.7 | <0.001 |
| Antihypertensive medication, % | 34.8 | 33.3 | 32.3 | 26.7 | 18.8 | <0.001 |
| ACE inhibitors, % | 3.6 | 2.8 | 3.1 | 2.8 | 2.1 | 0.01 |
| Fasting glucose, mg/dL | 109.6 ± 40 | 106.8 ± 32 | 107.6 ± 35 | 105.7 ± 33 | 102.9 ± 26 | <0.001 |
| Baseline eGFR, mL/min/1.73 m2 | 104.5 ± 16 | 103.5 ± 15 | 103.3 ± 15 | 101.8 ± 13 | 101.9 ± 12 | <0.001 |
| Baseline serum creatinine, mg/dL | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.9 |
| Serum magnesium, mEq/L | 1.61 ± 0.2 | 1.62 ± 0.2 | 1.63 ± 0.2 | 1.64 ± 0.2 | 1.66 ± 0.1 | <0.001 |
| Serum potassium, mmol/L | 4.35 ± 0.5 | 4.37 ± 0.5 | 4.39 ± 0.5 | 4.47 ± 0.5 | 4.56 ± 0.5 | 0.1 |
| Coffee consumptiona, 8-oz cups/d | 0 | 0.4 ± 0.3 | 1.2 ± 0.3 | 2.2 ± 0.2 | 4.9 ± 1.3 | <0.001 |
| Tea consumption, 8-oz cups/d | 0.5 ± 1.1 | 0.5 ± 1 | 0.6 ± 0.9 | 0.6 ± 1 | 0.5 ± 1 | <0.001 |
| Soft drink consumption, 8-oz cups/d | 0.4 ± 0.9 | 0.4 ± 0.4 | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.3 ± 0.7 | <0.001 |
| Alcohol intake statusc, % | <0.001 | |||||
| Never | 37.3 | 29.1 | 25.4 | 17.0 | 12.3 | |
| Former | 19.7 | 17.4 | 18.6 | 18.0 | 18.2 | |
| Current (moderate) | 6.1 | 8.0 | 10.0 | 11.9 | 14.2 | |
| Current (heavy) | 37.0 | 45.6 | 46.0 | 53.1 | 55.4 | |
| Total energy intakea, kcal/d | 1,463 ± 530 | 1,448 ± 494 | 1,488 ± 507 | 1,508 ± 526 | 1,559 ± 533 | <0.001 |
| DASH diet score (0–40) | 24.3 ± 5 | 24.4 ± 5 | 23.8 ± 5 | 23.7 ± 5 | 23.7 ± 5 | 0.001 |
Note: Values for categorical variables are given as number (percentage); for continuous variables, as mean ± standard deviation.
Dietary factors (coffee, total energy) were estimated using cumulative average intake. The average of visit 1 and visit 3 dietary data for those who did not develop kidney disease and who were not censored prior to visit 3. For those who developed kidney disease or were censored prior to visit 3, dietary data from visit 1 was used.
Physical activity index score was calculated based on intensity and time of sport and exercise during leisure time; 1-lowest and 5-highest.
Moderate alcohol consumption was defined as <1 drink/day for women and <2 drinks/day for men. Heavy alcohol consumption was defined as ≥1 drink/day for women and ≥2 drinks/day for men.
Categorical variables were analyzed using chi-square test. Continuous variables were analyzed using analysis of variance (ANOVA) test.
Abbreviations: ACE, angiotensin converting enzyme inhibitors; BMI, body mass index; BP, blood pressure; CHD, coronary heart disease; d, day; DASH, Dietary Approaches to Stop Hypertension; eGFR, estimated glomerular filtration rate; kcal/d, kilocalories per day; kg/m2; kilograms per meter squared; mEq/L, milliequivalents per litre; mg/dL, milligrams per deciliter; mmHg, millimeters of mercury; mmol/L, millimoles per liter; oz, ounce.
Association Between Coffee Consumption and CKD
A total of 3,845 (27%) participants developed CKD over a median follow-up of 24 years. Higher coffee consumption was associated with lower risk of incident CKD, and the findings were relatively consistent across the three models (Table 2). Compared to never drinkers, each category of coffee consumption was associated with 10–16% lower risk of CKD [<1 cup/day: 10% lower risk (HR, 0.90; 95% CI, 0.82–0.99); 1 to <2 cups/day: 10% lower risk (HR, 0.90; 95% CI, 0.82–0.99); 2 to <3 cups/day: 13% lower risk (HR: 0.87, 95% CI: 0.77–0.97); and ≥3 cups/day: 16% lower risk (HR, 0.84; 95% CI, 0.75–0.94); p for trend<0.001]. Each additional cup of coffee consumed per day was associated with 3% lower risk of incident CKD (HR, 0.97; 95% CI, 0.95–0.99). Compared to participants who never consumed coffee, participants who consumed any amount of coffee had an 11% lower risk of CKD (HR, 0.89; 95% CI, 0.82–0.96; p for trend<0.001).
Table 2.
Risk for Kidney Disease by Category of Daily Coffee Consumption
| Coffee Consumption Levela | ||||||
|---|---|---|---|---|---|---|
| Never (n=2,758) | <1 cup/d (n=3,024) | 1–<2 cups/d (n=3,578) | 2–<3 cups/d (n=2,102) | ≥3 cups/d (n=2,747) | p for trend | |
| CKD (composite definition)b | ||||||
| No. of events | 786 | 868 | 1,015 | 528 | 648 | |
| Incidence rate per 1,000 person-years | 14.5 | 13.9 | 13.9 | 12.5 | 11.5 | |
| Model 1 | 1.00 (reference) | 0.89 (0.81–0.98) | 0.88 (0.80–0.97) | 0.84 (0.75–0.94) | 0.80 (0.72–0.89) | <0.001 |
| Model 2 | 1.00 (reference) | 0.89 (0.81–0.98) | 0.86 (0.78–0.95) | 0.82 (0.73–0.91) | 0.74 (0.66–0.83) | <0.001 |
| Model 3 | 1.00 (reference) | 0.90 (0.82–0.99) | 0.90 (0.82–0.99) | 0.87 (0.77–0.97) | 0.84 (0.75–0.94) | <0.001 |
| CKD (visit-based definition)c | ||||||
| No. events | 412 | 509 | 548 | 283 | 351 | |
| Incidence rate per 1,000 person-years | 11.7 | 11.8 | 11.3 | 10.3 | 9.1 | |
| Model 1 | 1.00 (reference) | 1.01 (0.89–1.15) | 0.95 (0.84–1.08) | 0.89 (0.76–1.03) | 0.79 (0.69–0.92) | <0.001 |
| Model 2 | 1.00 (reference) | 1.01 (0.89–1.15) | 0.93 (0.82–1.06) | 0.88 (0.75–1.03) | 0.77 (0.66–0.89) | <0.001 |
| Model 3 | 1.00 (reference) | 1.03 (0.90–1.17) | 0.94 (0.83–1.08) | 0.95 (0.81–1.11) | 0.83 (0.72–0.97) | <0.001 |
Note: Unless otherwise indicated, values given as hazard ratio (95% confidence interval). Model 1: Adjusted for age, sex, race-center, education, total energy intake; model 2: model 1 + physical activity, smoking, alcohol status; DASH diet score; model 3: model 2 + diabetes status, BMI, systolic blood pressure, anti-hypertensive medication use, baseline eGFR.
Average of visit 1 and visit 3 coffee intake calculated for participants with no CKD events or with CKD event after visit 3; visit 1 coffee intake used for participants with CKD event between visits 1 and 3.
Defined as eGFR<60 mL/min/1.73 m2 accompanied by ≥25% eGFR decline, ICD-9/10 code related to CKD stage 3+ through ARIC surveillance or National Death Index, or ESRD identified by USRDS registry.
Defined as eGFR<60 mL/min/1.73 m2 accompanied by ≥30% eGFR decline relative to baseline.
Abbreviation: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; USRDS, US Renal Data System
The results for the visit-based definition for CKD (eGFR<60 mL/min/1.73 m2 and ≥30% eGFR decline) were generally consistent with the main results (Table 2). In the fully adjusted model, consuming ≥3 cups of coffee per day was significantly associated with a lower risk of incident CKD (HR for ≥3 cups/d vs. never, 0.83; 95% CI, 0.72–0.97). However, the association between coffee consumption and incident CKD was not statistically significant for the other coffee consumption categories (<1 cup/day, ≥1 to <2 cups/day, and ≥2 to <3 cups/day) compared to non-drinkers. Each additional cup of coffee consumed per day was associated with a 4% lower risk of incident CKD using the visit-based definition (HR, 0.96; 95% CI, 0.93–0.98, p<0.001).
Association Between Coffee Consumption and Advanced Kidney Disease
There were 281 (2%) cases of ESRD over a median follow-up of 25 years. Coffee consumption was nominally protective of risk of ESRD in Model 3 for all categories of coffee consumption, but this was not statistically significant (Table 3; Model 3 p-value for trend=0.5). In model 3, coffee consumption was associated with lower risk of kidney failure (HR for <1 cup/d vs. never, 0.71 [95% CI, 0.58–0.88] and HR for 1 to <2 cups/d vs. never, 0.79 [95% CI, 0.65–0.97]). However, there was no significant linear trend across coffee consumption categories for the kidney failure outcome (p=0.6).
Table 3.
Risk for Advanced Kidney Disease Outcomes by Category of Daily Coffee Consumption
| Coffee Consumption Levela | ||||||
|---|---|---|---|---|---|---|
| Never (n=2,758) | <1 cup/d (n=3,024) | 1–<2 cups/d (n=3,578) | 2–<3 cups/d (n=2,102) | ≥3 cups/d (n=2,747) | p for trend | |
| ESRDb | ||||||
| No. of events | 70 | 63 | 72 | 38 | 38 | |
| Incidence rate per 1,000 person-years | 1.2 | 0.9 | 0.9 | 0.8 | 0.6 | |
| Model 1 | 1.00 (reference) | 0.80 (0.57–1.12) | 0.79 (0.57–1.10) | 0.73 (0.48–1.10) | 0.69 (0.46–1.04) | 0.1 |
| Model 2 | 1.00 (reference) | 0.81 (0.58–1.14) | 0.76 (0.55–1.06) | 0.69 (0.45–1.05) | 0.59 (0.39–0.90) | 0.03 |
| Model 3 | 1.00 (reference) | 0.83 (0.59–1.16) | 0.84 (0.60–1.17) | 0.76 (0.50–1.16) | 0.83 (0.54–1.28) | 0.5 |
| Kidney Failurec | ||||||
| No. of events | 187 | 159 | 201 | 117 | 120 | |
| Incidence rate per 1,000 person-years | 3.3 | 2.4 | 2.6 | 2.7 | 2.1 | |
| Model 1 | 1.00 (reference) | 0.69 (0.56–0.85) | 0.75 (0.61–0.91) | 0.82 (0.64–1.04) | 0.73 (0.58–0.93) | 0.2 |
| Model 2 | 1.00 (reference) | 0.69 (0.56–0.86) | 0.72 (0.59–0.88) | 0.76 (0.60–0.97) | 0.62 (0.48–0.78) | <0.001 |
| Model 3 | 1.00 (reference) | 0.71 (0.58–0.88) | 0.79 (0.65–0.97) | 0.85 (0.67–1.08) | 0.82 (0.64–1.05) | 0.6 |
Note: Unless otherwise indicated, values given as hazard ratio (95% confidence interval). Model 1: Adjusted for age, sex, race-center, education, total energy intake; model 2: model 1 + physical activity, smoking, alcohol status, DASH diet score; model 3: model 2 + diabetes status, BMI, systolic blood pressure, anti-hypertensive medication use, baseline eGFR.
Average of visit 1 and visit 3 coffee intake calculated for participants with no ESRD or kidney failure events or with ESRD or kidney failure event after visit 3; visit 1 coffee intake used for participants with CKD event between visits 1 and 3.
Defined as cases of kidney transplant and dialysis, identified by the USRDS registry.
Defined by USRDS data, ICD-9/10 codes, or eGFR<15 mL/min/1.73 m2 during a study visit.
Abbreviation: ESRD, end-stage renal disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; USRDS, US Renal Data System
Subgroup Analyses
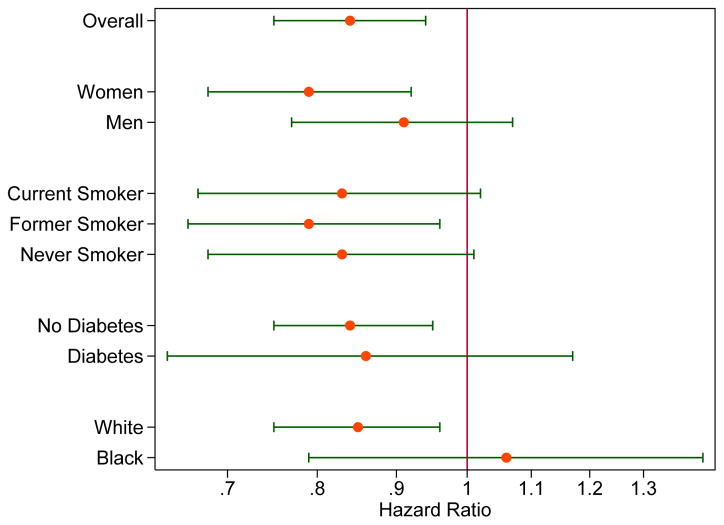
There was a significant inverse association between coffee and CKD among whites, but not among blacks (Table S1; p for interaction=0.003). We did not observe effect modification for the association between coffee consumption and incident CKD by sex (p=0.4), smoking (p=0.4), diabetes (p=0.7), physical activity (p=0.8), or DASH diet (p=0.2) (Figure 1).
Figure 1.
Adjusteda Risk for Incident Kidney Disease for Highest vs. Lowest Category of Coffee Consumption (≥3 cups of coffee per day versus never) According to Population Subgroups.
aHazard ratios (on logarithmic scale) for kidney disease are presented for ≥3 cups of coffee per day versus never, adjusted for age, sex, race-center, education, total energy intake, physical activity, smoking, alcohol status, DASH diet score, diabetes status, body mass index, systolic blood pressure, anti-hypertensive medication use, and baseline eGFR.
Discussion
Our analysis of 14,209 middle-aged adults in the U.S. suggests that increased consumption of coffee is associated with lower risk of CKD in the ARIC study. Men, whites, current smokers, and individuals without hypertension or diabetes were more likely to consume higher amounts of coffee per day. Inverse associations were observed for advanced kidney disease (ESRD and kidney failure), but these estimates were generally not statistically significant, which may be due to the relatively small number of events and correspondingly low power. Overall, higher amounts of coffee consumption were associated with a lower risk of kidney disease and may be beneficial for reducing the long-term risk of kidney disease. Furthermore, our results suggest that participants who drank any amount of coffee had an 11% reduced risk of incident CKD compared to those who never drank coffee. The association suggested a dose-response relationship, with the most robust associations among those consuming at least 3 cups of coffee per day. The results were consistent by sex, smoking status, and diabetes status but the association between blacks and whites was significantly different. The association between coffee and CKD among whites was similar to the main analysis; however, there was no clear association among blacks. The lack of association may be attributed to low power and low frequency of coffee consumption among blacks.
Several cross-sectional studies have suggested a significant protective association between coffee consumption and eGFR (23–26). A cross-sectional study involving Korean women found that ≥2 cups of coffee per day was associated with a lower odds of decreased kidney function (eGFR<60 mL/min/1.73 m2) compared to those who drank <1 cup per day (OR, 0.59; 95% CI, 0.37–0.95) (23). They found that the protective association was more robust among women who had diabetes compared to those who did not have diabetes. The Doetinchem Cohort Study found that individuals who drank > 6 cups of coffee per day had better kidney function compared to those who drank <1 cup per day [by 1.33 (95% CI, 0.24–2.43) mL/min/1.73 m2; P-trend=0.02] (25). In contrast, in our study, eGFR was lower by approximately 3 mL/min/1.73 m2 in the highest vs. lowest coffee consumption category. However, this difference is minimal and not clinically significant. Furthermore, cross-sectional analyses do not capture the temporal association between coffee and incident kidney disease.
Few longitudinal studies have been performed on the association between coffee and kidney outcomes. The existing studies have reported mixed results (9, 10, 13). In a prospective analysis of 185,855 nonwhite U.S. adults in the Multiethnic Cohort, consumption of at least 1 cup of coffee per day was found to be associated with a lower risk of kidney disease compared to never drinking coffee (HR for 1 cup/d, 0.88 [95% CI, 0.85–0.91]; HR for 2–3 cups/d, 0.82 [95% CI, 0.79–0.86]; HR for ≥4 cups/d, 0.42 [95% CI, 0.26–0.67]) (10). Our results contradict a recent meta-analysis that found no association between coffee and CKD (13). Among 14,898 individuals from 4 observational studies (2 from Japan, 1 from Korea, 1 from Italy), there was no statistically significant difference in CKD risk among those consuming more than one cup of coffee/day compared to those who never consumed coffee (relative risk, 0.71; 95% CI, 0.47–1.08). When the analysis was stratified by sex, the authors observed a stronger protective relationship among women; however, it was also not significant. When we stratified our analysis by sex, there appeared to be a stronger protective association between coffee and CKD among women compared to men, however there was no evidence of effect modification. The results of the meta-analysis may be due to an inability to distinguish between meaningful differences in coffee consumption categories (dichotomized as never or >1 cup/day in the meta-analysis vs. ranging from never to ≥3 cups/day in the present study). Furthermore, a large (N=90,317) prospective analysis of the U.S. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial found no significant association between coffee and mortality due to kidney disease (9). Consumption of ≥4 cups/d compared to never drinkers was inversely associated and coffee consumption for categories <1 cup/d, 1 cup/d, and 2–3 cups/d were directly associated with kidney disease mortality. This null finding could be due in part to limited statistical power because there were very few deaths due to kidney disease in their study (n=108).
The biological mechanism by which coffee may influence kidney disease risk is unclear and the chemical makeup of coffee is highly complex. However, there are several speculations regarding how the components in coffee may affect kidney disease. One hypothesis is that the anti-diabetic effect of coffee may improve glycemia and lower the risk of diabetic nephropathy (23). Several studies have found coffee to be inversely associated with diabetes (3, 4). One explanation for this association is the chlorogenic acid and other natural constituents of coffee beans that inhibit glucose absorption, reducing oxidative stress and liver glucose output (8, 23, 27–29). In our study, there were fewer individuals with diabetes in higher coffee consumption groups, confirming the previously found inverse associations between coffee and diabetes. However, we found no effect modification by diabetes on coffee and CKD (p=0.7). Another explanation for coffee being protective of kidney disease is that the components within coffee (caffeic acid, hydroxyhydroquinone, quinides, niacin, trionelline, magnesium, and chlorogenic acid) may protect the glomerular endothelium from oxidative stress and reduce insulin resistance and systematic inflammation (13, 30). Coffee consumption has been found to have an inverse association with hyperuricemia, which may be an explanation for slower progression to CKD (31, 32). Caffeine has been suggested to increase eGFR and renal blood flow so it is possible that caffeine enhances excretion of uric acid; however, there is not enough evidence to confirm this hypothesis (32).
Our study has several strengths. We used a large, community-based population. We also used average coffee consumption at visits 1 and 3 when applicable. Furthermore, we had well-measured outcomes for incident CKD and more advanced kidney disease (ESRD) and performed sensitivity analyses for both outcomes.
Limitations included self-reported coffee consumption, which is likely associated with some degree of misclassification. However, our exposure was categorized into specific categories so we could capture a wide range of coffee intake. Reverse causality is a potential concern for observational studies. However, we excluded participants with prevalent CKD at baseline, conducting a prospective analysis with baseline coffee consumption and subsequent kidney disease. Another limitation is the small sample size for the analysis of advanced kidney outcomes, which reduced the statistical power for detecting a significant association between coffee and ESRD. We did not have data on albuminuria or proteinuria at visit 1, which would have been useful in assessing kidney damage. Another limitation is that we do not know whether participants who consumed less coffee drank a higher amount of other types of fluids (tea, soft drinks) or if participants who drank higher amounts of coffee were replacing their consumption of other fluids with coffee. Our dietary assessment (food frequency questionnaire) measured frequency and quantity of coffee consumed but did not ask specifically about type of coffee beans, how it was prepared, or how much sweetener and milk were added to coffee. Preparation of coffee should be investigated in future studies because different brewing methods for different types of beans have been found to influence the chemical composition of coffee (33–35). For example, brewing time and water pressure influence the extraction of antioxidants and chlorogenic acid (34). Furthermore, unfiltered coffee has been suggested to have adverse effects on lipids whereas filtered coffee does not significantly change LDL cholesterol or triglyceride concentrations (35).
In this prospective study of 14,209 middle-aged blacks and whites in the U.S., we found that coffee consumers had a reduced risk of incident CKD, which was consistent in population subgroups (by sex, smoking, diabetes) but not between blacks and whites. Our results provide further support for the 2015–2020 Dietary Guidelines for Americans, which state that 3–5 cups of coffee per day can be incorporated into a healthy lifestyle (2). Future research should evaluate whether coffee preparation methods and sugar and milk added to coffee influence the renal protection association of coffee observed in the present study.
Supplementary Material
Table S1. Risk for kidney disease outcomes by category of daily coffee consumption stratified by race.
Acknowledgments
Support: Dr. Rebholz is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782). EAH is supported by a grant from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (training grant T32 HL007024). The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, HHSN2682017000021). The funders had no role in the study design; collection, analysis, and interpretation of these data; writing the report; and the decision to submit the report for publication.
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Authors’ Contributions: Research idea and study design: EAH, ES, MEG, LMS, JC, CMR; data analysis/interpretation: EAH; CMR; mentorship: CMR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: The authors declare no that they have relevant financial interests.
Disclaimer: Some of the data reported here have been supplied by the U.S. Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loftfield E, Freedman ND, Dodd KW, Vogtmann E, Xiao Q, Sinha R, et al. Coffee Drinking Is Widespread in the United States, but Usual Intake Varies by Key Demographic and Lifestyle Factors. J Nutr. 2016;146(9):1762–8. doi: 10.3945/jn.116.233940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services and U.S. Department of Agriculture. [Accessed on 25 Oct. 2017];2015–2020 Dietary Guidelines for Americans. Available from: http://health.gov/dietaryguidelines/2015/guidelines/
- 3.Floegel A, Pischon T, Bergmann MM, Teucher B, Kaaks R, Boeing H. Coffee consumption and risk of chronic disease in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany study. Am J Clin Nutr. 2012;95(4):901–8. doi: 10.3945/ajcn.111.023648. [DOI] [PubMed] [Google Scholar]
- 4.Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37(2):569–86. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129(6):643–59. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhoo-Pathy N, Peeters PH, Uiterwaal CS, Bueno-de-Mesquita HB, Bulgiba AM, Bech BH, et al. Coffee and tea consumption and risk of pre- and postmenopausal breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Breast Cancer Res. 2015;17:15. doi: 10.1186/s13058-015-0521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q, Luo ML, Li H, Li M, Zhou JG. Coffee consumption and risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:13410. doi: 10.1038/srep13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding M, Satija A, Bhupathiraju SN, Hu Y, Sun Q, Han J, et al. Association of Coffee Consumption With Total and Cause-Specific Mortality in 3 Large Prospective Cohorts. Circulation. 2015;132(24):2305–15. doi: 10.1161/CIRCULATIONAHA.115.017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loftfield E, Freedman ND, Graubard BI, Guertin KA, Black A, Huang WY, et al. Association of Coffee Consumption With Overall and Cause-Specific Mortality in a Large US Prospective Cohort Study. Am J Epidemiol. 2015;182(12):1010–22. doi: 10.1093/aje/kwv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SY, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Association of Coffee Consumption With Total and Cause-Specific Mortality Among Nonwhite Populations. Ann Intern Med. 2017;168(4):228–35. doi: 10.7326/M16-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunter MJ, Murphy N, Cross AJ, Dossus L, Dartois L, Fagherazzi G, et al. Coffee Drinking and Mortality in 10 European Countries: A Multinational Cohort Study. Ann Intern Med. 2017;167(4):236–47. doi: 10.7326/M16-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wouters OJ, O’Donoghue DJ, Ritchie J, Kanavos PG, Narva AS. Early chronic kidney disease: diagnosis, management and models of care. Nat Rev Nephrol. 2015;11(8):491–502. doi: 10.1038/nrneph.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijarnpreecha K, Thongprayoon C, Thamcharoen N, Panjawatanan P, Cheungpasitporn W. Association of coffee consumption and chronic kidney disease: A meta-analysis. Int J Clin Pract. 2017;71(1) doi: 10.1111/ijcp.12919. [DOI] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 15.Willett W. Nutritional Epidemiology. 3. New York, NY: Oxford University Press; 2013. [Google Scholar]
- 16.Grams ME, Rebholz CM, McMahon B, Whelton S, Ballew SH, Selvin E, et al. Identification of incident CKD stage 3 in research studies. Am J Kidney Dis. 2014;64(2):214–21. doi: 10.1053/j.ajkd.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lustgarten JA, Wenk RE. Simple, rapid, kinetic method for serum creatinine measurement. Clin Chem. 1972;18(11):1419–22. [PubMed] [Google Scholar]
- 18.Parrinello CM, Grams ME, Couper D, Ballantyne CM, Hoogeveen RC, Eckfeldt JH, et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem. 2015;61(7):938–47. doi: 10.1373/clinchem.2015.238873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebholz CM, Coresh J, Ballew SH, McMahon B, Whelton SP, Selvin E, et al. Kidney Failure and ESRD in the Atherosclerosis Risk in Communities (ARIC) Study: Comparing Ascertainment of Treated and Untreated Kidney Failure in a Cohort Study. Am J Kidney Dis. 2015;66(2):231–9. doi: 10.1053/j.ajkd.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER, 3rd, et al. DASH (Dietary Approaches to Stop Hypertension) Diet and Risk of Subsequent Kidney Disease. Am J Kidney Dis. 2016;68(6):853–61. doi: 10.1053/j.ajkd.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu JY, Roy JA, Xie D, Yang W, Shou H, Anderson AH, et al. Statistical Methods for Cohort Studies of CKD: Survival Analysis in the Setting of Competing Risks. Clin J Am Soc Nephrol. 2017;12(7):1181–9. doi: 10.2215/CJN.10301016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim BH, Park YS, Noh HM, Sung JS, Lee JK. Association between Coffee Consumption and Renal Impairment in Korean Women with and without Diabetes: Analysis of the Fourth Korea National Health and Nutrition Examination Survey in 2008. Korean J Fam Med. 2013;34(4):265–71. doi: 10.4082/kjfm.2013.34.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trovato GM, Pirri C, Martines GF, Trovato F, Catalano D. Coffee, nutritional status, and renal artery resistive index. Ren Fail. 2010;32(10):1137–47. doi: 10.3109/0886022X.2010.516853. [DOI] [PubMed] [Google Scholar]
- 25.Herber-Gast GC, van Essen H, Verschuren WM, Stehouwer CD, Gansevoort RT, Bakker SJ, et al. Coffee and tea consumption in relation to estimated glomerular filtration rate: results from the population-based longitudinal Doetinchem Cohort Study. Am J Clin Nutr. 2016;103(5):1370–7. doi: 10.3945/ajcn.115.112755. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima K, Hirose K, Ebata M, Morita K, Munakata H. Association between habitual coffee consumption and normal or increased estimated glomerular filtration rate in apparently healthy adults. Br J Nutr. 2010;103(2):149–52. doi: 10.1017/S0007114509991681. [DOI] [PubMed] [Google Scholar]
- 27.Svilaas A, Sakhi AK, Andersen LF, Svilaas T, Strom EC, Jacobs DR, Jr, et al. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J Nutr. 2004;134(3):562–7. doi: 10.1093/jn/134.3.562. [DOI] [PubMed] [Google Scholar]
- 28.Arion WJ, Canfield WK, Ramos FC, Schindler PW, Burger HJ, Hemmerle H, et al. Chlorogenic acid and hydroxynitrobenzaldehyde: new inhibitors of hepatic glucose 6-phosphatase. Arch Biochem Biophys. 1997;339(2):315–22. doi: 10.1006/abbi.1996.9874. [DOI] [PubMed] [Google Scholar]
- 29.van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294(1):97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- 30.Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2011;51(4):363–73. doi: 10.1080/10408390903586412. [DOI] [PubMed] [Google Scholar]
- 31.Pham NM, Yoshida D, Morita M, Yin G, Toyomura K, Ohnaka K, et al. The relation of coffee consumption to serum uric Acid in Japanese men and women aged 49–76 years. J Nutr Metab. 2010;2010 doi: 10.1155/2010/930757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi HK, Curhan G. Coffee, tea, and caffeine consumption and serum uric acid level: the third national health and nutrition examination survey. Arthritis Rheum. 2007;57(5):816–21. doi: 10.1002/art.22762. [DOI] [PubMed] [Google Scholar]
- 33.Wolska J, Janda K, Jakubczyk K, Szymkowiak M, Chlubek D, Gutowska I. Levels of Antioxidant Activity and Fluoride Content in Coffee Infusions of Arabica, Robusta and Green Coffee Beans in According to their Brewing Methods. Biol Trace Elem Res. 2017;179(2):327–33. doi: 10.1007/s12011-017-0963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caprioli G, Cortese M, Sagratini G, Vittori S. The influence of different types of preparation (espresso and brew) on coffee aroma and main bioactive constituents. Int J Food Sci Nutr. 2015;66(5):505–13. doi: 10.3109/09637486.2015.1064871. [DOI] [PubMed] [Google Scholar]
- 35.Rebello SA, van Dam RM. Coffee consumption and cardiovascular health: getting to the heart of the matter. Curr Cardiol Rep. 2013;15(10):403. doi: 10.1007/s11886-013-0403-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Risk for kidney disease outcomes by category of daily coffee consumption stratified by race.