Abstract
Background
Although pre- and perioperative statin therapy improve postoperative outcomes in several populations, few data examine their association with survival following abdominal aortic aneurysm (AAA) repair. In addition, no data exist regarding the benefits of starting statins in patients with AAA not currently taking them.
Methods
We performed a registry-based study of all patients undergoing repair of AAAs in the Vascular Quality Initiative between 2003 and 2017 without documented statin intolerance. In our primary analysis, we evaluated the association between preoperative statin therapy and long-term mortality, 30-day mortality, and in-hospital myocardial infarction and stroke. As a secondary analysis, we studied the cohort of patients not taking a statin preoperatively, and compared their long-term mortality based on whether or not they were discharged on a statin. To account for non-random assignment to treatment, we constructed propensity scores and applied inverse probability weighting.
Results
We identified 40,452 AAA repairs, of which 37,950 fit our entry criteria (29,257 endovascular [EVAR] and 8,693 open). Overall, 25,997 patients (69%) were taking a statin preoperatively, with EVAR patients more frequently taking a statin than those undergoing open repair (69% compared to 66%, P<.001). After propensity weighting, preoperative statin therapy was not associated with 30-day death; in-hospital stroke or MI. However, patients taking statins preoperatively experienced higher adjusted one-year (94% vs 90%) and five-year survival (85% vs 81%) from the date of surgery compared to those who were not (P<.001 overall), although subgroup analysis showed this only applied to intact or symptomatic aneurysms. Of the 11,941 patients not taking a statin preoperatively and discharged alive, 2,910 (24%) started on a statin prior to discharge. In our secondary analysis of the subset of patients not on statins preoperatively, those initiated on a statin prior to discharge experienced higher survival at one year (94% vs 91%) and five years (89% vs 81%) (P<.001 overall) than those who remained off statin therapy, with the greatest absolute long-term survival difference in patients with rupture (87% vs 62%, P<.001 overall).
Conclusions
Preoperative statin therapy is associated with higher long-term survival, but not perioperative mortality and morbidity, in patients undergoing AAA repair, and initiating statin therapy in previously statin-naïve patients is associated with markedly improved survival. All patients with AAAs without contraindications should receive statin therapy. In patients not taking a statin at the time of AAA repair, clinicians should consider initiating one prior to discharge.
Introduction
Prior studies demonstrated the benefit of preoperative statin use following cardiac and noncardiac surgery, and a recent, large analysis of patients in the Veteran’s Affairs Surgical Quality Improvement Program showed decreased perioperative mortality and complications in patients taking statins perioperatively.1–9 Based on these works, the American College of Cardiology/American Heart Association (ACC/AHA) recommend continuing statins in patients undergoing vascular surgery, and state that, “perioperative initiation of statin therapy is reasonable for patients undergoing vascular surgery” (a Class IIa recommendation).10 However, the recommendations do not address whether clinicians should initiate statins postoperatively in patients not previously taking them.
Furthermore, in studies of statin use in noncardiac surgery, vascular surgery patients comprised only a fraction of the study populations, with a small subset undergoing repair of abdominal aortic aneurysms (AAA). The vascular patients included in the aforementioned trials were a heterogeneous group, ranging from those undergoing carotid endarterectomies and stents, to peripheral interventions and bypasses, to AAA. In studies that directly examined patients undergoing AAA repair, the benefits of statins were less clear. Several small studies of mostly patients undergoing open AAA repair demonstrated improved survival with statins.3,9,11–13 However, a more contemporary review of patients undergoing high-risk vascular procedures, including over 3,000 AAA repairs, demonstrated no benefit to preoperative antiplatelet and statin use.14
Although the proportion of patients taking statins increased in recent years, a substantial number are not on therapy at the time of surgery.14–17 This is especially true in patients presenting with a ruptured AAA, a condition that carries with it exceedingly high morbidity and mortality.18–20 In a Danish registry, only 11% of patients with ruptured aneurysms were taking a statin at the time of presentation.12 As these patients present emergently, there is no time to initiate a statin preoperatively, but to our knowledge, no data exist on the benefit of initiating a statin postoperatively in this high-risk population.
Therefore, we utilized a large, national database to achieve two goals: to determine the association between preoperative statin use, perioperative cardiovascular events and long-term mortality following AAA repair, as well as the association between de novo initiation of statins postoperatively and long-term survival.
Methods
Subjects
We identified all patients who underwent AAA repair in the Society for Vascular Surgery’s Vascular Quality Initiative (VQI) between 2003–2017. We excluded patients without preoperative statin information (N = 204, <1%), patients with statin intolerance (N = 825, 2%), those with prior aortic interventions, repairs with a suprarenal clamp site, and we only included a patient’s first intervention. The VQI is a cooperative quality improvement initiative developed in 2002 to improve outcomes in vascular surgery, and collects data from 412 centers in 46 states and Canada. Over 200 variables are entered by vascular surgeons and trained nurses. Data include patient demographics, comorbid conditions, perioperative complications, one-year follow-up, and long-term mortality through linkage to the Social Security Death Index through May 12, 2017. The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived the need for patient consent due to the nature of the design and minimal risk to human subjects.
Definitions and variables
The VQI records statin usage as a binary variable irrespective of dose. We classified center volume by the average yearly volume of AAA repairs in each center since their initiation into the VQI, excluding their lowest volume year to account for centers that entered into the VQI later in the year. After calculating this adjusted average yearly volume by center, we classified centers into quartiles of volume. Chronic kidney disease (stage 3 or greater) was classified as a glomerular filtration rate (GFR) < 60 mL/min/1.73m2 by the Modification of Diet in Renal Disease (MDRD) formula.
Hospital Volume
Outcomes
The primary outcome was overall survival through the end of 2017, and secondary outcomes were 30-day mortality; and in-hospital myocardial infarction (MI), or stroke. We performed subgroup analyses stratified by type of repair (open versus endovascular - EVAR) and presentation (ruptured aneurysm versus intact or symptomatic). The VQI defines postoperative MI as either electrocardiographic changes consistent with MI or troponin elevation, with or without clinical symptoms. Stroke included both minor and major strokes as defined by the VQI.
Statistical Analysis
In our primary analysis, we assessed the association of preoperative statin use with outcomes in patients undergoing AAA repair in the overall cohort and stratified by type of repair (EVAR versus open), and presentation (intact or symptomatic versus ruptured). We conducted our analyses on an intention-to-treat basis (i.e. conversions from EVAR to open were classified as EVAR, and patients listed as taking statins at discharge but not at one year follow-up were considered to be on statins). As a secondary analysis, we studied the cohort of patients not taking a statin preoperatively, and compared their long-term mortality based on whether or not they were discharged on a statin (henceforth referred to as de novo statin use). For this secondary analysis, we excluded patients who died in the hospital.
To account for nonrandom assignment of treatments, we constructed propensity scores and used them to perform inverse probability weighting, with separate propensity scores for the primary and secondary analyses. We generously introduced covariates into our model, including age, race, sex, aortic diameter, surgery year, cerebrovascular or peripheral vascular disease, hypertension, chronic kidney disease, coronary artery disease (CAD), diabetes, chronic obstructive pulmonary disease, open vs endovascular approach, body mass index, urgency of operation (intact versus symptomatic versus ruptured), congestive heart failure, preoperative aspirin use, preoperative beta blocker use, angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) use, and smoking status. Our propensity score for our secondary analysis also included postoperative MI and stroke. The propensity scores enabled us to create inverse probability weights (the inverse of the probability of receiving the treatment that the subject received). We tested these scores for adequacy of overlap by plotting the distribution of propensity score distributions between treated and untreated groups. After weighting, the standardized differences were all less than 10% (the usual threshold), indicating minimal imbalance (in fact, our standardized differences were all < 3.6%). We then used these weights to compare the risk of primary and secondary outcomes between treatment arms. For binary outcomes, we applied weighted logistic regression. We constructed weighted Kaplan-Meier curves for long-term outcomes and performed comparisons using Cox regression and the Wald test.
Results
Statin Usage
We identified 40,452 AAA repairs, of which 37,950 fit our entry criteria (29,257 EVAR and 8,693 open). Overall, 25,997 (69%) were taking a statin preoperatively, with patients undergoing EVAR more likely to be taking a statin than those undergoing open repair (69% compared to 66%, P < .001). There were 3,074 (8%) patients with ruptured aneurysms, 1,295 (42%) took a statin preoperatively. From 2003 to 2009, statin use steadily increased from 45% to 75%, but subsequently remained between 67-70% for the remainder of the study period. Baseline characteristics of the study population are presented in Table I. Patients taking statins had significantly higher prevalence of diagnosed comorbidities, and more often presented with intact aneurysms (87% versus 74%, P < .001), and smaller aneurysms (median diameter 5.5 cm [Interquartile range 5.1–6.1] vs. 5.7 cm [5.1 – 6.7], P < .001). There was no significant difference in the usage of statins between high and low volume centers. Of the 11,941 patients not a statin preoperatively and discharged alive, 2,910 (24%) were discharged on a statin.
Table I.
Baseline Demographics of the Study Cohort. SD: Standardized Differences. ACE/ARB: angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB)
| On Statin Preoperatively (n = 25,997) | Not On Statin Preoperatively (n = 11,953) | SD After Weighting | ||
|---|---|---|---|---|
|
| ||||
| Characteristic | n (%) unless specified | P Value | % | |
| Age (median [IQR]) | 73 [67 – 79] | 72 [66 – 79] | < .001 | −1.1% |
| Male sex | 20,935 (81%) | 9,234 (77%) | < .001 | −0.9% |
| White race | 23,707 (91%) | 10,742 (90%) | < .001 | −0.2% |
| EVAR | 20,298 (78%) | 8,959 (75%) | < .001 | −0.2% |
| Aortic Diameter (median [IQR]) | 55 [51 – 61] | 57 [51 – 67] | < .001 | 0.3% |
| Urgency: | < .001 | 0.7% | ||
| Intact | 22,632 (87%) | 8,797 (74%) | ||
| Symptomatic | 2,037 (8%) | 1,356 (11%) | ||
| Ruptured | 1,295 (5%) | 1,779 (15%) | <.001 | 1.7% |
| Center Volume Quartile | ||||
| 1 st (lowest) | 6,587 (25%) | 3,030 (25%) | ||
| 2nd | 6,290 (24%) | 3,168 (27%) | ||
| 3rd | 6,798 (26%) | 3,020 (25%) | ||
| 4th (highest) | 6,272 (24%) | 2,715 (23%) | ||
| Hypertension | 22,644 (87%) | 8,770 (73%) | < .001 | −0.6% |
| Diabetes | 5,767 (22%) | 1,557 (13%) | < .001 | −2.7% |
| Coronary Artery Disease | 8,933 (34%) | 1,936 (16%) | < .001 | −0.6% |
| Congestive Heart Failure | 3,196 (12%) | 965 (8%) | < .001 | −1.7% |
| COPD | 8,541 (33%) | 3,858 (32%) | 0.14 | −1.2% |
| Chronic Kidney Disease | 9,327 (36%) | 4,047 (34%) | < .001 | −1.1% |
| Dialysis | 29 (0.1%) | 22 (0.2%) | 0.053 | −1.1% |
| Vascular Disease | 3,588 (14%) | 946 (8%) | < .001 | −3.1% |
| Smoking | 22,701 (87%) | 10,166 (85%) | < .001 | −0.2% |
| BMI | < .001 | −0.4% | ||
| Underweight | 555 (2%) | 463 (4%) | ||
| Normal | 6,809 (26%) | 3,808 (32%) | ||
| Overweight | 10,148 (39%) | 4,272 (36%) | ||
| Obese | 3,410 (29%) | 8,485 (33%) | ||
| Medicaid/Self-pay | 617 (3%) | 464 (5%) | < .001 | 0.4% |
| Aspirin | 18,892 (73%) | 5,105 (43%) | < .001 | −0.8% |
| Any Antiplatelet Agent | 19,662 (76%) | 5,316 (44%) | < .001 | −0.8% |
| Beta-Blocker | 16,557 (64%) | 4,905 (41%) | < .001 | .0.5% |
| ACE/ARB | 10,475 (50%) | 2,836 (30%) | < .001 | −1.3% |
Preoperative Statin Use
30-Day and Perioperative Outcomes
While in-hospital; 1,211 patients experienced MIs; and 183 were diagnosed with strokes. Within 30 days, there were 1,390 deaths. In the unadjusted analysis not accounting for confounding by differences in baseline clinical characteristics, there was no difference in thirty- day mortality between patients taking a statin preoperatively compared to those not taking one after repair of intact/symptomatic (1.5% vs 1.6%, P = .41) or ruptured aneurysms (28% vs 28%, P = .66). Patients on statins experienced slightly higher rates of in-hospital MI after repair of intact/symptomatic aneurysm (2.1% vs 1.8%, P = .02), and similar rates of stroke (0.30% vs 0.40%, P = .16). Rate of perioperative events did not differ after rupture (stroke 3% vs 3%, P = .82; MI 16% vs 17%, P = .84). After accounting for confounding by baseline clinical characteristics using inverse probability weighting, there was no association between preoperative statin use and any of the perioperative outcomes studied (Table II). However, patients undergoing repair of intact or symptomatic aneurysms experienced lower odds of 30-day death if taking a statin (OR 0.74 [0.56 – 0.99], P = .045).
Table II.
Adjusted Perioperative Outcomes with Preoperative Statin Therapy
| Overall | Intact | Ruptured | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Odds Ratio [95% C.I.] | P | Odds Ratio [95% C.I.] | P | Odds Ratio [95% C.I.] | P | |
| Death | 0.91 [0.78 – 1.06] | 0.24 | 0.74 [0.56 – 0.99] | 0.045 | 1.04 [0.83 – 1.29] | 0.33 |
| MI | 1.04 [0.85 – 1.26] | 0.71 | 0.98 [0.72 – 1.32] | 0.87 | 1.09 [0.83 – 1.43] | 0.54 |
| Stroke | 0.74 [0.48 – 1.15] | 0.18 | 0.69 [0.36 – 1.34] | 0.27 | 0.69 [0.37 – 1.29] | 0.24 |
Long-term Survival
Overall, 5,569 patients died, at a median of 1.67 years postoperatively, with a median follow-up of 2.9 years. After accounting for confounding by baseline clinical characteristics using inverse probability weighting, adjusted one-year (94% vs 90%) and five-year survival (85% vs 81%) from the time of the operation were higher in patients taking statins preoperatively compared to those who were not (Figure 1a) (P < .001 overall). However, statin use was associated with lower mortality only in patients with intact or symptomatic aneurysms (one-year: 96% vs 94%; five-year: 86% vs 84%, P < .001 overall) (Figure 1b, 1c). This association was consistent for patients undergoing EVAR as well as open repair (Figure 1d) (both P < .001). Long-term survival after repair of ruptured aneurysms did not differ between statin users and non-users, whether undergoing EVAR or open repair (P > .05 for all comparisons).
Figure 1.
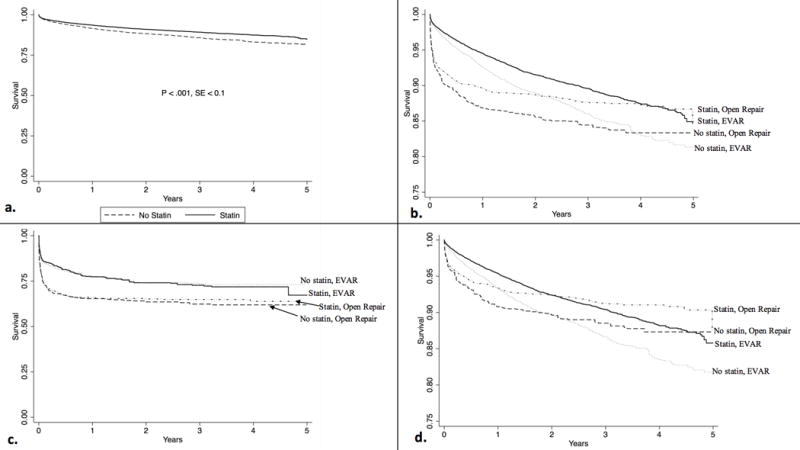
Adjusted long-term survival by preoperative statin use. a. Long-term survival in the overall cohort b. Long-term survival, stratified by open vs EVAR. P < .001 for all comparisons c. Long-term survival, stratified by open vs EVAR, in patients presenting with rupture. P = .72 for statin use overall, P = .89 for statin use in EVAR patients, P = .75 for statin use in open repair d. Long-term survival, stratified by open vs EVAR, in patients presenting with intact or symptomatic aneurysms. P < .001 for all comparisons
De Novo Statin Therapy
We then examined the cohort of patients not taking a statin at the time of surgery. After propensity-weighting, patients newly started on a statin prior to discharge (de novo statin therapy) experienced higher adjusted survival at one year (94% vs 91%) and five years (89% vs 81%) from the time of surgery compared to those who were not (Figure 2a) (P < .001 overall). De novo statin therapy was associated with higher survival in patients undergoing repair of intact/symptomatic (adjusted five-year survival 90% vs 83%, P < .001) and ruptured aneurysms (adjusted five-year survival 86% vs 63%, P < .001) (Figure 2b, 2c).
Figure 2.
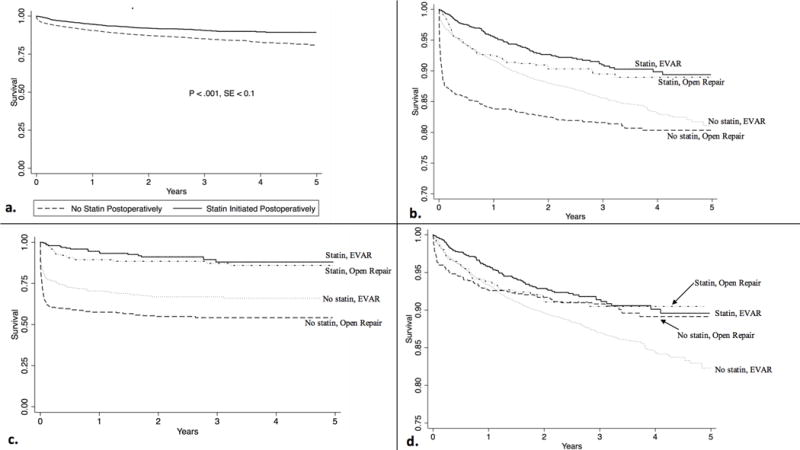
Adjusted long-term survival by postoperative statin initiation. a. Long-term survival in the overall cohort. b. Long-term survival, stratified by open vs EVAR. P < .001 for statin use in both EVAR and open c. Long-term survival, stratified by open vs EVAR, in patients presenting with rupture. P < .001 for statin use in both EVAR and d. Long-term survival, stratified by open vs EVAR, in patients presenting with intact or symptomatic aneurysms. P = 0.73 for statin use after open repair. P < .001 for statin use after EVAR
Interestingly, EVAR patients undergoing repair of ruptured or intact/symptomatic aneurysms experienced higher survival with de novo statin therapy (P < .001 for all subgroups). However, in the patients who underwent open surgery, de novo statin therapy was only associated with higher survival in patients presenting with ruptured (adjusted five-year survival 85% vs 57%, P < .001), but not intact/symptomatic aneurysms (adjusted five-year survival 90% vs 89%, P = .99) (Figure 2d).
Statin Compliance
The registry recorded information on whether patients were taking statins at one-year follow-up for 19,037 patients (50%); 15,469 (81%) of whom were discharged on statins, and 3,568 (19%) who were not. Fully 89% of the statin cohort remained on a statin, and 25% of the cohort not discharged on a statin received a prescription for statins prior to their follow-up visit.
Discussion
In this registry-based study of a large, contemporary database, statin therapy was associated with higher long-term, but not perioperative, survival after AAA repair. Preoperative statin therapy was associated with higher long-term survival only in patients undergoing repair of intact, but not ruptured aneurysms. In the cohort of patients not taking statins prior to surgery, those started on a statin prior to discharge experienced significantly higher survival than those who were not.
Abdominal aortic aneurysm is highly associated with coronary artery disease, and the National Institutes of Health classified AAA as a CAD risk equivalent in their Adult Treatment Panel III (ATP III) Guidelines.21,22 Although AAA is not caused by atherosclerosis, it is highly associated with the presence of atherosclerosis in other vascular territories, similar to the association between CAD and diabetes.16 Between one-third and one-half of all patients with AAA have either diagnosed or undiagnosed heart disease.23–27 In manifestations of atherosclerosis such as coronary artery disease, peripheral arterial disease and cerebrovascular disease, or diseases with a high correlation with atherosclerosis such as diabetes, the ACC/AHA recommend that patients receive statin therapy.16 However, despite the high correlation between AAA and CAD, the 2013 ACC/AHA Lipid Management Guidelines made no recommendations for statin therapy in patients with AAA, and did not list AAA as a component of, or marker for, atherosclerotic cardiovascular disease.16
Our results suggest that these recommendations may need to be re-examined. The association we found between long-term, but not short-term outcomes, implies that statins are not modifying the perioperative milieu, but rather that the need for AAA repair is a marker of elevated risk. Consequently, we hypothesize that statin therapy in those patients does not affect their immediate postoperative course, but provides secondary risk reduction similar to that seen in other atherosclerotic populations such as stroke, MI and peripheral arterial disease.16,28
In addition to atherosclerotic risk modification, limited evidence suggests that statins reduce aneurysm growth and risk of rupture.12,29,30 Indeed, patients in our study taking statins were one-third as likely to present with rupture, and had smaller diameter aneurysms at time of presentation. It is possible that statin use is merely a surrogate marker for adequate medical care. However, this finding, in combination with the correlation between AAA and CAD and the significant risk reduction associated with statin therapy in our study, suggests that clinical societies should consider recommending statins for patients with AAAs without contraindications.
Prior studies of perioperative statin initiation yielded mixed results. The Short Term Atorvastatin Regime for Vasculopathic Subjects (STAR VaS) Trial randomized 60 high risk patients undergoing noncardiac surgery to atorvastatin for one week preoperatively and one week postoperatively, or placebo.31 There was no difference in the primary endpoint at 30 days between the two groups (reduction in C-reactive protein levels). Durazzo et al. randomized 100 patients scheduled to undergo vascular surgery to atorvastatin or placebo for 45 days, with surgery performed at 30 days after randomization.32 In this small study, the statin group experienced a significant reduction in the composite endpoint of death from cardiac cause, nonfatal MI, unstable angina and stroke. However, these trials were small, excluded ruptured aneurysms, randomized only patients undergoing open repair, and lacked long-term follow-up. A recent Cochrane review concluded there was insufficient evidence that short-term statin therapy initiated in the perioperative period resulted in either benefit or harm.7
A contemporary randomized trial of perioperative statin initiation is unlikely to be feasible, given the prevalence of statin use in this population (69% in this study), and thus we must extrapolate from registry-based studies such as ours. Fortunately, this large, robust database provided an ideal study population, with about 12,000 patients who were not taking statins at the time of surgery, 2,000 of whom started a statin prior to discharge. The results of de novo statin therapy in this population were striking, with a 9% absolute risk reduction in the risk of death at 5 years and an even more remarkable 30% lower mortality in patients who presented with rupture. This last point is especially notable given that preoperative statins were not associated with improved outcomes in patients with ruptured aneurysms. Patients with ruptured aneurysms were the least likely to be taking statins at the time of presentation, and thus have the greatest potential to benefit.
The only subgroup in which statin initiation was not associated with benefit was patients undergoing open repair of intact or symptomatic aneurysms. This likely results from selection bias, as surgeons generally perform elective, open repair in the healthiest patients. This is especially true in the subset of patients we studied, which was limited to infrarenal aneurysm repairs where the majority of patients are candidates for EVAR. Indeed, as we only were able to capture long-term mortality, future efforts could focus on long-term cardiovascular outcomes such as stroke or myocardial infarction. Although this healthy subset experienced low rates of long-term mortality regardless of statin usage, we may be underpowered to detect such a rare outcome, and statins may be associated with lower rates of long-term major cardiovascular events in this population. The association between statins and higher survival in all but the healthiest cohort, lends weight to the theory that statin therapy is not modifying the perioperative course, but rather that the need to undergo AAA repair is a marker of generalized atherosclerosis. Our results indicate that clinicians should consider initiating statins on all patients with aneurysms without contraindications.
This study must be interpreted in the context of its design. With only 50% follow-up regarding statin use, we lack complete records on patient compliance with their statin prescriptions. Previous reports show that up to 60% of patients discontinue their statins within a year.33,34 However, as more evidence emerges about the benefits of statins, and professional societies place more emphasis on their use, contemporary rates are likely higher. Indeed, of those who did present to follow-up, 89% of those discharged on a statin at discharge reported compliance, and 25% of patients not discharged on a statin received a prescription prior to follow-up. Of those patients taking statins, we lack data on the intensity of statin therapy, which prior studies demonstrate is associated with long-term outcomes as well.16,28 The VQI also fails to capture the cause of death in most patients, and so we cannot fully elucidate the protective mechanism of statins in this population. Future studies that capture cause of death and long-term cardiovascular outcomes would be helpful in answering these questions. In addition, this is a cohort of patients who underwent surgery at hospitals in the VQI (representing about 15% of the overall repairs in the United States), so these results may not be generalizable to the larger population of patients with AAAs.35 Lastly, there is likely selection bias, with preoperative and postoperative statin use serving as an indicator of adequate medical care. Our propensity weighting at least partially adjusts for this bias, but there may be residual confounding.
Conclusions
Preoperative statin therapy is associated with higher long-term survival, but not perioperative mortality and morbidity, in patients undergoing AAA repair. Additionally, initiating a statin prior to discharge in previously statin-naïve patients is associated with markedly higher survival. All patients with AAAs without contraindications should receive statin therapy. In patients not on a statin at the time of AAA repair, therapy should be initiated prior to discharge.
Take Home Message
In 37,950 patients undergoing AAA repair, preoperative statin use was associated with higher adjusted one year (94% vs 90%) and five year (85% vs 81%) survival (P<0.001) compared to those who were not on a statin, while those started on a statin postoperatively also had a one year (94% vs 91%) and five year (89% vs 81%) survival benefit (P<0.001).
Recommendation
This study suggests that patients undergoing AAA repair should be on a statin preoperatively or started on day one postoperatively prior to discharge, ti improve long-term survival
Acknowledgments
TO, SD and KS are supported by the Harvard-Longwood Research Training in Vascular Surgery NIH T32 Grant 5T32HL007734-22
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iannuzzi JC, Rickles AS, Kelly KN, Rusheen AE, Dolan JG, Noyes K, et al. Perioperative pleiotropic statin effects in general surgery. Surgery. 2014 Mar;155(3):398–407. doi: 10.1016/j.surg.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 2.London MJ, Schwartz GG, Hur K, Henderson WG. Association of Perioperative Statin Use With Mortality and Morbidity After Major Noncardiac Surgery. JAMA Intern Med. 2016 Dec 19; doi: 10.1001/jamainternmed.2016.8005. [DOI] [PubMed] [Google Scholar]
- 3.McNally MM, Agle SC, Parker FM, Bogey WM, Powell CS, Stoner MC. Preoperative statin therapy is associated with improved outcomes and resource utilization in patients undergoing aortic aneurysm repair. J Vasc Surg. 2010 Jun;51(6):1390–6. doi: 10.1016/j.jvs.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg. 2015;61(2):519–532.e1. doi: 10.1016/j.jvs.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Westin GG, Armstrong EJ, Bang H, Yeo K-K, Anderson D, Dawson DL, et al. Association Between Statin Medications and Mortality, Major Adverse Cardiovascular Event, and Amputation-Free Survival in Patients With Critical Limb Ischemia. J Am Coll Cardiol. 2014 Feb;63(7):682–90. doi: 10.1016/j.jacc.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn EW, Slottosch I, Wahlers T, Liakopoulos OJ. Preoperative statin therapy for patients undergoing cardiac surgery. In: Liakopoulos OJ, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2015. [DOI] [PubMed] [Google Scholar]
- 7.Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. In: Lewis SR, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2013. p. CD009971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galinanes EL, Reynolds S, Dombrovskiy VY, Vogel TR. The impact of preoperative statin therapy on open and endovascular abdominal aortic aneurysm repair outcomes. Vascular. 2015 Aug 1;23(4):344–9. doi: 10.1177/1708538114552837. [DOI] [PubMed] [Google Scholar]
- 9.de Bruin JL, Baas AF, Heymans MW, Buimer MG, Prinssen M, Grobbee DE, et al. Statin therapy is associated with improved survival after endovascular and open aneurysm repair J Vasc Surg. 2014 Jan;59(1):39–44.e1. doi: 10.1016/j.jvs.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association. Task Force on Practice Guidelines. Circulation. 2014 Dec 9;130(24):2215–45. doi: 10.1161/CIR.0000000000000105. [DOI] [PubMed] [Google Scholar]
- 11.Galiñanes EL, Reynolds S, Dombrovskiy VY, Vogel TR. The impact of preoperative statin therapy on open and endovascular abdominal aortic aneurysm repair outcomes. Vascular. 2015 Aug;23(4):344–9. doi: 10.1177/1708538114552837. [DOI] [PubMed] [Google Scholar]
- 12.Wemmelund H, Høgh A, Hundborg HH, Thomsen RW, Johnsen SP, Lindholt JS. Statin use and rupture of abdominal aortic aneurysm. Br J Surg. 2014 Jul;101(8):966–75. doi: 10.1002/bjs.9517. [DOI] [PubMed] [Google Scholar]
- 13.McNally MM, Agle SC, Parker FM, Bogey WM, Powell CS, Stoner MC. Preoperative statin therapy is associated with improved outcomes and resource utilization in patients undergoing aortic aneurysm repair. J Vasc Surg. 2010;51(6):1390–6. doi: 10.1016/j.jvs.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 14.De Martino RR, Beck AW, Hoel AW, Hallett JW, Arya S, Upchurch GR, et al. Preoperative antiplatelet and statin treatment was not associated with reduced myocardial infarction after high-risk vascular operations in the Vascular Quality Initiative. J Vasc Surg. 2016 Jan;63(1):182–9.e2. doi: 10.1016/j.jvs.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.London MJ, Schwartz GG, Hur K, Henderson WG, ED K, NJ S, et al. Association of Perioperative Statin Use With Mortality and Morbidity After Major Noncardiac Surgery. JAMA Intern Med. 2016 Dec 19;314(17):1818–31. doi: 10.1001/jamainternmed.2016.8005. [DOI] [PubMed] [Google Scholar]
- 16.Stone NJ, Robinson J, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. Circulation. 2013 Nov 12; [Google Scholar]
- 17.De Martino RR, Hoel AW, Beck AW, Eldrup-Jorgensen J, Hallett JW, Upchurch GR, et al. Participation in the Vascular Quality Initiative is associated with improved perioperative medication use, which is associated with longer patient survival. J Vasc Surg. 2015 Apr;61(4):1010–9. doi: 10.1016/j.jvs.2014.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schermerhorn ML, Bensley RP, Giles KA, Hurks R, OʼMalley AJ, Cotterill P, et al. Changes in Abdominal Aortic Aneurysm Rupture and Short-Term Mortality, 1995–2008. Ann Surg. 2012 Oct;256(4):651–8. doi: 10.1097/SLA.0b013e31826b4f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giles KA, Pomposelli F, Hamdan A, Wyers M, Jhaveri A, Schermerhorn ML. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg. 2009 Mar;49(3):543–50-1. doi: 10.1016/j.jvs.2008.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimerink JJ, van der Laan MJ, Koelemay MJ, Balm R, Legemate DA. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013 Oct;100(11):1405–13. doi: 10.1002/bjs.9235. [DOI] [PubMed] [Google Scholar]
- 21.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002 Jul 16;106(3):388–91. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106(25) [PubMed] [Google Scholar]
- 23.Durieux R, Van Damme H, Labropoulos N, Yazici A, Legrand V, Albert A, et al. High Prevalence of Abdominal Aortic Aneurysm in Patients with Three-vessel Coronary Artery Disease. Eur J Vasc Endovasc Surg. 2014;47(3):273–8. doi: 10.1016/j.ejvs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Hernesniemi JA, Vänni V, Hakala T. The prevalence of abdominal aortic aneurysm is consistently high among patients with coronary artery disease. J Vasc Surg. 2015 Jul;62(1):232–240.e3. doi: 10.1016/j.jvs.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 25.Van Kuijk JP, Flu WJ, Dunckelgrun M, Bax JJ, Poldermans D. Coronary artery disease in patients with abdominal aortic aneurysm: a review article. J Cardiovasc Surg (Torino) 2009 Feb;50(1):93–107. [PubMed] [Google Scholar]
- 26.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009 Oct;50(4):S2–49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Brown OW, Hollier LH, Pairolero PC, Kazmier FJ, McCready RA. Abdominal aortic aneurysm and coronary artery disease. Arch Surg. 1981 Nov;116(11):1484–8. doi: 10.1001/archsurg.1981.01380230098015. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell TFX, Deery SE, Darling JD, Shean KE, Mittleman MA, Yee GN, et al. Adherence to lipid management guidelines is associated with lower mortality and major adverse limb events in patients undergoing revascularization for chronic limb-threatening ischemia. J Vasc Surg. 2017 May 12; doi: 10.1016/j.jvs.2017.03.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baxter BT, Terrin MC, Dalman RL. Medical Management of Small Abdominal Aortic Aneurysms. Circulation. 2008;117(14) doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein LH, Berger J, Tranquilli M, Elefteraides JA. Effect of Statin Drugs on Thoracic Aortic Aneurysms. Am J Cardiol. 2013 Oct 15;112(8):1240–5. doi: 10.1016/j.amjcard.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 31.Neilipovitz DT, Bryson GL, Taljaard M. STAR VaS - Short Term Atorvastatin Regime for Vasculopathic Subjects: a randomized placebo-controlled trial evaluating perioperative atorvastatin therapy in noncardiac surgery. Can J Anesth Can d’anesthésie. 2012 Jun 13;59(6):527–37. doi: 10.1007/s12630-012-9702-z. [DOI] [PubMed] [Google Scholar]
- 32.Durazzo AE, Machado FS, Ikeoka DT, De Bernoche C, Monachini MC, Puech-Leão P, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004 May;39(5):967–75. doi: 10.1016/j.jvs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM, et al. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007 Oct 11;357(15):1477–86. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 34.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008 Apr;155(4):772–9. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Beck AW, Sedrakyan A, Mao J, Venermo M, Faizer R, Debus S, et al. Variations in Abdominal Aortic Aneurysm Care: A Report From the International Consortium of Vascular Registries. Circulation. 2016 Dec 13;134(24):1948–58. doi: 10.1161/CIRCULATIONAHA.116.024870. [DOI] [PMC free article] [PubMed] [Google Scholar]


