Abstract
Objective
Peripheral artery disease (PAD) is an increasing health concern with rising incidence globally. Previous studies show an association between PAD incidence and depression. The objective of the study was to determine the association of comorbid depression with PAD outcomes (amputation and all-cause mortality rates) in veterans.
Methods
An observational retrospective cohort of 155,647 patients with incident PAD (2003-2014) from Nationwide US Veterans Health Administration hospitals was conducted using the national Veterans Affairs corporate data warehouse. Depression was measured using concurrent ICD-9 diagnosis codes six months before or after PAD diagnosis. The main outcomes were incident major amputation and all-cause mortality. Crude associations were assessed with Kaplan Meier plots. The effects of depression adjusted for covariates were analyzed using Cox proportional hazards models.
Results
Depression was present in 16% of the cohort, with the occurrence of 9,517 amputations and 63,287 deaths (Median follow-up 5.9 years). Unadjusted HRs of comorbid depression for amputations and all-cause mortality were 1.32 (1.25, 1.39) and 1.02 (0.99, 1.04), respectively. After adjusting for covariates in Cox regression models, a diagnosis of comorbid depression at the time of PAD diagnosis was associated with a 13% higher amputation [HR 1.13; 95% CI (1.07, 1.19)] and 17% higher mortality [HR 1.17; 95% CI (1.14, 1.20)] risk as compared to patients with no depression. On stratification by use of antidepressants, depressed patients not taking antidepressants had a 42% higher risk of amputation [HR 1.42 (1.27, 1.58)] as compared to those without depression. Patients taking antidepressants for depression still had increased risk of amputation but only 10% higher compared to those without depression [HR 1.10 (1.03, 1.17)]. Interstingly, patient taking antidepressants for other indications also had a higher risk of amputation compred to those not having depression or not on antidepressants [ HR 1.08 (1.03, 1.14)]. Having any diagnosis of depression or the need for antidepressants increased the mortality risk by 18-25% in the PAD cohort compared to those without depression and not on antidperessants for any other indication.
Conclusions
PAD patients with comorbid depression have a significantly higher risk of amputation and mortality than PAD patients without depression. Furthermore, untreated depression was associated with an increased amputation risk in the PAD population more so than depression or other mental illness being treated by antidepressants. The underlying mechanisms for causality, if any, remain to be determined. The association of antidepressant treatment use with amputation risk should prompt further investigations into possible mechanistic links between untreated depression and vascular dysfunction.
Table of contents summary
In this population-based study of 155,647 veterans with incident PAD from 2003-2014, co-morbid depression was associated with an increased risk of death and amputation, particularly among depressed patients not taking antidepressants. The finding that use of anti-depressantss could mitigate the increased amputation risk in PAD patients with co- morbid depression warrants further investigation.
INTRODUCTION
Peripheral artery disease (PAD) is a significant health issue with a rising incidence globally1. Patients with PAD continue to be threatened with an unacceptably high risk for cardiovascular (CV) events2, 3 and suffer significant morbidity related to claudication and limb loss, resulting in a decreased quality of life4, 5. Inflammation and endothelial dysfunction play an important role in the pathophysiology of PAD. While circulating inflammatory markers are associated with disease progression and mortality6, impaired endothelial function independently predicts future CV events.7, 8 More recent evidence suggests that depression, which is associated with inflammation and endothelial dysfunction9, should join smoking, diabetes, hyperlipidemia, and hypertension as recognized risk factors for PAD10, 11.
Although there has been recognition of the role of depression in the development and progression of CV disease, the relationship remains less well characterized in the PAD population12–16. It has been previously demonstrated that patients with depressive symptoms had a higher risk of prospective PAD occurrence over a period of 7 years10. Depression in the setting of PAD also leads to decreased functional outcomes and walking capacity17, 18, worse patency rates after revascularization19, and increased progression of PAD20. McDermott et al also recently demonstrated a higher association for all-cause mortality and CV mortality in PAD patients with depression21.
The impact of co-morbid depression on the risk of amputation in patients with PAD is less understood. In addition, the effect of antidepressant treatment on outcomes in this population has not been studied. The present analysis sought to characterize the association of co-morbid depression and of antidepressant treatment with the risk of limb loss and mortality in the PAD population.
METHODS
Sample and Database
Incident PAD patients were identified using Veterans Health Administration (VHA) data from 2003-2014 (N=155,647) using a validated algorithm [ICD-9 diagnosis code for PAD and any one of three criteria: 1) Two ankle brachial indices (ABIs) in 14 months, 2) two visits to a vascular surgeon/clinic in 14 months, or 3) any PAD procedure code.]22 Patients with a PAD diagnosis code in 2 years prior (2000-2002) were excluded to capture incident cases. A comprehensive list of covariates were obtained: age at PAD diagnosis (estimated using date of birth), sex, race, and socioeconomic status (SES) [median household income of the patient’s most recent residential ZIP code tabulation area (ZCTA)], body mass index (BMI), serum creatinine and comorbidities such as diabetes mellitus (DM), hypertension, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), atrial fibrillation (AF), carotid disease, chronic kidney disease (CKD), and end-stage renal disease (ESRD). Smoking status (using a validated method23) and use of the following medications were assessed: antiplatelet agents, statins, cilostazol, antiglycemics, and antidepressants. All covariates were measured within a 6-month timeframe (before or after) of the PAD diagnosis date from the national Veterans Affairs (VA) Corporate Data Warehouse (CDW) and VA Medical SAS administrative databases.
Study Exposure and Outcome
Co-morbid depression was defined as having an ICD-9 diagnosis code of 293.83, 296.2×, 296.3×, 300.4, or 311 either at least once in an inpatient setting or at least twice within 14 months in an outpatient setting within 6 months before or after of the PAD diagnosis date (See Supplemental table I, definition based on data from prior VA studies)24–26. Antidepressant use was obtained from the VA pharmacy files for prescriptions filled at a VA pharmacy as well as the non-VA medication file – which holds data on medications filled by veterans and covered by the VA regardless of where it was filled. Antidepressants were classified as selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCA), monoamine-oxidase inhibitor (MAOI), and others (see supplemental Table I for the full list). Patients could be on multiple medications, but no differentiation was made between subjects on one or more drugs within a given category. In patients without a diagnosis for depression, we did not explore the indication for use of antidepressant medications for the purpose of this analysis [such as smoking cessation, post traumatic stress disorder (PTSD), obsessive –compulsive disorder (OCD), anxiety disorders, social phobia, panic disorder, bulimia nervosa, premenstrual dysphoric disorder, borderline personality disorder, obesity etc].
The outcomes of interest were incident major amputation (below and above knee) and death after the PAD diagnosis date. Specific amputation codes are defined in supplemental Table I, while long-term survival of the cohort was extracted from the VA vital status file. The follow-up continued through outcome occurrence, death (censoring event in the case of amputations), or December 31, 2015 (whereupon the subject was censored). Patients with prior amputations were included in the analysis but incident amputation was defined as the first amputation after the PAD diagnosis date.
Statistical Analysis
Demographic and clinical variables were assessed for the entire cohort and stratified by depression. Continuous variables were expressed as means (± standard deviations (SD)) or as medians (± interquartile ranges (IQR)) if they were not normally distributed. These were compared using independent sample t-tests or the Wilcoxon rank sum test. Discrete variables were compared using χ2 tests for proportions. Proportions of missing data were also calculated with no variables missing over 15% except smoking (36.6%). Kaplan-Meier curves were used to compare overall survival (OS) by depression status. Cumulative incidence function (CIF) curves, instead of KM curves, were used to compare amputation-free survival (AFS) by depression status so as to account for death as a competing risk for patients since it precludes them from experiencing an amputation. Treating death as independent or non-informative censoring (as done in KM curves) would produce overestimation of the probability of amputation. Cause- specific Cox proportional hazards regression models were then constructed to calculate adjusted hazard ratios (HR) and 95% confidence intervals (CI) for amputation and death by depression status alone, and then while allowing for interaction by antidepressant use. The models adjusted for the covariates listed in Table I. All variables were found to meet the proportional hazards assumption via log-log survival curves for amputation and mortality. Wald confidence limits were constructed for all hazard ratios. The statistical analysis was done using SAS version 9.4 (SAS Institute, Cary, NC). Two-sided p-values < 0.05 were considered statistically significant. This study was approved by the Emory University IRB and Atlanta VAMC Research and Development Committee. Informed consent was waived for a retrospective cohort study design with no human subject contact and minimal privacy risks.
Table I.
Demographics of full cohort and stratifications by depression for N = 155,647 patients meeting the PAD algorithm from 2003-2014.
| Variable | All | Depression | No Depression | p-value |
|---|---|---|---|---|
| PAD patients in study, No. | 155,647 | 24,895 | 130,752 | |
| Age, Mean (SD), years | 66.7 (9.9) | 63.6 (9.4) | 67.3 (9.8) | <0.0001 |
| Sex, % Male | 98 | 96 | 98 | <0.0001 |
| Median Followup time (years) | 5.9 | 5.5 | 6.0 | <0.0001 |
| Race, % | 0.0234 | |||
| White | 83 | 83 | 83 | |
| Black | 16 | 16 | 16 | |
| Other | 1 | 1 | 1 | |
| Smoking, % | <0.0001 | |||
| Current | 45 | 50 | 44 | |
| Former | 41 | 37 | 42 | |
| Never | 14 | 13 | 14 | |
| BMI, Mean (SD), kg/m2 | 28.6 (6.2) | 29.1 (6.7) | 28.5 (6.1) | <0.0001 |
| Creatinine, Median (IQR), mg/dL | 1.1 (0.9-1.4) | 1.1 (0.9-1.3) | 1.1(0.9-1.4) | 0.0002 |
| Comorbidities, % | ||||
| Diabetes | 45.5 | 48.8 | 44.9 | <0.0001 |
| Hypertension | 84.2 | 85.0 | 84.1 | 0.0002 |
| CAD | 46.3 | 49.4 | 45.7 | <0.0001 |
| CHF | 16.4 | 19.3 | 15.9 | <0.0001 |
| COPD | 8.5 | 11.2 | 8.0 | <0.0001 |
| AF | 12.0 | 12.1 | 12.0 | 0.68 |
| Carotid Disease | 63.8 | 66.8 | 63.2 | <0.0001 |
| CKD or ESRD | 7.4 | 8.0 | 7.2 | <0.0001 |
| Statins, % | 72.1 | 73.7 | 71.7 | <0.0001 |
| Antiplatelets, % | 79.4 | 80.5 | 79.1 | <0.0001 |
| Antidiabetic medications, % | 39.7 | 43.0 | 39.1 | <0.0001 |
| Cilostazol, % | 8.0 | 7.0 | 8.2 | <0.0001 |
| Antidepressant (% taking any) | 36.2 | 84.7 | 26.9 | <0.0001 |
| Median Household Income of Residential Zip Code, % | 0.02 | |||
| <=$25,000 | 3.3 | 3.4 | 3.3 | |
| $25,001-$40,000 | 27.6 | 28.0 | 27.5 | |
| $40,001-$75,000 | 59.4 | 59.4 | 59.4 | |
| $75,001+ | 9.8 | 9.3 | 9.9 | |
| PAD severity, % | <0.0001 | |||
| Not Specified (per ICD-9 codes) | 68.5 | 68.0 | 68.6 | |
| Claudication | 20.2 | 19.5 | 20.3 | |
| Rest Pain | 3.9 | 4.0 | 3.9 | |
| Ulceration/Gangrene | 7.3 | 8.5 | 7.1 |
Abbreviations: SD, standard deviation; IQR, interquartile range; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; CKD, chronic kidney disease; ESRD, end stage renal disease; mg, milligram; dL, deciliter.
RESULTS
The cohort consisted of 155,647 veterans with incident clinical PAD and a median follow-up of 5.9 years. The majority of the cohort was male (97.9%) with a mean age of 66.7 years (SD 9.9). There were 9,517 amputations and 63,287 deaths identified during the follow-up period. Of the total, 24,895 (16.0%) carried a diagnosis of depression. The demographics of the cohort are listed in Table I. Amongst patients with depression, 21,090 (84.7%) were on antidepressants [15,353 (61.7%) were on SSRI, 2,373 (9.5%) were on SNRI, 2,565 (10.3%) were on TCAs, 13 (0.1%) were on MAOI and 11,349 (45.6%) were on other meds] while 3,805 (15.3%) were not on antidepressants. Amongst patients who did not carry a diagnosis of depression, 27% were on some form of antidepressant medication likely for other indications (e.g. PTSD, OCD, smoking cessation etc).
Unadjusted associations of depression with amputation and mortality
In unadjusted Cox models, the risk of amputation was 32% higher [HR 1.32 (1.25, 1.39)] for patients with depression as compared to those without. Depression had no association with mortality risk [HR 1.02 (0.99, 1.04)] in unadjusted analysis. Additionally, the association of depression with amputation and mortality was further explored by stratifying by use of antidepressants. CIF curves showed that AFS among those with depression was worse for those not taking antidepressants as compared to those without depression as well as those with depression on antidepressants (Gray’s test, p<0.0001) [Figure I]. In terms of absolute risk, the 1- year, 3-year and 5-year AFS for patients with depression and not on antidepressants was 93.6%, 91.5% and 89.8%, respectively, compared to 97.8%, 96.3% and 95.2% AFS for patients without depression, respectively. Patients with depression on antidepressants or those without a diagnosis of depression but on antidepressants for other indications still had a significantly worse AFS as compared to those without depression (p<0.001). The absolute AFS at 1-year, 3-years and 5- years was 96.9%, 95.3% and 94.0% for depressed patients on antidepressants and 97.4%, 95.7% and 94.5% for those on antidepressants for other indications but better than those with untreated depression. With respect to mortality [Figure II], patients with untreated depression had a significantly lower OS [89.5% at 1 year, 76.3% at 3 years and 64.3% at 5 years] compared to the other three groups: patients without depression [94.4% at 1 year, 83.30% at 3 years and 71.5% at 5 years], patients on antidepressants for other indications [94.0% at 1 year, 82.2% at 3 years and 70.9% at 5 years] or those with depression but taking antidepressants [94.0% at 1 year, 81.9% at 3 years and 70.1% at 5 years] (p<0.001). Patients with depression on antidepressants had no significant difference in mortality as compared to those without depression, whether on antidepressants or not (p= 0.08).
Figure I. Cumulative incidence function (CIF) curves to compare amputation-free survival (AFS) by depression status (A) and use of antidepressants (B).
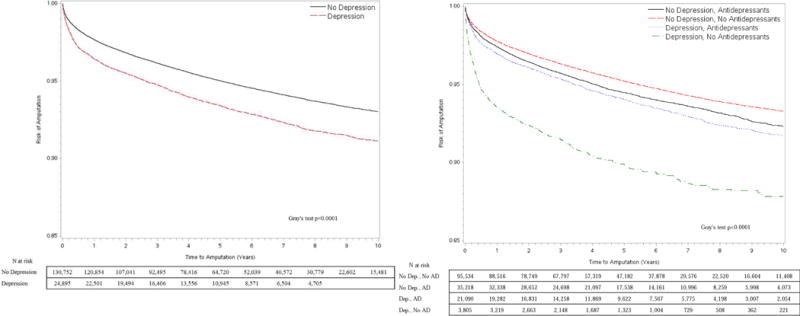
Death was a competing risk and subjects were censored on December 31, 2015.
Figure II. Kaplan Meier (KM) curves to compare Overall Survival (OS) by depression status (A) and use of antidepressants (B).
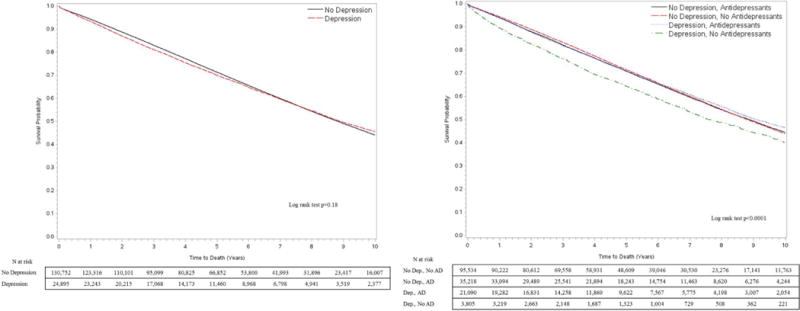
Subjects were censored on December 31, 2015.
Adjusted associations of depression with amputation and mortality
After adjusting for covariates (outlined in methods) in a Cox model [Table II], the hazard of death was 17% higher for depressed subjects versus non-depressed subjects [HR 1.17, 95% CI 1.14-1.20]. Additionally, the risk of amputation was 13% higher for depressed subjects [HR 1.13, 95% CI 1.07-1.19]. Female sex [HR for death 0.82, HR for amputation 0.44], higher BMI [HR for death 0.97, HR for amputation 0.96], Statin use [HR for death 0.77, HR for amputation 0.74], antiplatelet use [HR for death 0.71, HR for amputation 0.87], and Cilostazol use [HR for death 0.92, HR for amputation 0.77] were all associated with lower risks of death and amputation. Black race (vs white) was a significant predictor for increased risk of amputation [HR 1.42] but had a survival advantage in terms of mortality [HR 0.83] while low SES [HR for death 1.37, HR for amputation 1.15] had independently higher risk of mortality and amputation. Traditional atherosclerotic risk factors were associated with higher risk of mortality and amputation such as diabetes [HR for death 1.18, HR for amputation 1.74], hypertension [HR for death 1.11, HR for amputation 1.27] and current smoking [HR for death 1.28, HR for amputation 1.17]. Of note, CHF [HR for death 1.91, HR for amputation 1.52] and CKD/ESRD [HR for death 1.47, HR for amputation 1.68] were also significant predictors of poor outcomes in PAD. PAD severity in terms of late presentation as rest pain or tissue loss (vs claudication) had a very strong association with death and major amputation [HR for death 1.19 and 1.71 respectively, HR for amputation 2.58 and 8.19 respectively].
Table II.
Cox proportional hazards model for effect of depression on mortality and amputations in PAD patients (N=129,157).
| Variable | Death | Amputation |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Depression (Ref. No Depression) | 1.17 (1.14, 1.20) | 1.13 (1.07, 1.19) |
| Age (1-year increase) | 1.05 (1.05, 1.05) | 0.98 (0.98, 0.98) |
| Race | – | – |
| White | Ref. | Ref. |
| Black | 0.83 (0.81, 0.85) | 1.42 (1.35, 1.50) |
| Other | 0.87 (0.81, 0.95) | 1.14 (0.94, 1.37) |
| Sex (Ref. Male) | 0.82 (0.76, 0.88) | 0.44 (0.35, 0.54) |
| Smoking | ||
| Never | Ref. | Ref. |
| Current | 1.28 (1.23, 1.32) | 1.17 (1.07, 1.28) |
| Former | 1.08 (1.03, 1.12) | 0.95 (0.86, 1.05) |
| Unknown | 1.14 (1.10, 1.19) | 1.00 (0.91, 1.10) |
| BMI (1 kg/m2 increase) | 0.97 (0.97, 0.97) | 0.96 (0.96, 0.97) |
| Creatinine (1 mg/dL increase) | 1.09 (1.09, 1.10) | 1.02 (1.00, 1.03) |
| Comorbidities (Ref. = No) | ||
| Diabetes | 1.18 (1.14, 1.23) | 1.74 (1.58, 1.91) |
| Hypertension | 1.11 (1.08, 1.14) | 1.27 (1.18, 1.37) |
| CAD | 1.20 (1.18, 1.23) | 1.05 (1.00, 1.10) |
| CHF | 1.91 (1.87, 1.95) | 1.52 (1.44, 1.61) |
| COPD | 1.34 (1.31, 1.38) | 0.91 (0.84, 0.99) |
| AF | 1.37 (1.33, 1.40) | 1.27 (1.18, 1.35) |
| Carotid Disease | 1.09 (1.07, 1.11) | 1.16 (1.10, 1.22) |
| CKD or ESRD | 1.47 (1.42, 1.53) | 1.68 (1.54, 1.83) |
| Statin (Ref. None) | 0.77 (0.75, 0.78) | 0.74 (0.71, 0.78) |
| Antiplatelets (Ref. None) | 0.71 (0.69, 0.72) | 0.87 (0.83, 0.93) |
| Antiglycemics (Ref. None) | 1.17 (1.13, 1.21) | 1.36 (1.24, 1.49) |
| Cilostazol (Ref. No) | 0.92 (0.89, 0.95) | 0.77 (0.69, 0.85) |
| Antidepressants (Ref. None) | – | – |
| Median Income (Household) of Residential ZCTA (Ref. >$75,000) | ||
| <=$25,000 | 1.37 (1.30, 1.45) | 1.15 (1.01, 1.31) |
| $25,001-$40,000 | 1.16 (1.12, 1.19) | 1.15 (1.05, 1.25) |
| $40,001-$75,000 | 1.09 (1.06, 1.12) | 1.04 (0.96, 1.13) |
| PAD Severity (Ref. = Claudication) | ||
| Not Specified | 1.01 (0.99, 1.04) | 1.16 (1.07, 1.24) |
| Rest Pain | 1.19 (1.13, 1.25) | 2.58 (2.31, 2.88) |
| Ulceration/Gangrene | 1.71 (1.65, 1.77) | 8.19 (7.59, 8.85) |
Abbreviations: HR, hazard ratio; CI, confidence interval, SD, standard deviation; IQR, interquartile range; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; CKD, chronic kidney disease; ESRD, end stage renal disease; mg, milligram; dL, deciliter; ZCTA, zip code tabulation area.
Interaction of depression and use of antidepressants and its association with amputation and mortality
There was a statistically significant interaction between depression and antidepressant medications with regards to amputation (p<0.0001) [Table III, supplemental Table II]. After adding an interaction term to stratify by antidepressant use, depressed subjects not taking antidepressants had significantly elevated hazards of amputation [HR 1.42, 95% CI 1.27-1.58] relative to non-depressed patients and not on any antidepressants. Depressed patients on antidepressants as well as nondepressed patients but on antidepressants for other indications had a moderately higher risk of amputations compared to the non-depressed PAD patients [HR 1.08, 95% CI 1.03-1.14 and HR 1.10, 95% CI 1.03-1.17, respectively], but not to the same degree as untreated depression. Mortality risk was 18-25% higher for patients with depression (with or without use of antidperessants) or on antidepressants for other indications compared to those who were not depressed and not needing antidepressants for any other diagnoses. However, between these three groups, there was no significant difference in mortality for PAD patients [No depression on antidepressants for other indications (HR 1.18, 95% CI 1.16-1.21), depression with antidepressant use (HR 1.22, 95% CI 1.19-1.26) and without antidepressant use (HR 1.25, 95% CI 1.18-1.33)].
Table III.
Cox proportional hazard model results for effect of depression on mortality and amputations: unadjusted hazards, primary model, primary model with interaction for antidepressants.
| Mortality | Amputation | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
|
Unadjusted hazards (N=129,157) | ||
| Depression, Yes vs. No | 1.02 (0.99, 1.04) | 1.32 (1.25,1.39) |
|
Primary modela (N=129,157) | ||
| Depression, Yes vs. No | 1.17 (1.14, 1.20) | 1.13 (1.07, 1.19) |
|
Primary modela, with interaction by antidepressant status (N=129,157) | ||
| Depression and Antidepressants interaction | ||
| No Depression, No Antidepressants | Ref. | Ref. |
| No Depression, Antidepressants for other indications | 1.18 (1.16, 1.21) | 1.08 (1.03, 1.14) |
| Depression, Antidepressants | 1.22 (1.19, 1.26) | 1.10 (1.03, 1.17) |
| Depression, No Antidepressants | 1.25 (1.18, 1.33) | 1.42 (1.27, 1.58) |
Abbreviations: HR, hazard ratio; CI, confidence interval.
Model adjusting for age at entry into cohort, year of entry into cohort, race, sex, socio-economic status, body mass index, PAD severity (claudication, unspecified, rest pain, ulceration/gangrene), comorbidities [Diabetes mellitus, hypertension, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, atrial fibrillation, carotid disease, chronic kidney disease and end stage renal disease], antiplatelet medications, cilostazol, antiglycemics, statins, serum creatinine.
DISCUSSION
In this large cohort of veterans with incident PAD, the present study found that patients with co- morbid depression had a significantly higher risk of amputation (13%) and all-cause mortality (17%) than PAD patients without depression, after adjusting for covariates. There was a significant interaction of antidepressant use on the risk of amputation in PAD patients with depression but not so much on mortality. Being on prescription antidepressants for depression was associated with a significantly lowered risk for amputation (10% higher than non-depressed patients, not on antidepressants) in contrast to those with depression and not on antidepressants (42% higher than non-depressed patients, not on antidepressants). Interestingly, patients on antidepressants for other diagnoses (e.g. PTSD, OCD, anxiety etc) were also at a slightly higher risk of amputation (8% higher than non-depressed, not on antidepressants). Having any diagnosis of depression or the need for antidepressants increased the mortality risk by 18-25% in the PAD cohort. The data underline the negative impact of depression on prognosis of patients with PAD. Although causality is not established, the results presented also highlight the importance of further investigating depressive treatment options in the care of PAD patients suffering from depression.
Association between PAD and Depression
While the evidence base for the relationship between depression and heart disease is growing12–16, the relationship between depression and PAD is still less well understood. In the Atherosclerosis Risk in Communities Study, Wattanakit et al. demonstrated that depression and anger proneness were associated with greater incident PAD independent of other PAD risk factors27. Using data from a large elderly Asian population, Wong et al. demonstrated that depressive symptoms were associated with subclinical PAD even after adjusting for stroke and other cardiovascular diseases28. McDermott et al. found that depression was present in 22% of patients with PAD, and an increasing number and severity of depressive symptoms were associated with greater impairment in lower extremity functioning29. Other investigators have demonstrated that PAD patients with greater depressive symptoms have more dramatic annual declines in functional performance30, including reduced walking distance and fast walking velocity. Smolderen et al. found that depression was associated with less improvement in health status after undergoing revascularization31. Cherr and colleagues demonstrated that patients with depression are at increased risk for cardiovascular events and progression of contralateral PAD after undergoing lower extremity revascularization20. The results of the current study provide further evidence of a clear association between depression and PAD by demonstrating that depression is a significant risk factor for the endpoints of amputation and mortality in the management of PAD. In the cohort, depressed patients initially did not appear to have any mortality difference in unadjusted KM analysis. However, they were on average younger than non-depressed patients with more prevalence of current smoking and other comorbidities such as diabetes, CAD, CHF, COPD and kidney disease. Upon adjustment for these confounders, there was a strong association in terms of 17% increased risk of mortality with depression. To our knowledge, it is the first time that the association of depression on PAD outcomes has been demonstrated in such a large cohort and we also demonstrate an intriguing association between anti-depressant treatment and amputation. These findings deserve further investigation as they have the potential to improve health outcomes in a large population.
Mechanistic Pathways
The association between depression and PAD adverse outcomes may likely to be explained by a composite of behavioral and biological mechanisms as has been previously reported10. In the Cardiovascular Health Study, 25% of cardiovascular mortality in patients with depression was attributed to physical inactivity32, supporting findings by Whooley et al. that adjusting for behavioral mediators, particularly physical inactivity, eliminated the 31% association between depression and cardiovascular events in patients with stable CAD33. In a prospective cohort of veterans, the Heart and Soul Study found that 12% of patients with depressive symptoms were shown to have prevalent PAD compared to 7% of patients without depressive symptoms10. After exclusion of patients with a self-reported history of PAD, incident PAD events occurred in 7% of patients with depressive symptoms and 5% of patients without depressive symptoms. Factors explaining >5% of the association between depression and incident PAD events included race/ethnicity, diabetes, congestive heart failure, high-density lipoprotein, triglyceride levels, serum creatinine, inflammation (CRP, IL-6, TNF-α), smoking, and levels of physical activity.
Several investigators have reported the association of depression with different physiological systems, which may have mechanistic implications for the present study’s findings. These include autonomic dysfunction due to excessive sympathetic or insufficient parasympathetic activity34, 35, alterations in the hypothalamic-pituitary-adrenal axis resulting in elevated cortisol level and dexamethasone nonsuppression36, 37, and platelet hyperaggregability related to elevated plasma levels of platelet factor 4 and beta thromboglobulin.38–40 Endothelial dysfunction associated with reduced levels of endothelial progenitor cells and attenuated flow mediated dilation41–43 may also be involved as well as a heightened inflammatory response indicated by elevated inflammatory markers such as CRP, TNF-a, and IL-6.44–47 Investigations thus far support a relative contribution of both physiological and behavioral factors driving the association between depression and vascular disease. The results support the role of more research to better understand the mechanisms involved in the reported association in order to help direct treatment regimens.
Role of Anti-depressants
The findings from this study suggest that antidepressant therapy may alleviate the morbid burden of depression on PAD outcomes related to limb loss but not mortality, although causality remains to be determined. Possible explanations for these findings include a physiological effect of anti-depressants on limb loss in PAD (true causality) or residual confounding from care utilization. In terms of healthcare access and utilization, depressed patients were more likely to be Caucasian, present with tissue loss at PAD diagnosis, and slightly higher chance of being in the lowest SES stratum. However, they had higher prescription rates of antiplatelet, statin and diabetic medications but lower prescription of cilostazol. Despite adjustment for these markers, there was significant effect modification of amputation risk by treatment of depression. Furthermore, nondepressed patients who were on antidepressants for other indications had a moderately higher risk of amputations compared to the non-depressed PAD patients [HR 1.10] which was similar for treated depressed patients [HR 1.08]. This group of patients likely had other mental illnesses necessitating use of antidepressants. There are newer studies suggesting the increased vascular dysfunction in disorders such as PTSD that are treated with antidperessants48, 49. Some of the mechanisms of action of antidepressants involve pathophysiological processes in PAD and CV diseases, such as platelet activation.50 In fact, the impairment of primary hemostasis induced by SSRIs may result in a reduction in thrombotic risk.51
The present study’s findings bring more data in an area where controversy remains related to the effect of antidepressants on the incidence of CV events. In fact, several randomized studies have taken place on the use of antidepressant in patients with CV disease, with mixed results.52 It is felt that a large trial on the use of antidepressant therapy vs. placebo would bring ethical concerns because depression in itself requires treatment. With regards to PAD, studies examining the role of antidepressants are limited (or non-existent) and the present study qualifies the potential benefit antidepressants may have in PAD patients with depression. Future studies are needed to support this finding that antidepressants are related to decreased limb loss in patients with PAD with depression and whether depression should be more aggressively treated in patients with cardiovascular disease. Also future studies are needed in ascertaining the risk of other mental illnesses and their treatement (PTSD, OCD, anxiety etc) on PAD outcomes.
Limitations
This study has several limitations. First, this is an observational study using administrative data, which come from clinical care records, and the analysis may be susceptible to residual confounding. A significant effort was made to account for accurate PAD diagnosis as well as performing a comprehensive Cox model. Additionally, careful handling of missing data were done to minimize and investigate the possibility of bias. Right censoring was done at occurrence of outcomes (death/amputation) [which are fairly accurately reported given the multiple data sources used] or the end of the followup in the study but we could miss the occurrence of amputations in a small percentage of patients who sought all their care outside the VA system and did not use VA coverage (fee-basis). The study did not assess for severity of depression or response to treatment, which represents another limitation. With regards to treatment of depression, it is possible that patients treated for depression were treated with psychotherapy which was not captured in the analysis. Amongst patients on antidepressants we couldn’t assess medication compliance. If there is a direct medication effect on amputation risk, data on compliance could help delineate that effect. We also did not investigate the indications for use of anti-depressants in patients who did not carry a diagnosis of depression. We therefore cannot comment on the role of other mental illnesses on PAD amputation risk but it would be something to explore in future manuscripts. Additionally, the study is based on VHA data and it is overwhelmingly comprised of male patients. Therefore, results may differ in a non-VA or female population.
CONCLUSION
The clinical significance of co-morbid depression on PAD is becoming more recognized. The findings of the present study demonstrate that PAD patients with depression are at a higher risk of long-term death and amputation, and that amputation risk may be modifiable based on treatment of depression. The 2016 American Heart Association guidelines for the management of patients with lower extremity PAD have called for new medical therapies for patients PAD.53 We suggest raised awareness related to mental health issues in this population. We also press for continued investigation into mechanisms involved in worse outcomes for those with depression (mechanisms specific to both overall survival and limb salvage) as well as treatment strategies to alleviate this risk.
Supplementary Material
Supplemental Table I. ICD-9 diagnosis and CPT codes used to define comorbidities and amputations; list of antidepressants.
Supplemental Table II. Full results of Cox proportional hazards model for effect of depression on death and major amputations in PAD patients, stratified by antidepressant use. (N=129,157).
Abbreviations: HR, hazard ratio; CI, confidence interval, SD, standard deviation; IQR, interquartile range; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; CKD, chronic kidney disease; ESRD, end stage renal disease; mg, milligram; dL, deciliter; ZCTA, zip code tabulation area.
Take Home Message
In 155,647 veterans with peripheral artery disease (PAD), co-morbid depression was present in 16% and it was associated with increased risk of death and amputation, particularly among depressed patients not taking antidepressants.
Recommendation
The authors suggest that all patients with PAD be evaluated for depression and receive treatment if depression is identified.
Acknowledgments
This work was supported by start-up funds from the University of California San Francisco and the Northern California Institute for Research and Education. Additional support came from Award Number KL2RR024130 from the National Center for Research Resources, Award Number 1K23HL122446-01 from the National Institute of Health/NHLBI, and a Society for Vascular Surgery Seed Grant and Career Development Award (Grenon). Furthermore, the work was also supported by an American Heart Association Mentored Clinical and Population Research Award (15MCPRP25580005), NIH-NIA Award Number 1R03AG050930, and an American Geriatric Society/Society for Vascular Surgery (SVS) Foundation Jahnigen Career Development Award (Arya). This material is the result of work supported with resources and the use of facilities at the Atlanta VA Medical Center, Decatur GA.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The work does not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The funding organizations were not involved in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation, review or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note to editor: Abstract presented at the Association for Academic Surgery Meeting (2017) at Las Vegas, Feb 9th 2017.
Author conflict of interest: none.
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Grenon SM, Vittinghoff E, Owens CD, Conte MS, Whooley M, Cohen BE. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: insights from the Heart and Soul Study. Vascular medicine. 2013;18:176–84. doi: 10.1177/1358863X13493825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114:688–99. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 4.Sampson UK, Fowkes FG, McDermott MM, Criqui MH, Aboyans V, Norman PE, et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart. 2014;9:145–158 e21. doi: 10.1016/j.gheart.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vascular medicine. 2008;13:15–24. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 6.Vidula H, Tian L, Liu K, Criqui MH, Ferrucci L, Pearce WH, et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med. 2008;148:85–93. doi: 10.7326/0003-4819-148-2-200801150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. Journal of the American College of Cardiology. 2003;41:1769–75. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 8.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–72. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 9.van Dooren FE, Schram MT, Schalkwijk CG, Stehouwer CD, Henry RM, Dagnelie PC, et al. Associations of low grade inflammation and endothelial dysfunction with depression – The Maastricht Study. Brain Behav Immun. 2016;56:390–6. doi: 10.1016/j.bbi.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Grenon SM, Hiramoto J, Smolderen KG, Vittinghoff E, Whooley MA, Cohen BE. Association between depression and peripheral artery disease: insights from the heart and soul study. Journal of the American Heart Association. 2012;1:e002667. doi: 10.1161/JAHA.112.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grenon SM, Cohen BE, Smolderen K, Vittinghoff E, Whooley MA, Hiramoto J. Peripheral arterial disease, gender, and depression in the Heart and Soul Study. Journal of vascular surgery. 2014;60:396–403. doi: 10.1016/j.jvs.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lett H, Ali S, Whooley M. Depression and cardiac function in patients with stable coronary heart disease: findings from the Heart and Soul Study. Psychosom Med. 2008;70:444–9. doi: 10.1097/PSY.0b013e31816c3c5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McManus D, Pipkin SS, Whooley MA. Screening for depression in patients with coronary heart disease (data from the Heart and Soul Study) Am J Cardiol. 2005;96:1076–81. doi: 10.1016/j.amjcard.2005.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otte C, Marmar CR, Pipkin SS, Moos R, Browner WS, Whooley MA. Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: The Heart and Soul Study. Biol Psychiatry. 2004;56:241–7. doi: 10.1016/j.biopsych.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the Heart and Soul Study. Biol Psychiatry. 2007;62:314–20. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wulsin LR, Musselman D, Otte C, Bruce E, Ali S, Whooley MA. Depression and whole blood serotonin in patients with coronary heart disease from the Heart and Soul Study. Psychosom Med. 2009;71:260–5. doi: 10.1097/PSY.0b013e31819cc761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smolderen KG, Aquarius AE, de Vries J, Smith OR, Hamming JF, Denollet J. Depressive symptoms in peripheral arterial disease: a follow-up study on prevalence, stability, and risk factors. J Affect Disord. 2008;110:27–35. doi: 10.1016/j.jad.2007.12.238. [DOI] [PubMed] [Google Scholar]
- 18.McDermott MM, Greenland P, Guralnik JM, Liu K, Criqui MH, Pearce WH, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18:461–7. doi: 10.1046/j.1525-1497.2003.20527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherr GS, Wang J, Zimmerman PM, Dosluoglu HH. Depression is associated with worse patency and recurrent leg symptoms after lower extremity revascularization. Journal of vascular surgery. 2007;45:744–50. doi: 10.1016/j.jvs.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 20.Cherr GS, Zimmerman PM, Wang J, Dosluoglu HH. Patients with Depression are at Increased Risk for Secondary Cardiovascular Events after Lower Extremity Revascularization. Journal of General Internal Medicine. 2008;23:629–634. doi: 10.1007/s11606-008-0560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott MM, Guralnik JM, Tian L, Kibbe MR, Ferrucci L, Zhao L, et al. Incidence and Prognostic Significance of Depressive Symptoms in Peripheral Artery Disease. Journal of the American Heart Association. 2016;4:e002959. doi: 10.1161/JAHA.115.002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arya S, Binney Z, Khakharia A, Brewster LP, Wilson PWF, Goodney PP. Peripheral arterial disease (PAD) variability in diagnosis: The Veterans Affairs (VA) population experience. Association of VA Surgeons 40th Annual Surgical Symposium. 2016 [Google Scholar]
- 23.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2011;13:1233–9. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherrer JF, Virgo KS, Zeringue A, Bucholz KK, Jacob T, Johnson RG, et al. Depression increases risk of incident myocardial infarction among Veterans Administration patients with rheumatoid arthritis. General hospital psychiatry. 2009;31:353–9. doi: 10.1016/j.genhosppsych.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Richardson LK, Egede LE, Mueller M, Echols CL, Gebregziabher M. Longitudinal effects of depression on glycemic control in veterans with Type 2 diabetes. General hospital psychiatry. 2008;30:509–14. doi: 10.1016/j.genhosppsych.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Chermack ST, Zivin K, Valenstein M, Ilgen M, Austin KL, Wryobeck J, et al. The prevalence and predictors of mental health treatment services in a national sample of depressed veterans. Medical care. 2008;46:813–20. doi: 10.1097/MLR.0b013e318178eb08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wattanakit K, Williams JE, Schreiner PJ, Hirsch AT, Folsom AR. Association of anger proneness, depression and low social support with peripheral arterial disease: the Atherosclerosis Risk in Communities Study. Vascular medicine. 2005;10:199–206. doi: 10.1191/1358863x05vm622oa. [DOI] [PubMed] [Google Scholar]
- 28.Wong SY, Woo J, Hong AW, Leung JC, Leung PC. Clinically relevant depressive symptoms and peripheral arterial disease in elderly men and women. Results from a large cohort study in Southern China. J Psychosom Res. 2007;63:471–6. doi: 10.1016/j.jpsychores.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott MM, Greenland P, Guralnik JM, Liu K, Criqui MH, Pearce WH, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18:461–7. doi: 10.1046/j.1525-1497.2003.20527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruo B, Liu K, Tian L, Tan J, Ferrucci L, Guralnik JM, et al. Persistent depressive symptoms and functional decline among patients with peripheral arterial disease. Psychosom Med. 2007;69:415–24. doi: 10.1097/PSY.0b013e318063ef5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smolderen KG, Safley DM, House JA, Spertus JA, Marso SP. Percutaneous transluminal angioplasty: association between depressive symptoms and diminished health status benefits. Vascular medicine. 2011;16:260–6. doi: 10.1177/1358863X11415568. [DOI] [PubMed] [Google Scholar]
- 32.Win S, Parakh K, Eze-Nliam CM, Gottdiener JS, Kop WJ, Ziegelstein RC. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the Cardiovascular Health Study. Heart. 2011;97:500–5. doi: 10.1136/hrt.2010.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–88. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. 2004;57:353–8. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, et al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25:2117–24. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 36.Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 37.Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154:1497–503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- 38.Laghrissi-Thode F, Wagner WR, Pollock BG, Johnson PC, Finkel MS. Elevated platelet factor 4 and beta-thromboglobulin plasma levels in depressed patients with ischemic heart disease. Biol Psychiatry. 1997;42:290–5. doi: 10.1016/S0006-3223(96)00345-9. [DOI] [PubMed] [Google Scholar]
- 39.Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. 2000;140:57–62. doi: 10.1067/mhj.2000.109978. [DOI] [PubMed] [Google Scholar]
- 40.Ormonde do Carmo MB, Mendes-Ribeiro AC, Matsuura C, Pinto VL, Mury WV, et al. Major depression induces oxidative stress and platelet hyperaggregability. J Psychiatr Res. 2015;61:19–24. doi: 10.1016/j.jpsychires.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Cooper DC, Tomfohr LM, Milic MS, Natarajan L, Bardwell WA, Ziegler MG, et al. Depressed mood and flow-mediated dilation: a systematic review and meta-analysis. Psychosom Med. 2011;73:360–9. doi: 10.1097/PSY.0b013e31821db79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serebruany VL, Glassman AH, Malinin AI, Sane DC, Finkel MS, Krishnan RR, et al. Enhanced platelet/endothelial activation in depressed patients with acute coronary syndromes: evidence from recent clinical trials. Blood Coagul Fibrinolysis. 2003;14:563–7. doi: 10.1097/00001721-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. Journal of the American College of Cardiology. 2005;46:656–9. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 44.Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- 45.Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 46.Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009;23:936–44. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosom Med. 2003;65:362–8. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- 48.Grenon SM, Owens CD, Alley H, Perez S, Whooley MA, Neylan TC, et al. Posttraumatic Stress Disorder Is Associated With Worse Endothelial Function Among Veterans. Journal of the American Heart Association. 2016;5:e003010. doi: 10.1161/JAHA.115.003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clausen AN, Aupperle RL, Sisante JF, Wilson DR, Billinger SA. Pilot Investigation of PTSD, Autonomic Reactivity, and Cardiovascular Health in Physically Healthy Combat Veterans. PloS one. 2016;11:e0162547. doi: 10.1371/journal.pone.0162547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serebruany VL, Glassman AH, Malinin AI, Nemeroff CB, Musselman DL, van Zyl LT, et al. Sertraline AntiDepressant Heart Attack Randomized Trial Study G Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation. 2003;108:939–44. doi: 10.1161/01.CIR.0000085163.21752.0A. [DOI] [PubMed] [Google Scholar]
- 51.de Abajo FJ. Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging. 2011;28:345–67. doi: 10.2165/11589340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Elderon L, Whooley MA. Depression and cardiovascular disease. Prog Cardiovasc Dis. 2013;55:511–23. doi: 10.1016/j.pcad.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;2016 doi: 10.1161/CIR.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table I. ICD-9 diagnosis and CPT codes used to define comorbidities and amputations; list of antidepressants.
Supplemental Table II. Full results of Cox proportional hazards model for effect of depression on death and major amputations in PAD patients, stratified by antidepressant use. (N=129,157).
Abbreviations: HR, hazard ratio; CI, confidence interval, SD, standard deviation; IQR, interquartile range; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; AF, atrial fibrillation; CKD, chronic kidney disease; ESRD, end stage renal disease; mg, milligram; dL, deciliter; ZCTA, zip code tabulation area.


