Abstract
Background
Many studies have focused on the association between a single measurement of blood pressure (BP) and the risk of adverse outcomes. However, the association of BP trajectories during young adulthood with subsequent decline in renal function has not been well-defined.
Study design
Observational cohort study.
Setting and Participants
3,429 participants of the Coronary Artery Risk Development in Young Adulthood (CARDIA) study enrolled between the ages of 18 and 30 years.
Predictors
BP slope during the first 10 years of participation in CARDIA, derived from linear mixed models incorporating all repeated BP measures.
Outcome
Decline in estimated glomerular filtration rate (eGFR) during the interval between the 10th and 20th years of CARDIA participation using cystatin C measured at years 10, 15, and 20.
Results
Mean age of CARDIA participants at year 0 was 25.1 years, 56% were women, and 53% were white. Every 10 mmHg higher level of systolic (SBP) and diastolic (DBP) BP in year 10 was associated with a change in eGFR of −0.09 (95% CI, −0.13 to −0.06) and −0.07 (95% CI −0.12 to −0.03) mL/min/1.73m2 per year, respectively. Every 10 mmHg increase in SBP slope between years 0 and 10 was associated with a subsequent −0.52 (95% CI, −1.02 to −0.03) mL/min/1.73 m2 per year change in renal function after adjustment for comorbidities and SBP at year 10. Similarly, every 10 mmHg increase in DBP slope between years 0 and 10 was associated with a subsequent change in renal function of −0.65 (95% CI, −1.23 to −0.07) mL/min/1.73 m2 per year, after adjustment for comorbidities and DBP in year 10.
Limitations
Observational design
Conclusions
During young adulthood, increasing SBP and DBP are associated with a higher rate of subsequent renal function decline, independent of BP measured at the beginning of eGFR assessment.
Keywords: blood pressure, SBP, DBP, hypertension, BP slope, kidney function, estimated glomerular filtration rate (eGFR), eGFR trajectory, young adulthood, modifiable risk factor, renal outcome, renal function
Introduction
The majority of studies of hypertension and its related complications have been conducted in individuals over 40 years of age.1–4 In addition, studies focused on the implications of elevated BPs during young adulthood have primarily evaluated the associations between elevated BP and risk of cardiovascular outcomes. For example, one-time BP measurements taken during early life have been shown to predict the risk of subsequent carotid intimal thickness, cardiovascular disease, and mortality in later adulthood.5–8 Whether exposure to elevated BPs during early life may also associate with adverse renal outcomes is less well known.9 To our knowledge, no prior large population based study has examined the association between BP levels during young adulthood and risk of subsequent renal function decline.
The Joint National Committee currently recommends treating adults over the age of 18 once blood pressure (BP) levels exceed the threshold of 140/90 mm Hg,10 although recent guidelines have lowered the threshold for the definition of hypertension and recommended treatment to even lower targets.11 Most BP guidelines have not considered BP trajectories in their recommendations for treatment, even though longitudinal changes in BPs over time have been shown to be associated with an increased risk for intermediate cardiovascular outcomes.12 For example, a recent study in the Coronary Artery Risk Development in Young Adults (CARDIA) study demonstrated that BP trajectories during early adulthood add to the prognostication of coronary artery calcification in later life beyond the consideration of one-time BP measurements.12 Another recent study in CARDIA demonstrated that cumulative exposure to elevated systolic BP was associated with a higher subsequent risk of albuminuria.13 However, less is currently known about the association between BP trajectories and risk of renal function decline in middle adulthood. This knowledge is important, as it would help clarify whether clinicians should focus on trends in BP over time to identify young patients who may be at-risk for kidney function decline in later life and who are candidates for primordial prevention. These data could also guide recommendations regarding whether repeated BP screening (for determination of BP trajectory) may be useful in otherwise asymptomatic young adults.
The main objective of this study was to evaluate the association between changes in BP during young adulthood (between ages 18–40 years) and subsequent kidney function decline in later life within CARDIA.14 We hypothesized that higher BPs during young adulthood would be associated with faster kidney function decline. We also hypothesized that changes in BP during young adulthood (even at BP levels currently not considered to meet the definition for hypertension) would remain strongly associated with future renal function decline, even after accounting for BPs taken at the time-point closest to the period of renal function trajectory assessment.
Methods
Participants
We included data from participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. CARDIA is a prospective cohort study designed to understand the development of cardiovascular risk factors in young adults.14
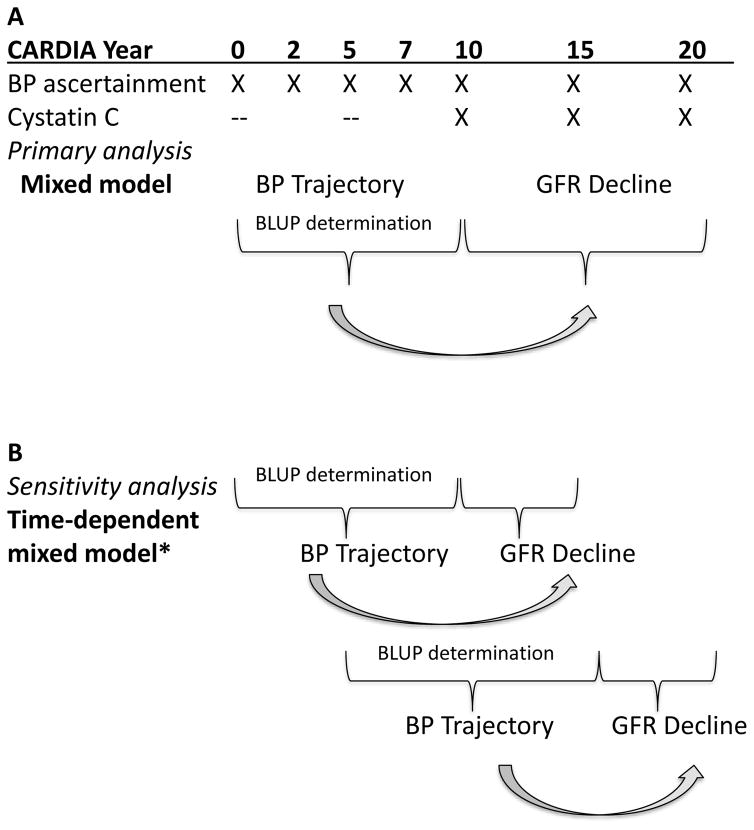
CARDIA recruited 5,115 black and white adults who were 18–30 years of age at time of enrollment between 1985–1986 at four US cities: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. Details of CARDIA have been previously described.14 Briefly, we used data from participant examinations conducted at years 0, 2, 5, 7, 10, 15 and 20. Of relevance to this study, participants had BP assessments at each visit, and renal function measures (urinary albumin, serum creatinine, and cystatin C) were obtained starting at year 10 (1995–1996) with each visit (Figure 1). For our study, we included 3,429 participants for analysis. The derivation of the cohort included for analysis is shown in Figure S1. All participants provided informed consent, and the institutional review boards of each participating center approved this study.
Figure 1.
Study design and approach in A) primary analysis and B) sensitivity analysis using a time-updated approach.
*In this approach, BP between years 5–10 will predict eGFR decline between years 10–15, and BP between years 10–15 will predict eGFR decline between years 15–20. BLUP = best linear unbiased predictor
Blood pressure measurement
At each exam, BPs were measured three times using random zero mercury sphygmomanometry at each examination after five minutes of quiet rest in the sitting position by a trained technician. The average of the second and third BP measurements were used as the visit BP.
Measurement of estimated glomerular filtration rate (eGFR)
Renal function was determined based on cystatin C measurements performed at the same time using frozen sera collected at years 10, 15, and 20 by nephelometry using the N Latex Cystatin C Kit (Dade Behring, now Siemens) at University of Minnesota. Cystatin C measurements were recalibrated for drift as previously described.15 We estimated GFR using the equation 76.7 x cystatin C−1.19 given the improved accuracy of cystatin C over serum creatinine based estimates in higher eGFR ranges (>60 mL/min/1.73 m2) and reduced bias by age and race.16 We estimated GFR trajectories with stored sera because measures performed at the same time in the same laboratory from frozen samples are better suited for estimation of trajectories and less likely to be subject to assay drift over time.
Covariates
Age, sex, and race were determined by self-report at baseline visit. Socioeconomic determinants were measured using self-reported participant income (categorized as <$25,000, $25,000–50000, and >$50,000 per year), employment (full-time, part-time, or unemployed), and highest level of education (categorized as less than high school, completed high school, or college) at year 0 or 10. Smoking status was determined by self-report at year 10. Diabetes, body mass index (BMI), and anti-hypertensive medication use were evaluated at year 10 (in Approach 1) or years 10 and 15 (in Approach 2). Diabetes was defined as fasting blood glucose ≥ 126 mg/dL or use of medications for diabetes.
Statistical analysis
To define individual BP slopes, we used best linear unbiased predictions (BLUPs) derived from linear mixed models (LMMs) fit to all available BP measures (years 0, 2, 5, 7, and 10) to estimate the participant-specific rate of change in BP over this 10-year interval. These LMMs included year, a four-level categorization of race and sex, and their interaction as fixed effects, as well as random intercepts and slopes, with unstructured covariance. We also calculated an analogous BLUP estimate of the slope from years 5 to 15, using measures obtained at years 5, 7, 10, and 15 (Figure 1).
To determine the independent association of BP slope in young adulthood with subsequent renal function decline in later adulthood, we used two approaches. In approach 1, we divided the cohort into two time periods: years 0–10 were used for BP determination, and years 10–20 were used for renal function determination (Figure 1a). We modeled renal function decline using a LMM for repeated measures of eGFRcys at years 10, 15, and 20. In these models, average trajectories were estimated specific to race and sex. The statistical association of BP change in years 0–10 with eGFRcys was estimated using an interaction between the BP change (BLUP) and a linear term in time (Figure 1a). We examined the association of BP change with change in eGFRcys both using BP change as an independent predictor, and in models with BP change adjusted for year 10 observed BPs.
To confirm the relative associations of year 10 BPs and BP change to the rate of renal function decline, we also used a data-driven approach. Specifically, we determined changes in eGFRcys per year between years 10–20 based upon tertiles of the BP slope between years 0–10 and tertiles of year 10 BP measurements.
Our first analysis rests on the assumption that the BP change during years 0–10 has a constant relationship with change in eGFRcys during years 10–20. To relax this assumption in approach 2, we assessed the associations of the BP slopes determined between years 0–10 and 5–15 with repeated measures of renal function decline over the intervals from year 10 to 15 and from year 15 to 20, respectively (Figure 1b). In this model, we included the average of the two period-specific BP measures as well as departures from those averages, which respectively capture between- and within-person effects, and we report the between-person effects in this study.
To address the possibility that changes in kidney function may be both a consequence and a cause of changes in BP, we used marginal structural models to assess the associations of the time-dependent changes in BP with yearly changes in eGFRcys, using stabilized inverse probability of treatment weights (sIPTWs) to control for eGFR at the previous visit (see Item S1 for details).
In each of these analyses, we estimated a sequence of nested models, first adjusting for sex, race, and observed year 10 BP in model 1; then adding education, income, employment, smoking, diabetes, body mass index, and anti-hypertensive medication use at year 10 in model 2 (our primary model); and finally adding presence of albuminuria at year 10 (as a categorical variable, < 30 versus ≥ 30 mg/g) in model 3.
We considered year 10 SBP and DBP separately as our predictors of interest and determined its association with subsequent renal function decline per year. To do so, we first calculated absolute eGFRcys change within nine categories, cross-tabulated by year 10 BP tertiles and BP slope tertiles. We were also interested in whether BP slope provides information above and beyond that of BP measurements taken at a single time-point closest to eGFR determination.
All analyses were conducted using Stata Version 14.2 (StataCorp; LLC).
Results
Participant characteristics
Characteristics of CARDIA participants at year 0 and 10 included in these analyses are shown in Table 1. At time of enrollment, mean age of participants was 25 years, approximately half of participants were white and female, and very few participants had co-morbidities such as diabetes or anti-hypertensive medication use (Table 1). Characteristics of participants at year 10 are also shown in Table 1. Approximately 6% of participants had diabetes and 3% were treated with anti-hypertensive therapy at Year 10. Mean follow-up duration in CARDIA study was 19.4 years.
Table 1.
Baseline characteristics of CARDIA participants included for analysis.
| Characteristic | Year 0 | Year 10 |
|---|---|---|
| Age (y) | 25.1 ± 3.6 | 35.1 ± 3.6 |
| Race/Sex | ||
| White male | 871 (25.4) | |
| White female | 961 (28.0) | |
| Black male | 661 (19.3) | |
| Black female | 936 (27.3) | |
| Education | ||
| Less than high school | 267 (7.8) | 195 (5.7) |
| Completed high school | 938 (27.4) | 747 (21.8) |
| College | 2224 (64.9) | 2487 (72.5) |
| Household incomea | ||
| <$25,000 per year | 1281 (37.4) | 885 (25.8) |
| $25,000–$50,000 per year | 1277 (37.3) | 1183 (34.5) |
| >$50,000 | 869 (25.4) | 1359 (39.7) |
| Employmentb | ||
| Full-time | 2716 (81.0) | 2952 (86.1) |
| Part-time | 531 (15.8) | 338 (9.9) |
| Unemployed | 105 (3.1) | 139 (4.1) |
| Current smoker | 923 (26.9) | 827 (24.1) |
| Diabetes* | 33 (1.0) | 215 (6.3) |
| Body mass index (kg/m2)c | 24.4 ± 4.9 | 27.4 ± 6.3 |
| Systolic blood pressure (mm Hg)d | 110 ± 10.8 | 110 ± 12.6 |
| Diastolic blood pressure (mm Hg)d | 68.6 ± 9.6 | 72.4 ± 10.1 |
| Anti-hypertensive therapy | 28 (0.8) | 110 (3.2) |
| eGFRe, mL/min/1.73m2 | 95.3 ± 17.5 | 99.0 ± 16.5 |
N=3429. Categorical values are given as count (percentage); continuous values as mean +/− standard deviation. eGFR, estimated glomerular filtration rate (based on creatinine); CARDIA, ____.
Missing in n=2.
Missing in n=77 at Year 0
Missing in n=11 at Year 0
Missing in n=4 at Year 10
Missing in n=32 at Year 0
Defined as fasting blood glucose ≥ 126 mg/dL or use of medication for diabetes.
Association between BP slope and subsequent renal function decline
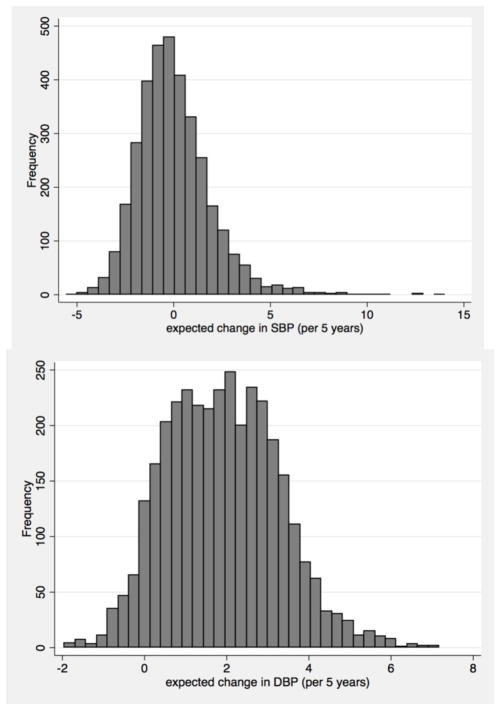
We first examined the distribution of our BP change (determined from BLUPs in LMM) between years 0–10. Very few participants had clinically significant declines in SBP, defined as > 5 mmHg decline over 5 years (Figure 2).
Figure 2.
Distribution of SBP and DBP slopes (in mm Hg) per 5 years between years 0–10.
We then cross-tabulated the eGFR change across nine categories, defined by tertiles of BP change and tertiles of year 10 BP, to examine model assumptions and to understand the relative contribution of one-time BPs versus BP change to subsequent eGFRcys changes (Table S1 and Table S2). Qualitatively, higher SBP and DBP slope tertiles were associated with a more rapid decline in eGFRcys decline after stratifying by year 10 BP, although there was some overlap in the confidence intervals,
Next, we evaluated the association between BP slope and eGFRcys change using Approach 1 (Figure 1), where we found that every 10 mmHg increase in SBP per five-year period was associated with faster annual decline in eGFRcys (−0.72 (95% CI, −0.98 to −0.46) mL/min/1.73 m2 per year) in Model 2, (Table 2). In this model, SBP change between years 0–10 remained statistically significantly associated with subsequent decline in renal function even after accounting for year 10 SBP (Table 2). Additional adjustment for albuminuria at year 10 in Model 3 did not substantially alter the effect size (Table 2).
Table 2.
Mixed models of the association between systolic blood pressure slope (Years 0–10) or one-time SBP (at Year 10) for the outcome of subsequent renal function decline.
| ΔeGFR, in mL/min/1.73m2 per year | |||
|---|---|---|---|
| Predictor | Model 1 | Model 2 | Model 3 |
| Approach 1a (Primary Analysis) | |||
| Model incorporating BP slope only (Years 0–10) | |||
| ΔSBP (per 10-mmHg increase over 5 years) | −0.76 (−1.01, −0.52) | −0.72 (−0.98, −0.46) | −0.71 (−0.99, −0.43) |
| Model incorporating one-time BP at Year 10 only | |||
| Year 10 observed SBP (per 10-mm Hg higher) | −0.10 (−0.13, −0.06) | −0.09 (−0.13, −0.06) | −0.09 (−0.13, −0.05) |
| Model incorporating BP slope (Years 0–10) and one-time BP measurement at Year 10 | |||
| ΔSBP (per 10-mmHg increase over 5 years) after accounting for Year-10 observed SBP | −0.55 (−1.05, −0.05) | −0.52 (−1.02, −0.03) | −0.48 (−1.01, 0.04) |
| Year-10 observed SBP (per 10-mm Hg higher) | −.03 (−.10, 0.03) | −.03 (−.10, 0.04) | −0.04 (−0.11, 0.04) |
| Approach 2b (Time-updated Analysis) | |||
| Model incorporating BP slope (Years 0–10 or 5–15) only | |||
| ΔSBP (per 10-mmHg increase over 5 years) | −0.90 (−1.21, −0.67) | −0.87 (−1.10, −0.63) | −0.87 (−1.12, −0.63) |
| Model incorporating one-time BP only | |||
| Observed SBP at start of interval* (per 10-mm Hg higher) | −0.14 (−0.18, −0.10) | −0.13 (−0.17, −0.09) | −0.13 (−0.17, −0.09) |
| Model incorporating one-time and change in BP | |||
| ΔSBP (per 10-mm Hg increase over 5 years) after accounting for Year-10 observed SBP | −0.66 (−1.15, −0.16) | −0.73 (−1.24, −0.23) | −0.72 (−1.25, −0.18) |
| Year-10 observed SBP (per 10 mm Hg higher) | −0.04 (−0.12, 0.04) | −0.02 (−0.11, 0.06) | −0.03 (−0.12, 0.06) |
eGFRcys, cystatin C–based estimated glomular filtration rate; SBP, systolic blood pressure; BP, blood pressure
Approach 1: BP change estimated from best linear unbiased predictor (BLUP) over years 0–10 as fixed covariate in linear mixed models for repeated eGFRcys at years 10, 15, and 20 as outcome. No. of patients in Model 1, 2, and 3 are 3425, 3423, and 3081, respectively.
Approach 2: Best linear unbiased predictor BP changes over years 0–10 and 10–15 as time-updated covariates in linear mixed models for changes in eGFRcys over years 10–15 and 15–20. No. of patients in Model 1, 2, and 3 are 3130, 3129, and 2818, respectively.
Model 1: Adjusted for age, sex, and race
Model 2: Model 1 + education, income, employment, smoking, BMI, diabetes, and anti-hypertensive medication use
Model 3: Model 2, with additional adjustment for albuminuria
Start at the interval is either Year 10 or Year 15 BP measurements
In Approach 1, every 10 mmHg increase in DBP per five-year period was associated with a statistically significantly faster annual decline in eGFRcys (−0.80 (95% CI, −1.21 to −0.40) mL/min/1.73 m2 per year) in Model 2 (Table 3). Similar to our results for SBP, DBP slope remained statistically significantly associated with subsequent renal function decline, even after accounting for year 10 DBP (Table 3).
Table 3.
Mixed models of the association between diastolic blood pressure slope (Years 0–10) or one-time DBP (at Year 10) for the outcome of subsequent renal function decline.
| ΔeGFR, in mL/min/1.73m2 per year | |||
|---|---|---|---|
| Predictor | Model 1 | Model 2 | Model 3 |
| Approach 1a (Primary Analysis) | |||
| Model incorporating BP slope (Years 0–10) only | |||
| ΔDBP (per 10-mmHg increase over 5 years) | −0.87 (−1.26, −0.48) | −0.80 (−1.21, −0.40) | −0.79 (−1.22, −0.36) |
| Model incorporating one-time BP at Year 10 only | |||
| Year-10 observed DBP (per 10-mmHg higher) | −0.08 (−0.13, −0.04) | −0.07 (−0.12, −0.03) | −0.07 (−0.12, −0.03) |
| Model incorporating one-time and change in BP | |||
| ΔDBP (per 10-mm Hg increase over 5 years) after accounting for Year-10 observed DBP | −0.61 (−1.19, −0.03) | −0.65 (−1.23, −0.07) | −0.63 (−1.25, −0.01) |
| Year-10 observed DBP (per 10 mm Hg higher) | −0.04 (−0.1, 0.02) | −0.02 (−0.09, 0.04) | −0.02 (−0.09, 0.04) |
| Approach 2b (Time-updated Analysis) | |||
| Model incorporating BP slope (Years 0–10 or 5–15) only | |||
| ΔDBP (per 10-mmHg increase over 5 years) | −0.88 (−1.26, −0.51) | −0.84 (−1.22, −0.46) | −0.83 (−1.23, −0.42) |
| Model incorporating one-time BP only | |||
| Observed DBP at start of interval* (per 10-mm Hg higher) | −0.13 (−0.18, −0.09) | −0.12 (−0.17, −0.07) | −0.12 (−0.1, −0.07) |
| Model incorporating one-time and change in BP | |||
| ΔDBP (per 10-mm Hg increase over 5 years) after accounting for Year-10 observed DBP | −0.26 (−0.80, 0.27) | −0.39 (−0.94, 0.17) | −0.35 (−0.94, 0.24) |
| Year-10 observed DBP (per 10-mm Hg higher) | −0.11 (−0.17, −0.04) | −0.08 (−0.15, −0.01) | −0.08 (−0.16, −0.01) |
eGFRcys, cystatin C–based estimated glomular filtration rate; DBP, diastolic blood pressure; BP, blood pressure
Approach 1: BP change estimated from best linear unbiased predictor (BLUP) over years 0–10 as fixed covariate in linear mixed models for repeated eGFRcys at years 10, 15, and 20 as outcome. No. of patients in Model 1, 2, and 3 are 3425, 3423, and 3081, respectively.
Approach 2: Best linear unbiased predictor BP changes over years 0–10 and 10–15 as time-updated covariates in linear mixed models for changes in eGFRcys over years 10–15 and 15–20. No. of patients in Model 1, 2, and 3 are 3130, 3129, and 2818, respectively.
Model 1: Adjusted for age, sex, and race
Model 2: Model 1 + education, income, employment, smoking, BMI, diabetes, and anti-hypertensive medication use
Model 3: Model 2, with additional adjustment for albuminuria
Start at the interval is either Year 10 or Year 15 BP measurements
When we allowed BP slopes to vary over time in a time-dependent manner (Approach 2, Figure 1), the associations between BP slope and subsequent renal function was similar to findings using our primary analysis (Model 2) for SBP (Table 2 and 3). However, DBP slope was no longer statistically significantly associated with subsequent renal function decline after accounting for year 10 DBP (Model 2, Table 3).
When marginal structural models were used to account for time-dependent confounding between categorized changes of SBP and lagged eGFRcys renal function decline in approach 3, lagged eGFR was associated with subsequent changes in both SBP (p=0.046) and DBP (p=0.002). However, changes in BP remained strongly associated with subsequent decreases in eGFRcys (see Item S1).
Association between one-time BP measurement and renal function decline
When we used one-time measures of observed BP as an independent predictor (year 10 SBP and DBP), these BPs were statistically significantly associated with renal function decline (Tables 2 and 3). Each 10 mmHg higher level of baseline SBP was associated with a faster annual decline in eGFR in model 2 (−0.09 (95% CI, −0.13 to −0.06) mL/min/1.73m2 per year). For DBP, each 10 mmHg higher level at baseline was also associated with a faster annual decline in eGFR in model 2 (−0.07 (95% CI, −0.12 to −0.03) mL/min/1.73m2 per year). However, when we added BP slope to these models, the association between one-time measures of SBP and subsequent renal function decline were attenuated (Table 3).
Discussion
Although BPs measured at a single time-point have been shown to be prognostic of the long-term risk for adverse cardiovascular outcomes and albuminuria,5, 6, 17, 18 the prognostic value of BP trajectory during longitudinal follow-up of younger individuals is less clear. In this study, we found that among young persons who were largely free of co-morbidities, BP changes over a ten-year period were associated with subsequent risk of renal function decline. These findings persisted even when we accounted for single time-point BPs taken at the time closest to the determination of renal function decline. Single time-point BPs were also independently associated with risk of subsequent renal function decline, but the association was no longer statistically significant in models that accounted for BP change over time.
There have been a number of studies that have suggested that BPs during early adulthood, BP trajectory, and cumulative BP exposure, are all associated with intermediate cardiovascular outcomes such as coronary artery calcification or carotid intimal-medial thickness.12, 19 Exposure to elevated BP in young adulthood has also been associated with a variety of other cardiovascular outcomes, including risk of systolic and diastolic cardiac dysfunction,20 subclinical atherosclerosis,12 coronary heart disease and stroke.21 However, most prior studies have focused on the associations between BP levels and adverse cardiovascular outcomes. To our knowledge, our study is novel in its examination of the association between BP levels and renal function decline. Although the magnitude of association between BP elevations during early adulthood and renal function decline was not large, the magnitude of these early declines in renal function have been associated with a higher risk of subsequent cardiovascular complications and warrant prevention.22, 23 Moreover, the accumulation of small decrements in renal function over time would become clinically meaningful over a lifetime.
Of interest, although we found that single time-point measures of BP during early adulthood were associated with subsequent renal function decline, this association was attenuated after accounting for BP changes over a ten-year period in our primary analyses. This finding contrasts with studies in middle-aged and older adults in the general population, in whom one-time BP measurements are strongly predictive of renal complications, including risk of end-stage renal disease.1, 3 However, these prior studies in older adults did not consider BP changes over time. Our data suggest that in early adulthood, the change in BP levels may contribute more information than BP levels at a single time-point to the assessment of risk for declines in renal function in later life. These findings may have important screening implications, as a single BP measurement in itself may not be fully informative of subsequent renal risk. Rising BP levels over time may potentially be a reason to screen and evaluate renal function in young adults less than 40 years of age. Our data contribute importantly to the accumulating literature on the detrimental effects of BP trajectories and the importance of primordial prevention. Our results also support the revised 2017 American Heart Association hypertension guidelines, which recently lowered the threshold for treatment of hypertension, given our observation that risk of adverse outcomes begins prior to the 140/90 mm Hg threshold in young adults.11
Some studies have suggested that with age, SBP increases linearly as DBP begins to fall, pulse pressure increases, and SBP, as opposed to DBP, becomes increasingly associated with adverse outcomes of interest.24–27 However, in younger populations, DBP has been more strongly associated with the risk of adverse cardiovascular outcomes in some, but not all studies.21, 28 Our primary analysis (using Model 2) confirms the qualitatively stronger association between DBP and early renal functional decline in a younger population without baseline kidney disease or hypertension.
There has been significant debate over the bi-directional relationship between BP and renal function loss. In a prior analysis in MESA (Multi-Ethnic Study of Atherosclerosis), lower eGFRcys was associated with risk of incident hypertension in individuals older than 45 years of age.29 The direction of the relationship between renal disease and hypertension in young individuals has not been clearly defined. In our study of young adults, we found that early declines in renal function were associated with subsequent elevations in BP. However, the adjusted associations between recent increases in BP and subsequent declines in eGFR were robust in analyses using marginal structural models to account for this complexity. In a separate study in CARDIA, cumulative exposure to higher SBP during young adulthood was also associated with higher mean albuminuria levels in mid-life.13 Taken together, our findings support the notion that changes in BP and eGFR are likely to have a bidirectional relationship. Further studies are needed to corroborate these findings in other young adult cohorts.
A particular strength of CARDIA is the availability of research-quality, repeated BP and renal function measures over two decades of follow-up. Our data are most applicable to young adults with preserved kidney function during early adulthood. In addition, because of the young age of the cohort at enrollment, the competing risk of death would be low. Limitations include the observational nature of the data, in which the causal relationship between BP and renal function decline cannot be definitively confirmed. In addition, we do not have measured GFR to confirm the changes in renal function estimated by cystatin C.
In conclusion, our results suggest that increasing BP levels over a ten-year time span in young adulthood are significantly associated with early renal function decline in individuals without decreased kidney function or clinical hypertension at baseline. Close monitoring of changes in BP levels over time during early adulthood may help identify individuals at higher risk for subsequent renal complications. Young adults who have continued rises in their BP levels over time, even in the absence of clinical hypertension, may warrant screening for kidney disease.
Supplementary Material
Figure S1. Flow diagram of cohort derivation.
Item S1. Detailed methods.
Table S1. Kidney function decline by tertile of SBP year 0–10 BLUP and SBP at year 10.
Table S2. Kidney function decline by tertile of DBP year 0–10 BLUP and DBP at year 10.
Acknowledgments
Support: CARDIA is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). EK was supported by NIH KL2 TR00014 and K23 HL131023. CP was funded by NIH 1R01AG046206 and American Heart Association Established Investigator Award 17IEA33410161.
Footnotes
Authors’ Contributions: Research idea and study design: EK, EV, MS and CP: data analysis and interpretation: EK, EV, MS; KBD, DR, HK, CL, NA, CP; statistical analysis: EV; supervision or mentorship EV; MS; CP; KBD, DR, HK, CL, and NA. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- 2.Ji B, Zhang S, Gong L, et al. The risk factors of mild decline in estimated glomerular filtration rate in a community-based population. Clinical biochemistry. 2013;46:750–754. doi: 10.1016/j.clinbiochem.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Archives of internal medicine. 2005;165:923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 4.Halbesma N, Brantsma AH, Bakker SJ, et al. Gender differences in predictors of the decline of renal function in the general population. Kidney international. 2008;74:505–512. doi: 10.1038/ki.2008.200. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Chen W, Srinivasan SR, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. Jama. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 6.Raitakari OT, Juonala M, Kahonen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Jama. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 7.Sundstrom J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ (Clinical research ed) 2011;342:d643. doi: 10.1136/bmj.d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miura K, Daviglus ML, Dyer AR, et al. Relationship of blood pressure to 25-year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Archives of internal medicine. 2001;161:1501–1508. doi: 10.1001/archinte.161.12.1501. [DOI] [PubMed] [Google Scholar]
- 9.Liu K, Colangelo LA, Daviglus ML, et al. Can Antihypertensive Treatment Restore the Risk of Cardiovascular Disease to Ideal Levels?: The Coronary Artery Risk Development in Young Adults (CARDIA) Study and the Multi-Ethnic Study of Atherosclerosis (MESA) Journal of the American Heart Association. 2015;4:e002275. doi: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 11.Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension (Dallas, Tex : 1979) 2017 Nov; doi: 10.1161/HYP.0000000000000065. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Allen NB, Siddique J, Wilkins JT, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. Jama. 2014;311:490–497. doi: 10.1001/jama.2013.285122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer H, Colangelo L, Lewis CE, et al. Cumulative Exposure to Systolic Blood Pressure During Young Adulthood Through Midlife and the Urine Albumin-to-Creatinine Ratio at Midlife. American journal of hypertension. 2017;30:502–509. doi: 10.1093/ajh/hpx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of clinical epidemiology. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 15.Peralta CA, Vittinghoff E, Bansal N, et al. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:261–266. doi: 10.1053/j.ajkd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoq S, Chen W, Srinivasan SR, Berenson GS. Childhood blood pressure predicts adult microalbuminuria in African Americans, but not in whites: the Bogalusa Heart Study. American journal of hypertension. 2002;15:1036–1041. doi: 10.1016/s0895-7061(02)03066-2. [DOI] [PubMed] [Google Scholar]
- 18.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. Journal of the American Society of Nephrology : JASN. 2006;17:1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 19.Pletcher MJ, Bibbins-Domingo K, Lewis CE, et al. Prehypertension during young adulthood and coronary calcium later in life. Annals of internal medicine. 2008;149:91–99. doi: 10.7326/0003-4819-149-2-200807150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishi S, Teixido-Tura G, Ning H, et al. Cumulative Blood Pressure in Early Adulthood and Cardiac Dysfunction in Middle Age: The CARDIA Study. Journal of the American College of Cardiology. 2015;65:2679–2687. doi: 10.1016/j.jacc.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Yano Y, Reis JP, Tedla YG, et al. Racial Differences in Associations of Blood Pressure Components in Young Adulthood With Incident Cardiovascular Disease by Middle Age: Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA cardiology. 2017;2:381–389. doi: 10.1001/jamacardio.2016.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry RM, Kostense PJ, Bos G, et al. Mild renal insufficiency is associated with increased cardiovascular mortality: The Hoorn Study. Kidney international. 2002;62:1402–1407. doi: 10.1111/j.1523-1755.2002.kid571.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Zuo L, Wang F, et al. Cardiovascular disease in early stages of chronic kidney disease in a Chinese population. Journal of the American Society of Nephrology : JASN. 2006;17:2617–2621. doi: 10.1681/ASN.2006040402. [DOI] [PubMed] [Google Scholar]
- 24.Franklin SS, Gustin Wt, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 25.Nichols WW, Nicolini FA, Pepine CJ. Determinants of isolated systolic hypertension in the elderly. Journal of hypertension. Supplement : official journal of the International Society of Hypertension. 1992;10:S73–77. [PubMed] [Google Scholar]
- 26.O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658. doi: 10.1161/01.HYP.0000153793.84859.b8. [DOI] [PubMed] [Google Scholar]
- 27.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. The New England journal of medicine. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 28.Franklin SS. The importance of diastolic blood pressure in predicting cardiovascular risk. Journal of the American Society of Hypertension : JASH. 2007;1:82–93. doi: 10.1016/j.jash.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Kestenbaum B, Rudser KD, de Boer IH, et al. Differences in kidney function and incident hypertension: the multi-ethnic study of atherosclerosis. Annals of internal medicine. 2008;148:501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram of cohort derivation.
Item S1. Detailed methods.
Table S1. Kidney function decline by tertile of SBP year 0–10 BLUP and SBP at year 10.
Table S2. Kidney function decline by tertile of DBP year 0–10 BLUP and DBP at year 10.