Abstract
The diagnosis of autosomal dominant polycystic kidney disease (ADPKD) relies on imaging criteria in the setting of a positive familial history. Molecular analysis, seldom used in clinical practice, identifies a causative mutation in >90% of cases, in the genes PKD1, PKD2, or rarely GANAB. We report the clinical and genetic dissection of a seven-generation pedigree, resulting in the diagnosis of two different cystic disorders. Using targeted next generation sequencing of 65 candidate genes in a patient with a ADPKD-like phenotype who lacked the familial PKD2 mutation, we identified a COL4A1 mutation (p.Gln247*), and made the diagnosis of HANAC syndrome (hereditary angiopathy with nephropathy, aneurysms, and muscle cramps). While four individuals had ADPKD-PKD2, various COL4A1-related phenotypes were identified in five patients, and three individuals with likely digenic PKD2/COL4A1 disease reached ESRD around 50 years of age, significantly earlier than observed for either monogenic disorder. Thus, using targeted next-generation sequencing as part of the diagnostic approach in patients with cystic diseases provides differential diagnoses and identifies factors underlying disease variability. As specific therapies are rapidly developing for ADPKD, a precise etiologic diagnosis should be paramount for inclusion in therapeutic trials and optimal patient management.
Index words: autosomal dominant polycystic kidney disease (ADPKD), PKD2, COL4A1, HANAC, genetic testing, targeted next-generation sequencing (TNGS), pedigree, case report, phenotypic variability
Introduction
Autosomal dominant polycystic kidney disease is characterized by progressive, bilateral cyst development with highly variable renal disease.1 PKD1 and PKD2 mutations are, respectively, identified in ~78% and ~15% of the pedigrees,2, 3 with mutation of a third gene, GANAB, occurring rarely (~0.3%); the genetic lesion in approximately 7% remains unresolved.4, 5 Genetic variability strongly influences the severity of ADPKD, with median age at ESRD of 58y in individuals with PKD1 truncating mutations, about 67y for those with PKD1 nontruncating mutations, and approximately 79y in those with mutations in PKD2.6 An ADPKD diagnosis is presently based on the conjunction of age-dependent imaging criteria with a positive familial history; molecular testing is rarely employed.6–9 However, the phenotypes associated with mutations in several genes can occasionally mimic ADPKD: PKHD1, causing autosomal recessive polycystic kidney disease (ARPKD); HNF1B, autosomal dominant tubulointerstitial disease (ADTKD-HNF1B); the tuberous sclerosis genes TSC1 and TSC2; and the autosomal dominant polycystic liver disease (ADPLD) genes (SEC63, PRKCSH, LRP5, ALG8, SEC61B).6 Mutations to COL4A1 can also cause bilateral renal cysts and decline in kidney function after 50y, as part of HANAC syndrome.10, 11 Here we report how genetic testing of a multigenerational “ADPKD” pedigree explains the marked intra-familial variability due to finding two distinct genetic disorders.
Case Report
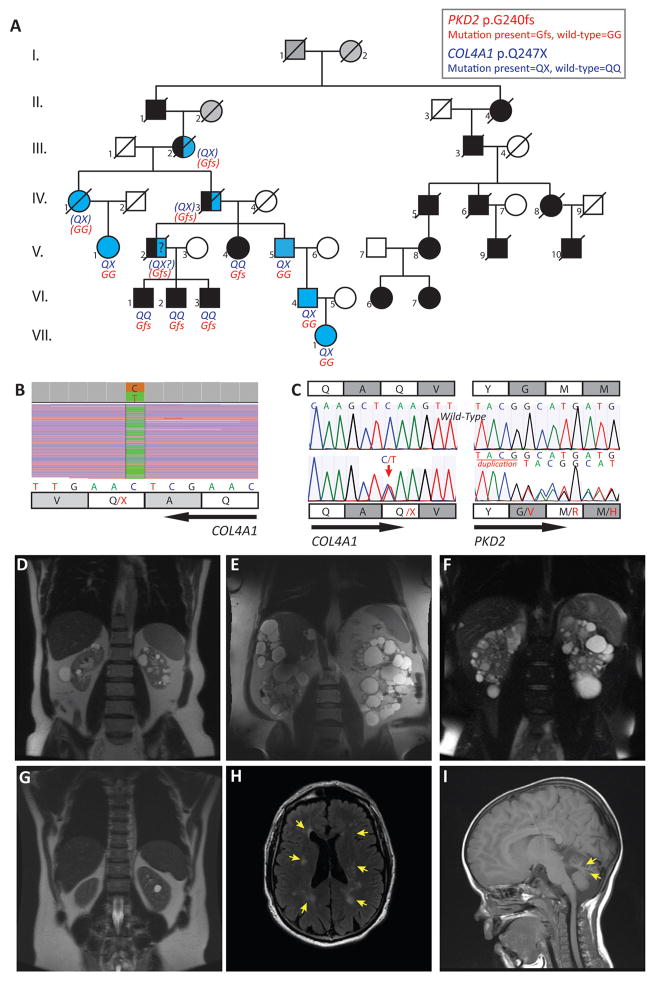
The index case, V.5, in pedigree M625 (Fig 1A), had experienced microscopic hematuria since 14y and was diagnosed with PKD at 21y (Table 1). His family history was significant for ADPKD in 4 generations. At 56y, he had more than 20 cysts per kidney, no liver cysts (Fig 1D) and an estimated glomerular filtration rate (eGFR) of 47ml/min/1.73m2 by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. The patient and three affected relatives participated in the HALT-PKD clinical trial; VI.2 was a participant in Study A (eGFR >60ml/min/1.73m2 at enrollment) and V.4, V.5, and VI.1 were participants in Study B (eGFR of 25–60ml/min/1.73m2).12, 13 Molecular analysis3 of PKD1 and PKD2 led to the identification of a frameshifting variant of PKD2 (c.715_718dupTACG [a duplication of the indicated 4-nucleotide sequence], predicted to lead to a frameshift at the glycine at amino acid 240 [p.Gly240fs]) in subjects V.4, VI.1 and VI.2 but was not detected in V.5 (Fig 1A, C). As opposed to that seen in the three mutation-positive individuals, height-adjusted total kidney volume (HtTKV) was normal in V.5 (Table 1, Fig 1D–F). At age 57y, V.5 was diagnosed with proximal stenosis of the left carotid artery and underwent endarterectomy. At reevaluation one year later for recurrent spells of dizziness and an episode of memory loss and confusion, gadolinium-enhanced MRI showed periventricular and subcortical leukoencephalopathy, consistent with chronic cerebral small-vessel disease (CSVD; Fig 1H). MR angiography of the carotid arteries was this time unremarkable.
Figure 1. Molecular and imaging data for Pedigree M625 with ADPKD-PKD2 and COL4A1-associated diseases.
A. Disease segregation in pedigree M625. Black symbols denote ADPKD-PKD2 affected individuals, blue symbols denote patients with COL4A1-associated diseases, and gray shading is used when the status of the individuals is unknown. Blue and black symbols are used in individuals with suspected digenic COL4A1-PKD2 disease. Slash over symbols denotes death. Genetic results were not available in III.2, IV.1, IV.3 but their status could be inferred from their medical records (IV.1 and IV.3) and/or from the dominant inheritance pattern of both diseases (III.2, IV.1, IV.3) (inferred genotype within parentheses). Subject II.1, who died at 63y, had a positive familial history of ADPKD (The ADPKD-affected descendants of II.4 are represented on the pedigree, and live in Europe, but no further information is available). Whether he also carried the COL4A1 variant is uncertain. Subject III.2 died at 46y from uremia; she was likely carrying both variants that she passed on to IV.1 and IV.3; for her husband (III.1), who died at age 72, no relevant past medical history was mentioned. B. IGV (integrative genomics viewer, Broad Institute) view of the next-generation sequencing of IV.6 shows COL4A1 variant c.739C>T (p.Gln247*) (reverse strand). Read depth: 2822, C=49%, T=51%. C. Sanger sequencing confirmation of heterozygous COL4A1 variant c.739C>T (p.Gln247*) in V.5, and PKD2 variant c.715_718dupTACG in VI.3. The wild-type sequences are shown for comparison. D. MRI, T2 weighted, of the COL4A1 subject V.5, 61y, showing bilateral renal cysts without kidney enlargement (HtTKV=200ml/m). E. MRI, T2 weighted, of PKD2 patient VI.1 at 53y, showing enlarged polycystic kidneys (HtTKV=1953 ml/m). F. MRI, T2, of PKD2 patient VI.2, who has moderately enlarged polycystic kidneys at 46y (HtTKV=470ml/m). G. MRI, T2, of COL4A1 individual VI.4 who has non-enlarged kidneys at 35y, with six cysts in the left kidney (the largest shown here measures 1.6 cm) and two millimeter sized cysts in the right kidney. H. Cerebral MRI of COL4A1 subject V.5, Axial FLAIR (fluid-attenuated inversion recovery) sequence demonstrating bilateral areas of signal hyperintensity in the centra semiovale (arrows). I. Cerebral MRI in COL4A1 subject VII.1 (4y), sagittal T1 weighted sequence showing marked volume loss in the superior cerebellar vermis (arrows).
Table 1.
Clinical presentations and genetic analyses of 12 affected family members
| Pt | Sex | eGFRa | HTNb | Microscopic hematuria b |
Proteinuriab,c | Kidney Morphology |
Myalgiab | Elevated CPKb,d |
CNS involvement | Cause of death |
Other conditionsb | Genetic resultsg |
Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV.1 | F | ESRD (71 y) | Y (50 y) | Y (70 y) | Y (70 y) | US at 71 y: atrophic RK (6.6 cm), 2 cysts in LK, 5 in RK | NA | NA | CSVD (CT scan, 71 y), TIA (36 y), recurrent ischemic strokes (70 y) | Stroke in 1995 (79 y | NA | NA | HANAC-like syndrome e |
| V.1 | F | 33 (67 y) | N (67 y) | Y (25 y) | Y (62 y): 0.7 | US at 59 y: atrophic RK (7.7cm), LK (9.3 cm), 2 cysts | Y (NA) | NA | Vertigo (64 y), normal non-enhanced CT (67 y) | - | Hypothyroidism (21 y), Gout (67 y) |
COL4A1: Q247* PKD2: WT |
HANAC-like syndrome |
| V.5 | M | 47 (67 y) | Y (57 y) | Y (14 y) | N (66 y) | MRI at 61 y: >15 cysts/kidney, no liver cyst, HtTKVf = 200 | Y (65 y) | NA | Migraines, dizzy spells since age 63; CSVD (MRI 58 y) | - | Gout (52 y), DM (65 y), Carotid endarteriectomy (57) |
COL4A1: Q247* PKD2: WT |
HANAC-like syndrome |
| VI.4 | M | 112 (32 y) | N (35 y) | Y (35 y) | N (35 y) | MRI at 35 y: 6 cysts in LK, 2 in RK | Y (32 y) | Y (32 y): 527 | Migraines since age 30, normal enhanced CT (32 y) | - | None | COL4A1: Q247* | HANAC-like syndrome |
| VII.1 | F | 129 (3 y) | N (4 y) | Y (3 y) | N (3 y) | NA | NA | NA | Global developmental delay, Hypotonia, absence epilepsy, MRI: thin cerebellar folia (4 y) | - | None | COL4A1: Q247* | COL4A1-related CNS disorder |
| III.2 | F | ESRD (48 y) | NA | NA | NA | NA | NA | ESRD (46 y) | NA | NA | Likely PKD2/COL4A1e | ||
| IV.3 | M | ESRD (51 y) | N (53 y) | Y (50 y) | Y (50 y) | Pyelography: enlarged kidneys, numerous renal cysts | Y (50 y) | NA | Migraines (50 y) | ESRD (51 y) | Tortuosity of the retinal arteries (50 y), Raynaud phenomena (50 y) | NA | Likely PKD2/COL4A1e |
| V.2 | M | ESRD (51 y) | Y (NA) | NA | NA | Non-enhanced CT at 56 y: Enlarged polycystic kidneys, polycystic liver | Y (56 y) | Y (55 y): 206 | Normal non-enhanced CT (56 y) | ID (57 y) | Gout (<50 y), hearing loss (56 y), aseptic necrosis of the femoral head (56 y), spontaneous cecum perforation (56 y) | NA | ADPKD-PKD2e; Possible PKD2/COL4A1 |
| V.4 | F | 30 (66 y) | N (59 y) | NA | N (59 y) | US: Enlarged polycystic kidneys, polycystic liver | N | NA | none | - | None |
PKD2: c.715_718dupTACG COL4A1: WT |
ADPKD-PKD2 |
| VI.1 | M | 21 (54 y) | Y (25 y) | N (55 y) | Y (53 y): 0.8 | MRI at 53 y: enlarged polycystic kidneys, HtTKVf=1953 | N | NA | none | - | T2DM (52 y); obesity, with BMI 40 kg/m2 |
PKD2: c.715_718dupTACG COL4A1: WT |
ADPKD-PKD2 |
| VI.2 | M | 56 (50 y) | Y (41 y) | NA | N (50 y) | MRI at 46 y: enlarged polycystic kidneys, HtTKVf=470 | N | NA | none | - | None |
PKD2: c.715_718dupTACG COL4A1: WT |
ADPKD-PKD2 |
| VI.3 | M | 36 (47 y) | Y (24 y) | N (47 y) | Y (34 y) | US at 31 y: LK 14.5 cm, LK 13.3 cm, >15 cysts/kidney | Y (47 y) | Y, under statins (33 y) | none | - | T1DM (11 y), MN (31 y) |
PKD2:c.715_718dupTACG COL4A1: WT |
ADPKD-PKD2 |
Expressed in mL/min/1.73 m2 on the basis of the last data available; obtained with the CKD-EPI equation for adults and with the Bedside Schwartz formula in the child.
Values in parentheses is age when first reported if present, or when last available data available if not present.
When available, proteinuria is expressed in grams per gram of urinary creatinine.
When available, expressed in UI/L
The patient is likely digenic carrier considering the familial history and the dominant inheritance of each condition.
Height adjusted total kidney volume, calculated by stereology and expressed in ml/m
WT: wild-type; Q247*: frame shift leading to stop codon instead of glutamine at amino acid 247; c.715_718dupTACG: a duplication of the indicated 4-nucleotide sequence, predicted to lead to a frameshift at the glycine at amino acid 240
List of abbreviations and definitions: CNS=central nervous system, CPK=Creatine Phosphokinase, CSVD=cerebral small-vessel disease, CT=computed tomography, LK=left kidney, RK=Right Kidney, NA= not available, US=ultrasound; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; pt, patient; TIA, transient ischemic attack; ID, infectious disease; MN, Membranous nephropathy;
We expanded the genetic analyses by rescreening subject V.5 by targeted next-generation sequencing to analyze all of the exons and flanking intronic sequences of PKD1, PKD2, PKHD1, GANAB, HNF1B, UMOD, PRKCSH, SEC63, LRP5, TSC1, TSC2, COL4A1, SEC61B and 52 candidate genes. Libraries were enriched using custom capture baits (SureSelect; Agilent) and paired-end sequencing of 150 base pairs was performed on an Illumina HiSeq4000. After alignment of the resulting FASTQ files to the human genome 19 (hg19) reference sequence and realignment and recalibration (using Genome Analysis Toolkit (GATK)), multi-sample variant calling was performed (with GATK Haplotype Caller) and variants filtered with Variant Quality Score Recalibration. Variant mining was performed with Golden Helix SNP & Variation Suite v.8. From this analysis we identified a cytosine to thymine substitution at nucleotide 739 of the coding sequence (c.739C>T) in exon 13 of COL4A1, predicted to lead to a nonsense mutation (introduction of a premature stop codon) at the glutamine at amino acid 247 (p.Gln247*); the nucleotide variant was confirmed by Sanger sequencing (Fig 1B, C). The diagnosis of HANAC-like syndrome was consistent with the clinical presentation, associating longstanding microscopic hematuria, renal cysts and CSVD; the patient also reported intermittent myalgia.
We broadened the familial investigation and identified several other family members with COL4A1-related phenotypes. Individual V.1 and her mother IV.1 had microscopic hematuria, mild proteinuria, atrophic cystic kidneys, and decreased kidney function/kidney failure (Table 1). The latter had a transient ischemic stroke at 36y, and recurrent strokes after 70y. Individual VII.1, a child, suffered from developmental delay associated with ataxia, global hypotonia, and absence epilepsy. She had moderate cerebellar hypoplasia (Fig 1I), and proved positive for the COL4A1 mutation, likely underlying her neurologic phenotype. Her father, VI.4, was subsequently diagnosed with HANAC-like syndrome; he had muscles cramps and elevated CPK since 32y, urinalysis revealed microhematuria, and an abdominal MRI showed bilateral kidney cysts (Fig 1G). Considering the dominant inheritance of PKD2 and COL4A1 related disease, the family history, and lack of phenotype in IV.4, IV.3, deceased at 51y from ESRD, was an obligate carrier for the COL4A1 mutation and likely had the PKD2 mutation. Although retrograde pyelography showed enlarged cystic kidneys, ADPKD was considered “unlikely to be the only cause to his renal insufficiency”, as he had systemic symptoms including severe Raynaud’s phenomenon, migraines, and myalgia and because his kidneys were “not massively enlarged”. Fundus examination showed, “a curly-cue extension of many of the arterioles bilaterally, probably congenital”. His mother, III.2, who died from uremia at 46y, was also likely carrying both the PKD2 and the COL4A1 mutations. Subject V.2 had ADPKD and started hemodialysis at 51y. Medical history was also significant for myalgia with elevated CPK, hearing loss, and gout. The ADPKD course was significantly more severe in V.2 than in his 3 sons and his sister, all with ADPKD-PKD2 and negative for the COL4A1 variant, suggesting that V.2 inherited both variants (Table 1).
Discussion
In the setting of a single family with multiple individuals fullfiling the ADPKD diagnosis criteria,8 we describe here how targeted next-generation sequencing revealed two separate genetic etiologies, ADPKD-PKD2 and COL4A1, clarifying the disease heterogeneity over six generations.
COL4A1 and COL4A2 encode procollagen type IV α1 and α2, which assemble to form the heterotrimeric helix α1/α1/α2, and are present in almost all basement membranes (BM). In the kidney, α1/α1/α2 is expressed in the Bowman capsule and the tubular BM, but replaced by the heterotrimer α3/α4/α5 in the glomerular BM after embryogenesis. Consistent with this expression pattern, COL4A1-related phenotypes encompass cerebrovascular, ophthalmologic, renal, cardiac, and muscular abnormalities.14 Less than eighty COL4A1 pedigrees have been reported to date, including eight with the full HANAC phenotype.10, 11, 14, 15 The disease presentation in the four HANAC-like patients reported is consistent with these previous descriptions. Interestingly, tortuosity of the retinal arteries, a hallmark of the COL4A1 vascular phenotype, was described in this family more than 50y ago. Unexpectedly, identifying the cause of subject V.5’s renal phenotype led to an etiological diagnosis in his 4 year-old granddaughter, affected by developmental delay and hypotonia. Simultaneous intrafamilial occurrence of these divergent phenotypes was not previously reported.11 HANAC syndrome is classically attributed to missense mutations affecting glycine residues within the COL4A1 triple-helix domain (a dominant-negative mechanism), while rare truncating mutations (haploinsufficiency), as in our pedigree, have mostly been reported with cerebrovascular disease.14, 16, 17 Noteworthy, we identified three deceased individuals with suggested (although unconfirmed) digenic PKD2/COL4A1 disease. All reached ESRD around 50y, significantly earlier than usually observed for either monogenic disorder, suggesting an aggravating effect of the COL4A1 variant on the PKD2 phenotype.6, 10, 14, 15, 17 Only a handful of digenic cystic diseases have been reported, involving PKD1, PKD2, or HNF1B,18, 19 but these cases are likely under-diagnosed, illustrating the value of targeted next-generation sequencing of multiple cystic genes.
As specific therapies become available for ADPKD, obtaining a precise diagnosis is a prerequisite to provide appropriate treatment. However, ADPKD imaging-based diagnostic criteria require a positive familial history, lacking in 10 to 25% of the patients, and genetic diagnostics are presently rarely performed in ADPKD patients, although they are part of the diagnostic algorithm of other kidney diseases.6 In our study, although the proband met the imaging criteria, he was affected by a totally different condition, phenocopying ADPKD. Inclusion of cases as presented herein in clinical trials, along with others with slowly progressing renal phenotypes, can reduce the likelihood of a positive outcome,20 supporting systematic genetic screening of ADPKD patients considered for treatment or inclusion in trials. While the high cost of ADPKD genetic testing has long been a disincentive, improvement of the screening protocols, together with the rapid development of specific next-generation sequencing approaches will undoubtedly increase its accessibility.6 The routine analysis of multiple cystic genes in ADPKD patients may eventually reveal the full genetic and phenotypic spectrum of cystic kidney diseases.
Acknowledgments
Support: NIDDK grants DK058816, the Mayo Translational PKD Center (DK090728), and Robert M. and Billie J. Pirnie, and an American Society of Nephrology Foundation Kidney Research Fellowship supports ECLG. The HALT-PKD studies were supported by NIDDK cooperative agreements (DK62402, DK62411, DK62410, DK082230, DK62408, and DK62401) by the National Center for Research Resources General Clinical Research Centers, and National Center for Advancing Translational Sciences Clinical and Translational Science Awards.
We thank the patients and their family members for their involvement in this study.
Footnotes
HALT Progression of Polycystic Kidney Disease Group Investigators: Kaleab Z. Abebe, PhD and Kyongtae T. Bae, MD, PhD (University of Pittsburgh), Robert W. Schrier, MD (University of Colorado, Denver), Arlene B. Chapman, MD, (University of Chicago), Ronald D. Perrone, MD (Tufts University), William E. Braun, MD (Cleveland Clinic), and Theodore I. Steinman, MD (Beth Israel Deaconess Medical Center).
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ong AC, Devuyst O, Knebelmann B, Walz G Diseases E-EWGfIK. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385:1993–2002. doi: 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 2.Cornec-Le Gall E, Audrezet MP, Rousseau A, et al. The PROPKD Score: A New Algorithm to Predict Renal Survival in Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2016;27:942–951. doi: 10.1681/ASN.2015010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heyer CM, Sundsbak JL, Abebe KZ, et al. Predicted Mutation Strength of Nontruncating PKD1 Mutations Aids Genotype-Phenotype Correlations in Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2016;27:2872–2884. doi: 10.1681/ASN.2015050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porath B, Gainullin VG, Cornec-Le Gall E, et al. Mutations in GANAB, Encoding the Glucosidase IIalpha Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am J Hum Genet. 2016;98:1193–1207. doi: 10.1016/j.ajhg.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Besse W, Dong K, Choi J, et al. Isolated polycystic liver disease genes define effectors of polycystin-1 function. J Clin Invest. 2017;127:1772–1785. doi: 10.1172/JCI90129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornec-Le Gall E, Torres VE, Harris PC. Brief Review: Genetic complexity of autosomal dominant polycystic kidney and liver diseases (ADPKD and ADPLD). [Published online ahead of print October 16, 2017] J Am Soc Nephrol. doi: 10.1681/ASN.2017050483. [DOI] [PMC free article] [PubMed]
- 7.Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pei Y, Hwang YH, Conklin J, et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2015;26:746–753. doi: 10.1681/ASN.2014030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman A, Devuyst O, Eckardt K, et al. Autosomal Dominant Polycystic Kidney Disease (ADPKD): Executive Summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88:17–27. doi: 10.1038/ki.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plaisier E, Gribouval O, Alamowitch S, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Eng J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 11.Plaisier E, Ronco P. COL4A1-Related Disorders. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews(R) Seattle (WA): 2009. updated 2016. [PubMed] [Google Scholar]
- 12.Torres VE, Abebe KZ, Chapman AB, et al. Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2267–2276. doi: 10.1056/NEJMoa1402686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrier RW, Abebe KZ, Perrone RD, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–2266. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meuwissen ME, Halley DJ, Smit LS, et al. The expanding phenotype of COL4A1 and COL4A2 mutations: clinical data on 13 newly identified families and a review of the literature. Genet Med. 2015;17:843–853. doi: 10.1038/gim.2014.210. [DOI] [PubMed] [Google Scholar]
- 15.Gale DP, Oygar DD, Lin F, et al. A novel COL4A1 frameshift mutation in familial kidney disease: the importance of the C-terminal NC1 domain of type IV collagen. Nephrol Dial Transplant. 2016;31:1908–1914. doi: 10.1093/ndt/gfw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeanne M, Gould DB. Genotype-phenotype correlations in pathology caused by collagen type IV alpha 1 and 2 mutations. Matrix Biol. 2017;57–58:29–44. doi: 10.1016/j.matbio.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plaisier E, Chen Z, Gekeler F, et al. Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3[IV] domain. Am J Med Genet A. 2010;152A:2550–2555. doi: 10.1002/ajmg.a.33659. [DOI] [PubMed] [Google Scholar]
- 18.Pei Y, Paterson AD, Wang KR, et al. Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet. 2001;68:355–363. doi: 10.1086/318188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergmann C, von Bothmer J, Ortiz Bruchle N, et al. Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol. 2011;22:2047–2056. doi: 10.1681/ASN.2010101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornec-Le Gall E, Blais J, Irazabal MV, et al. Can we further enrich ADPKD clinical trials for rapidly progressive patients? Application of the PROPKD score in the TEMPO trial [Published online ahead of print July 13, 2017] Nephrol Dial Transpl. doi: 10.1093/ndt/gfx188. https://10.1093/ndt/gfx188. [DOI] [PMC free article] [PubMed]