Abstract
Sweat contains a variety of lipid mediators, but whether they originate from the plasma filtrate or from the cutaneous sweat glandular tissues themselves is unknown. To explore this knowledge gap, we collected plasma and sweat from healthy men (n = 9) immediately before and 0.5, 2 and 4 h after oral administration of 400 mg ibuprofen. Of the over 100 lipid mediators assayed by liquid chromatography-tandem mass spectrometry, ~45 were detected in both plasma and sweat, and 36 were common to both matrices. However, baseline concentrations in each matrix were not correlated and metabolite relative abundances between matrices differed. Oral ibuprofen administration altered sweat lipid mediators, reducing prostaglandin E2, linoleoylethanolamide, and oleoylethanolamide, while increasing 11-hydroxyeicosatetraenoic acid, and causing transient changes in 9-nitrooleate, N-arachidonylglycine and 20-hydroxyeicosatetraenoic acid. Meanwhile, plasma N-acylethanolamide concentrations increased with ibuprofen administration. These results suggest that sweat and plasma differentially reflect biochemical changes due to oral ibuprofen administration, and that plasma is unlikely to be the predominant source of the sweat lipid mediator profile.
Keywords: Oxygenated lipids, endocannabinoids, metabolic profiling, ibuprofen
Introduction
Sweat is a complex hypotonic biofluid produced by the eccrine, apocrine and apoeccrine glands of the skin. This secretion is well known to contain water-soluble compounds including electrolytes, urea, lactate, metals and xenobiotics [1]. More recent characterizations show sweat to be a rich source of proteins, carbohydrates, organic acids, lipids [2-6], and lipid mediators including oxygenated lipids (i.e. oxylipins), endocannabinoids (EC) and EC-like compounds (ECLs), ceramides and sphingolipids [7, 8]. The recognition of sweat’s chemical complexity has spurred sweat based exploration beyond cystic fibrosis and forensic testing [9, 10]. Changes in sweat composition have been speculated to be associated with inflammatory, infectious, psychiatric and neoplastic diseases [3, 7, 11, 12].
It is generally believed that sweat composition is reflective of some combination of the systemic (plasma) and/or local (cutaneous) microenvironment [13]. Most evidence for this hypothesis comes from drug delivery studies. For instance, ketoconazole has been shown to rapidly partition from the bloodstream to eccrine sweat following oral consumption [14], whereas theophylline migrates trans-dermally to the skin surface where it can be amalgamated into sweat [15]. More recently, sweat gland epithelial cells were found to synthesize the endocannabinoids arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG) [16], thus demonstrating that sweat gland metabolic activities are not confined to filtration and electrolyte modulation. To properly interpret changes in metabolism of the sweat gland apparatus, it will be important to characterize the sources of the metabolites. This is particularly relevant in the area of lipid mediators, which are generally thought to be produced locally via biosynthetic pathways in response to extracellular stimuli and function similarly to local hormones or autacoids [17].
Lipid mediators are formed via a number of enzymatic pathways, and usually involve primary metabolism of polyunsaturated fatty acids (PUFAs) by enzymes such as cyclooxygenase (COX), lipoxygenase (LOX), cytochrome P450 (CYP), diacylglycerol lipase and N-acyltransferase, followed by secondary action of enzymes such as soluble epoxide hydrolase, prostaglandin synthase and leukotriene synthases [17] with or without the secondary release by lipases [17]. Sweat has been shown to contain COX-, LOX-, and CYP-derived metabolites [7] and there is well recognized cross-talk among these signaling pathways [18-20]. Non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen or aspirin reduce pain, inflammation, fever and clotting by inhibiting COX activity in various tissues, which in turn causes a decrease in prostaglandin and thromboxane formation from various PUFAs, predominantly arachidonic acid [21]. Ibuprofen demonstrates differential pharmacokinetics in human plasma and skin following both oral and topical administration [22], making it an excellent drug to probe the effects of systemic COX inhibition on the sweat lipid mediator profiles.
This study is primarily designed to test the effects of systemic COX inhibition on plasma and sweat lipid mediators as a means to evaluate the relative contribution of plasma to the sweat lipid mediator profile. A secondary study goal is to assess ibuprofen pharmacokinetics in plasma and sweat, in order to evaluate the plasma as a source of sweat xenobiotic content. Collectively, these findings will enhance our understanding of the metabolic dynamics of sweat as a potential source of clinically relevant biomarkers.
Materials and Methods
Subjects
This study is an extension of our previous work examining the stability and variability of the sweat lipid mediator profile, and uses the same subject population [8]. Of the 10 healthy male subjects who participated in our previous study, three subjects declined to return. Therefore, an additional four healthy male subjects aged 20-40 yr were recruited from the Sacramento, CA, USA metropolitan area between March and May 2017, resulting in a final enrollment of 11 subjects for this study.
Eligibility for participation was slightly modified from our previous inclusion and exclusion criteria, and was assessed by an in-person screening questionnaire completed at recruitment. Exclusion criteria included: a diagnosed disease for which the subject was currently taking medication; recent hospitalization, surgery or antibiotic therapy; regular consumption of prescription or over-the-counter medications such as statins, steroids, weight loss aids, or non-steroidal anti-inflammatory drugs; orthopedic or cardiovascular limitations that precluded participation in moderate exercise; and regular performance of physical activity defined as “vigorous” by the Centers for Disease Control and Prevention [23]. Written informed consent was obtained from all subjects prior to participation, and all study protocols were approved by the Institutional Review Board of the University of California-Davis (Protocol #929370). This study is registered with ClinicalTrials.gov as NCT02935894.
Of the 11 subjects enrolled, two did not produce sweat at all four collection times, thus their results were excluded from all statistical analyses. Baseline physiological characteristics of the nine remaining subjects are listed in Supplemental Table S1.
Study Design and Sample Collection
Subjects participated in one half-day study visit at the United States Department of Agriculture Western Human Nutrition Research Center in Davis, CA, USA. All study visits started between 07:00 and 07:30 to avoid potential circadian effects on lipid mediators. Prior to each study visit, subjects were asked to fast for 12 h, not to apply any creams or topical medications for 24 h, and to refrain from use of non-steroidal anti-inflammatory drugs such as aspirin, acetaminophen, or ibuprofen for 48 h. Subjects remained fasted during study visits, but ad libitum water intake was encouraged.
Immediately after subject arrival, blood and sweat samples were collected, after which subjects consumed one 400 mg dose of ibuprofen orally. The ibuprofen was obtained from the University of California-Davis Investigational Drug Services Pharmacy, Sacramento, CA, USA. Additional blood and sweat samples were collected 30 min, 2 h and 4 h after ibuprofen intake. Blood and sweat samples were collected from contralateral arms, and the arm from which the blood sample was collected was alternated at each subsequent collection timepoint. All sample collections took place in environmentally regulated rooms (22 ± 1 °C; 38 ± 7% relative humidity).
Blood samples were collected from the antecubital vein in Vacutainer® tubes containing K2-EDTA (Beckton Dickinson, Franklin Lakes, NJ, USA). Immediately after collection, samples were centrifuged at 4 °C for 10 min at 1300 × g and 0.2 mL aliquots of plasma were stored in methanol-rinsed 0.5 mL micro centrifuge tubes (Eppendorf, Hauppauge, NY) at −80 °C until analysis. Sweat samples were collected from a ~7 cm2 area on the volar forearm (5-10 cm above the wrist) by capillary action using Macroduct® sweat collectors (Wescor, Inc., Logan, UT, USA). Prior to collection, sweating was stimulated by pilocarpine iontophoresis using the Webster Sweat Inducer (Wescor, Inc.) and manufacturer-supplied proprietary gel discs reported to contain 0.5% (~18 mM) pilocarpine nitrate [24]. All sweat collection procedures were in accordance with previously published protocols [7, 8]. Collected sweat was exuded into methanol-rinsed 2 mL amber vials with Teflon-lined closures (Waters Corporation, Milford, MA, USA) by passing air through the collection tubing three times using a 250 μL gastight syringe (Hamilton, Reno, NV, USA). Samples were stored at −80 °C until analysis.
Analysis of Lipid Mediators and Ibuprofen
Plasma oxylipins, nitrolipids, free fatty acids, ECs, ECLs and ibuprofen were isolated from the matrix with methanolic acetonitrile. Specifically, 50 μL of plasma was enriched with 5 μL of anti-oxidant solution (0.2 mg/mL butylated hydroxytoluene/EDTA in 1:1 methanol/water), 10 μL of a 500 nM deuterated-oxylipin/EC/ECL surrogate solution in methanol, and 5 μL of a 20 ng/mL deuterated-NSAID surrogate solution in methanol. Protein precipitation was achieved by the forceful addition of 200 μL of a 1:1 (v/v) methanol/acetonitrile solution containing 100 nM each of the internal standards 1-cyclohexyl-3-ureido dodecanoic acid (Sigma Aldrich, St Louis, MO, USA) and 1-phenyl,3-ureido hexanoic acid (gift from B.D. Hammock, University of California-Davis). Samples were vortexed for 1 min at 1200 rpm, centrifuged at 6 °C for 5 min at 10000 × g, and the supernatant was filtered by centrifugation using Amicon® Ultrafree-MC Durapore PVDF 0.1 μm filters (Merck Millipore, Billerica, MA, USA). Filtered extracts were stored at −20 °C prior to analysis.
Sweat lipid mediators and ibuprofen were isolated from the matrix by direct evaporation using modifications of previously published protocols [7, 8]. Briefly, the collected sweat volume was determined for each sample using either a 50 μL or 100 μL gastight syringe (Hamilton), and samples were enriched with 5 μL the anti-oxidant, 2 μL of deuterated-oxylipin/EC/ECL surrogate and 5 μL of deuterated-NSAID surrogate solutions. Each sample was then mixed with 100 μL of methanol, enriched with 10 μL of 20% glycerol in methanol, and the solvent was removed under vacuum (GeneVac EZ-2 Personal Evaporator, SP Scientific, Warminster, PA, USA). Residues were reconstituted in 40 μL of the internal standard solution described above and stored at −20 °C prior to analysis.
Analytes were assayed by ultra-performance liquid chromatography tandem mass spectrometry, using an expansion of published protocols to include analysis of NSAIDs [8]. Briefly, 10 μL aliquots of the samples were injected onto a Shimadzu Nexera X2 series UPLC system (Columbia, MD, USA) and analytes were separated on a 2.1 × 150 mm, 1.7 μm BEH C18 column (Waters) and detected with an API 6500 QTrap (Sciex, Framingham, MA, USA) with positive-negative mode switching electrospray ionization. Chromatographic solvent gradients are listed in Supplemental Table S2A, and ionization voltages, mass spectrometry parameters, and retention times are listed in Supplemental Table S2B. Sample batches included method blanks and duplicate analysis of an in-house pooled laboratory reference material sample. Analytes were quantified using internal standard methodology with five- to seven-point calibration curves (r ≥ 0.997) and data were processed using Sciex MultiQuant version 3.0.2. All analyte abbreviations are fully expanded in Supplemental Table S3.
Statistical Analysis
Surrogate recoveries were unacceptable for five of the 36 plasma samples analyzed, and these analytical failures were randomly distributed by subject and timepoint. After excluding the failed samples, plasma data for at least seven subjects are reported and analyzed at each timepoint.
Plasma and sweat data were independently pretreated, and all data pretreatments were performed in JMP Pro 13 (SAS Institute, Inc., Cary, NC, USA). Prior to statistical analyses, data were curated such that analytes with >30% missing values across the data set were excluded. The remaining data were screened for outliers using Huber’s maximum likelihood type estimates to determine the center and spread of each analyte [25]. Missing data were imputed by least squares multivariate normal imputation with covariance shrinkage [26], after which data were transformed to normal using the procedures of Box and Cox [27], and normality was verified using the Shapiro-Wilk test [28].
Linear mixed models evaluating the relationship between metabolite concentrations and time with a random intercept for subject were performed in R version 3.1.0 using the lme4 package [29] after verification of population homoscedasticity at each timepoint in plasma and sweat by Bartlett’s test using JMP Pro 13. Statistical significance of the fixed effect of time was determined by likelihood ratio tests of the full model against a null model (i.e., without the fixed effect of time, but with random intercept for subject). Results of likelihood ratio test were considered significant if P < 0.05 and the false discovery rate (q) as estimated by the method of Storey and Tibshirani using the fdrtool package was < 0.2 [30, 31]. Differences between metabolite concentrations at each timepoint were assessed by Tukey’s post-hoc HSD test using the multcomp package [32].
Comparisons of metabolite patterns in plasma and sweat were performed in JMP Pro 13. Correlations between imputed but not transformed baseline (i.e. 0 h) lipid mediator concentrations in plasma and sweat were estimated using Kendall rank correlation coefficients for analytes detected in both matrices. Additionally, relative abundances of all detected lipid mediators in each matrix were estimated on both a per-class (e.g. relative abundance of total alcohols detected regardless of fatty acid precursor) and by fatty acid precursor within a lipid mediator class (e.g. individual abundances of alcohol metabolite isomers of arachidonic acid), and were compared using the Wilcoxon rank-sum exact test.
Results
Plasma and Sweat Demonstrate Different Baseline Lipid Mediator Profiles
A total of 43 lipid mediators were detected in plasma, and 45 were detected in sweat, of which 36 were common to both matrices (Supplemental Fig. S1). Despite the considerable overlap, individual plasma and sweat metabolites were not correlated (Supplemental Table S4). Plasma showed greater proportions of ECs and ECLs relative to sweat, whereas sweat had increased proportions of diols, triols and ketones (Fig. 1). Sweat also contained detectable levels of nitrolipids whereas plasma did not (Fig. 1). Additionally, the relative abundance of regioisomers within each of these classes differed between matrices (Supplemental Fig. S2). Collectively, these results suggest either different lipid mediator origins for each matrix, or in some cases, considerable modifications of plasma lipid metabolites by sweat gland epithelial cells.
Fig. 1. Relative abundances of lipid mediator classes are different in plasma and sweat at baseline.
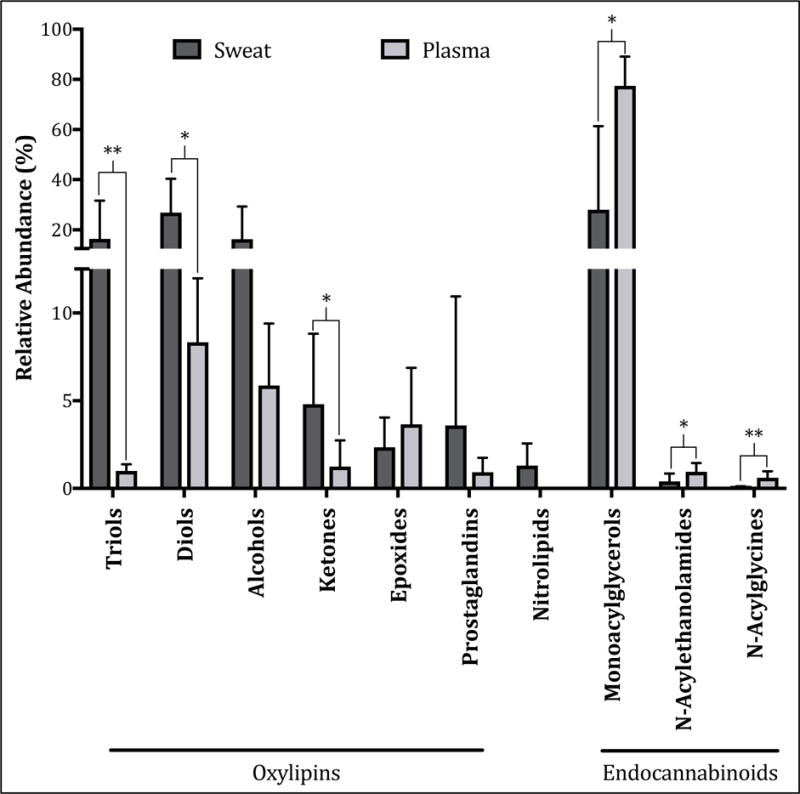
Sweat demonstrates increased proportions of diols, triols and ketones compared to plasma, and also demonstrates the presence of nitrolipids whereas plasma does not. By contrast plasma demonstrates increased proportions of endocannabinoids and endocannabinoid-like compounds. Data are reported as arithmetic mean ± standard deviation (n = 8 out of 9 subjects due to analytical failures in plasma samples), and matrix differences were evaluated using Wilcoxon’s rank-sum exact tests. * P < 0.05; ** P < 0.0005.
Ibuprofen Demonstrates Variable Pharmacokinetics in Plasma and is Not Detected in Sweat
On average, changes in plasma ibuprofen concentrations generally followed a typical pharmacokinetic profile, with the average tmax occurring ~2 h after consumption (Supplemental Table S4). However, examining individual subject pharmacokinetic profiles, it was apparent that one subject appeared to be a rapid absorber of ibuprofen (tmax ≈ 1 h), two subjects appeared to be delayed absorbers (tmax ≥ 4 h) and the remaining six subjects (i.e. 67% of the study population) were typical absorbers of ibuprofen (tmax ≈ 2 h) (Fig. 2). By contrast, ibuprofen concentrations in sweat were below the assay limit of detection (100 ng/mL) at all study timepoints (Supplemental Table S4), preventing the direct pharmacokinetic assessment of ibuprofen in sweat here.
Fig. 2. Ibuprofen demonstrates variable plasma pharmacokinetics in healthy adult human male subjects.
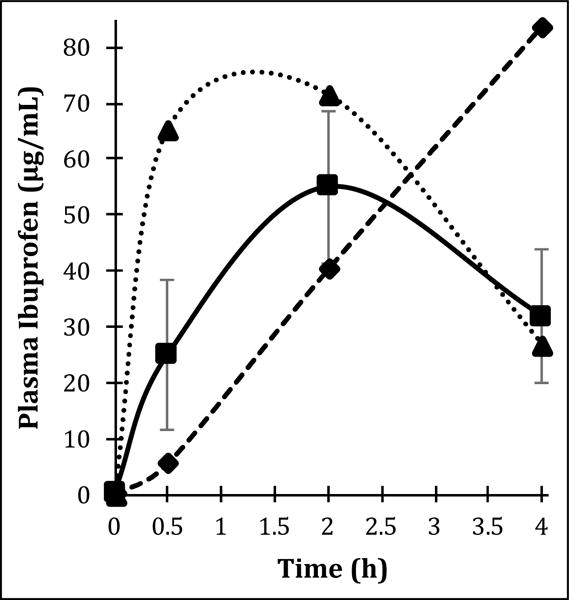
Out of nine subjects sampled, six demonstrate typical ibuprofen pharmacokinetics with tmax ≈ 2 h. One subject appeared to be a rapid absorber of ibuprofen with tmax ≈ 1 h, and two subjects appeared to be delayed absorbers of ibuprofen with tmax ≥ 4 h. Data are reported as arithmetic means and where shown, error bars represent standard deviation. ■ typical absorbers; ▲ rapid absorbers; ◆ delayed absorbers.
Ibuprofen Differentially Affects Plasma and Sweat Lipid Mediator Profiles
Oral administration of 400 mg ibuprofen affected the sweat lipid profile, with temporal changes observed in sweat concentrations of COX-derived PGE2 and 11-HETE (Fig. 3), nitric oxide synthase (NOS)-derived 9-nitrooleate (Fig. 4), CYP-derived 20-HETE (Fig. 4), and the ECLs NA-Gly, OEA and LEA (Fig. 5A). Many of these enzyme systems are known targets of ibuprofen. Inverse correlations or trends towards inverse correlations were also observed between normalized sweat concentrations of PGE2 and various COX and 15-LOX-derived lipid mediators (Table 1), further supporting sweat’s ability to reflect ibuprofen-induced lipid mediator changes. By contrast, ibuprofen administration increased plasma concentrations of OEA, AEA and DGLEA (Fig. 5B). A complete list of detected lipid mediator concentrations before and after ibuprofen intake are available in Supplemental Table S4, and representative chromatograms of the sweat lipid mediators affected by ibuprofen administration are available in Supplemental Fig. S3.
Fig. 3. Ibuprofen affects sweat concentrations of lipid mediators derived from cyclooxygenase (COX).
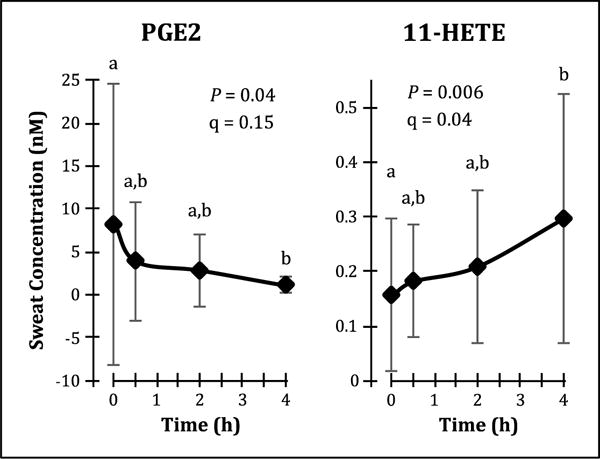
Sweat prostaglandin (PG) E2 concentrations decreased following oral ibuprofen administration, whereas 11-hydroxyeicosatetraenoic acid (HETE) concentrations increased. Data are presented as arithmetic mean ± standard deviation (n = 9), and temporal differences were evaluated using linear mixed models with Tukey’s post-hoc HSD. The false discovery rate (q) of the linear mixed models analysis was estimated according to the method of Storey and Tibshirani (2003).
Fig. 4. Ibuprofen affects sweat concentrations of nitric oxide synthase (NOS)-derived 9-nitrooleate and cytochrome P450 (CYP)-derived 20-hydroxyeicosatetraenoic acid (HETE).
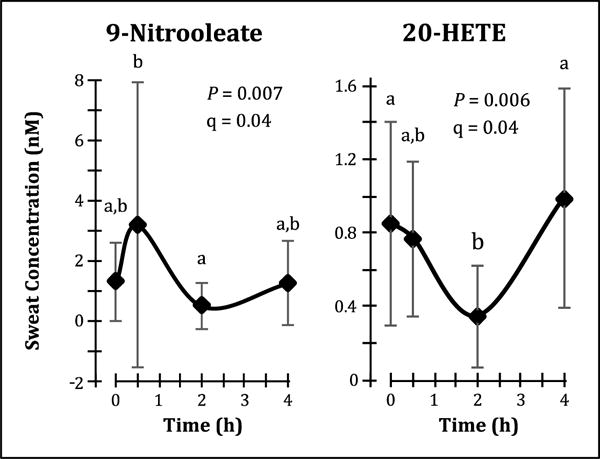
Oral administration of ibuprofen causes transient changes in sweat 9-nitrooleate and 20-HETE concentrations that appear to be inversely related. Data are presented as arithmetic mean ± standard deviation (n = 9), and temporal differences were evaluated using linear mixed models with Tukey’s post-hoc HSD. The false discovery rate (q) of the linear mixed models analysis was estimated according to the method of Storey and Tibshirani (2003).
Fig. 5. Ibuprofen differentially affects plasma and sweat endocannabinoids and endocannabinoid-like compounds.
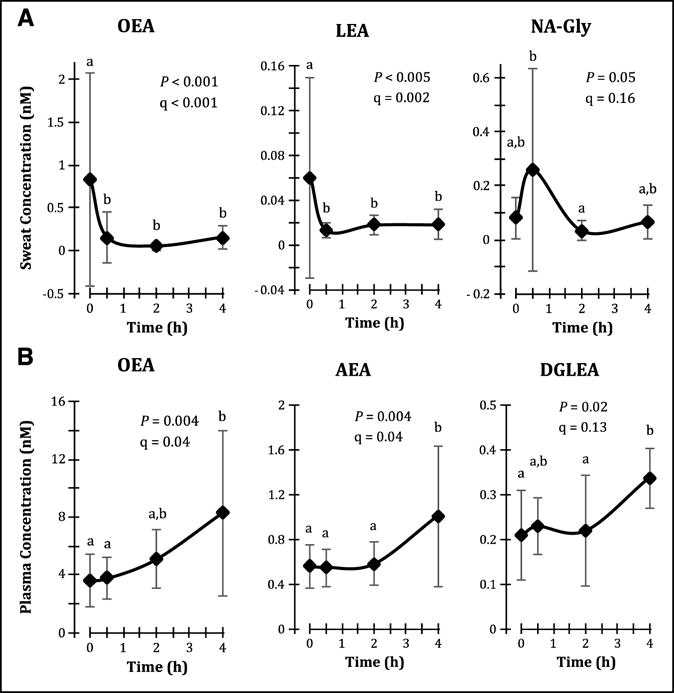
Oral ibuprofen administration results in (A) decreases in sweat OEA and linoleoylethanolamide (LEA) concentrations, and (B) increases in plasma concentrations of oleoylethanolamide (OEA), arachidonoylethanolamide (AEA, anandamide) and dihomo-gamma-linoleneoylethanolamide (DGLEA). Data are presented as arithmetic mean ± standard deviation (n = 9 for sweat, and n = 9 cumulatively for plasma, though each individual timepoint contains n = 7 or 8), and temporal differences were evaluated using linear mixed models with Tukey’s post-hoc HSD. The false discovery rate (q) of the linear mixed models analysis was estimated according to the method of Storey and Tibshirani (2003).
Table 1.
Correlations between sweat PGE2 concentrations and concentrations of COX and/or 12/15-LOX derived polyunsaturated fatty acid alcohols detected in sweat (n = 36).
| Lipid Mediator | R a | P b |
|---|---|---|
| 11-HETE | −0.230 | 0.18 |
| 12-HETE | 0.199 | 0.25 |
| 15-HETE | −0.243 | 0.15 |
| 13-HODE | −0.341 | 0.04 |
| 13-HOTE | −0.351 | 0.04 |
Correlations calculated by Pearson’s product-moment correlations using normalized lipid mediator data.
Reported P values are not adjusted for multiple comparisons.
Discussion
Currently, cutaneous research is largely dependent on tissue biopsies or tape strips, both of which are invasive procedures and have potential to cause considerable discomfort to the subject. Both techniques are also unsuitable for repeated temporal sampling, in part due to subject discomfort, but also because the affected or treated area is usually excised after the first biopsy. By contrast, sweat sampling offers a non-invasive and non-destructive alternative to profile the skin, and the presence of lipid mediators in sweat and their demonstrated relevance as markers of inflammatory skin disease opens new avenues for non-invasive sampling and diagnostics in cutaneous research. However, lipid mediators are generally produced in and act on the local microenvironment [17]. In order to properly interpret changes in lipid mediator concentration due to either therapeutic intervention or disease, we must first know more about where they come from, or at least how they are influenced by changes in associated metabolism. The origins of sweat lipid mediators have not been previously elucidated, but studies of xenobiotic excretion suggest that plasma and the sweat gland apparatus are both good sources of sweat components [13-15]. As both sites are rich in lipid mediators, they may both contribute to the sweat lipid mediator profile as well, or at least affect sweat gland cellular pathobiology and sweat lipid profiles. It is already known that the sweat gland apparatus itself is capable of lipid mediator production, particularly the endocannabinoids AEA and 2-AG [16]. In this study, we evaluate the ability of sweat to reflect biochemical changes due to systemic COX inhibition, and also consider the relative contribution of plasma to the sweat lipid mediator profile.
The non-competitive inhibitory effects of ibuprofen on COX are well known, particularly reduction of prostaglandin and thromboxane formation [21], and this is manifested in sweat by reduced PGE2 concentrations. However, COX also demonstrates LOX activity which is enhanced with NSAID administration, particularly the formation of 11-HETE and 15-HETE but also possibly 13-HODE and 13-HOTE [33, 34]. Sweat 11-HETE concentrations increased following ibuprofen intake and were significantly correlated to decreases in sweat PGE2 concentrations, and while sweat 15-HETE, 13-HODE and 13-HOTE did not change significantly, they also showed a strong inverse correlation to sweat PGE2 concentrations. By contrast, plasma levels of these analytes did not change with ibuprofen intake, which is not surprising since subjects were generally healthy and activation of COX metabolism was not induced by external stressors such as exercise or platelet stimulation [35-37].
Ibuprofen is also a known inhibitor of fatty acid amide hydrolase (FAAH), the enzyme that catabolizes N-acylethanolamides [38], which may explain increases in plasma AEA, OEA and DGLEA after ibuprofen administration. The decreases in sweat OEA and LEA concentrations following ibuprofen intake appear contrary to FAAH inhibition, but other pathways exist for the degradation of N-acylethanolamides. One possibility is alcohol dehydrogenase (ADH) 7-catalyzed oxidation of N-acylethanolamides to N-acylglycines [39], which would also explain the observed changes in sweat NA-Gly concentration, and similar but not significant changes in sweat NO-Gly. Additionally, since ADH7 is a class IV ADH, it is only present in extrahepatic tissues [40], which might also explain why N-acylethanolamides in plasma do not display similar behavior.
The effects of ibuprofen on NOS are not as well known, but previous studies have demonstrated that ibuprofen can also enhance inducible NOS activity at low concentrations, and inhibit both the inducible and constitutive forms of NOS at higher concentrations [41]. Consistent with these reports, sweat levels of the NOS metabolite 9-nitrooleate increased initially, decreased as plasma ibuprofen reached its peak, and finally increased again as plasma ibuprofen declined. Similar changes in other measured sweat nitrolipids did not reach significance. The underlying mechanism is unknown, but it is interesting that changes in sweat NA-Gly concentrations following ibuprofen intake matched those of sweat 9-nitrooleate, and that palmitoylglycine has previously been demonstrated to modulate nitric oxide production in mice [42]. The speculation that modulation of NOS activity by ibuprofen occurs through its effects on N-acylglycines warrants further research.
Finally, the changes in sweat 20-HETE concentrations following ibuprofen intake are unexpected, since ibuprofen is not known to affect CYP4A, the enzyme system responsible for 20-HETE formation from arachidonic acid. However, nitric oxide is known to inhibit CYP4A activity [43], and changes in sweat 20-HETE concentrations are counter to those of 9-nitrooleate. Interestingly, 20-HETE is an important regulator of renal vascular tone as well as ion transport in the renal proximal tubule [44, 45], and it is possible that 20-HETE plays a similar role in modulating blood flow and ion transport in the sweat gland, which may explain the relatively high concentrations of sweat 20-HETE.
The differential effects of ibuprofen on plasma and sweat lipid mediators, along with the lack of correlations between baseline lipid mediator concentrations in the two matrices, suggest that either plasma is not the primary contributor of sweat lipid mediators, or that the sweat gland tissues significantly modify lipid mediators filtered from plasma. Moreover, we find that the relative abundance of sweat lipid mediator classes are similar to those reported in skin tissues [46]. It is known that the sweat electrolyte profile, while primarily derived from plasma, is modified by differential absorptions of electrolytes by the sweat gland epithelia [47]. Differences in the relative abundances of lipid mediator classes and between the relative abundance of isomers derived from the same precursors suggest either the localized production of these mediators by the sweat gland epithelial cells or an impact of the surrounding cutaneous matrix and cells on sweat gland metabolism. For instance, the pattern of arachidonate-derived diols in sweat were dominated by the 5,6-DiHETrE isomer, while the 14,15- and 11,12-DiHETrEs dominated the plasma profile. The presence of lipid mediators and lipid mediator-forming enzymes in non-sweat gland cutaneous tissue cells have been well documented [46, 48], and these and other non-lipid mediators can be expected to modify the pathophysiology of the cutaneous sweat gland apparatus.
Regardless of their source, the finding of lipid mediators in sweat is in itself surprising if their transport from tissue or plasma is considered a passive process. Previous studies have indicated that basic analytes passively diffuse more readily from blood into sweat due to the differences in pH between the matrices (sweat pH = 4-6; blood pH = 7.4), and that a low octanol-water partition coefficient (log P or KOW), typically < 3, increases the likelihood that a molecule will passively diffuse into sweat from either tissue or blood [49, 50]. By contrast, lipid mediators typically have pKa ~4-5 and log P ~3-6 [51]. Since ibuprofen has pKa = 4.85 and log P = 3.97 [51], this may also explain why we were unable to detect ibuprofen in sweat despite others showing peak penetration of ibuprofen into the skin within 3 h after oral administration [22]. Alternative routes by which lipid mediators may be secreted into sweat include active transport or facilitated diffusion. Active transporters and/or transport proteins for prostaglandins and endocannabinoids are known [52, 53], suggesting these routes may be available for lipid mediator transport into sweat. More recently, exosomes were found in sweat [54], representing yet another potential route for lipid mediator delivery into sweat.
Regardless of their origin, it is interesting to speculate on what role lipid mediators and their associated enzymes play in sweat and sweat gland biology. Our initial study reporting the presence of lipid mediators in sweat demonstrated that sweat ceramide profiles differ in individuals with and without atopic dermatitis [7], and that similar changes have been shown in the skin [55]. This led us to speculate that the sweat lipid mediators reflected the mediator profiles of the surrounding cutaneous tissue [7]. However, work by Czifra et al. demonstrated that endocannabinoids regulate cell proliferation, apoptosis, lipid synthesis and expression of cytoskeleton proteins via the mitogen-activated protein kinase pathway in cultured NCL-SG3 sweat gland epithelial cells, and that these cells are capable of producing the endocannabinoids responsible for modulating these processes [16]. Finally, Fujii et al. have previously demonstrated that inhibition of COX and NOS has no effect on sweat production, though they did not evaluate the impact of inhibiting these pathways on sweat content [56]. Therefore, it would appear that the prostanoids either play no functional role in sweat production, or that they do not influence sweat volume, but regulate sweat gland cell biology as shown in a variety of other cell types [17]. It is also possible that sweat lipid mediators regulate blood flow and ion transport across the coil of the sweat gland, in a manner similar to their role in the nephron of the kidney [44, 45]. Without conducting appropriate studies, it is difficult to know with certainty what roles sweat lipid mediators play, but understanding their role is key in determining potential applications for the technology presented in this and other works. Our hope is that sweat metabolomics investigations will supplement, if not replace, skin biopsy and tape stripping as a primary sample collection technique in cutaneous research and diagnosis.
A major limitation of this study is that no control experiments were conducted in the absence of ibuprofen, making it difficult to discriminate whether observed sweat lipid mediator changes are truly due to ibuprofen consumption, or whether stress, time or individual variation could have contributed as well. This is particularly relevant as published studies examining circadian effects on prostanoids in human saliva and urine indicate peak prostanoid concentrations between 07:00 and 08:00 with steady decreases in concentrations over the subsequent 12 h [57, 58]. However, in a previous study, we observed no differences in lipid mediator concentrations when sweat was collected from the volar forearm of healthy individuals between either 09:00 and 14:00 or 14:00 and 18:00 [7], which appears to mitigate potential impacts of circadian variation. Furthermore, we have previously established that sweat lipid mediator concentrations in healthy individuals are temporally stable over a two-week period and that inter- and intra-individual variability of sweat lipid mediators are comparable to those observed in other commonly analyzed matrices such as skin or plasma [8]. Finally, the fact that the observed changes can be associated with biological pathways known to be affected by ibuprofen are highly suggestive of an ibuprofen-mediated response.
Other limitations of this study include a small sample size, complicated by population variance in ibuprofen uptake. Additionally, we were only able to sample subjects at four timepoints, as additional sample collections within a 24 h period would require catheterization, which would have increased study cost and subject discomfort. While the sampling timepoints were chosen keeping typical ibuprofen pharmacokinetics and subject burden in mind, it is possible that additional and/or later samplings of plasma and sweat would demonstrate additional ibuprofen-associated changes in lipid mediator concentrations or strengthen currently observed correlations between lipid mediators that indicate the effects of ibuprofen on COX. Additionally, subject lifestyle (e.g. diet, medication and cosmetic use) was not controlled in this study, which while offering a better representation of real-world conditions, may increase variance in the data.
In conclusion, results from this study suggest that like plasma, the sweat lipid mediator profile is capable of reflecting changes induced by systemic ibuprofen administration. However, it seems unlikely that plasma is the predominant source of sweat lipid mediators. Future work is needed to further characterize the degree to which the sweat gland epithelium modifies and contributes to the overall sweat lipid mediator profile, the degree to which the sweat gland cell functions can be influenced by constituents of the surrounding cutaneous tissue pathobiology, and the transport mechanisms by which lipid mediators are amalgamated into sweat.
Supplementary Material
Highlights.
Basal plasma and sweat lipid mediator composition is different
Oral ibuprofen altered COX-derived oxylipins and nitrolipids in sweat but not plasma
Oral ibuprofen increased plasma but decreased sweat acylethanolamides
Plasma ibuprofen pharmacokinetics revealed high inter-individual variability
Sweat lipid mediators appear to be produced in the skin, not collected from plasma
Acknowledgments
The authors gratefully acknowledge the assistance and advice provided by E.L. Bonnel in obtaining IRB approval for this study and registering this study with ClinicalTrials.gov; by R.K. Sivamani and P.B. Trovitch in obtaining ibuprofen for administration to study subjects; by T.L. Pedersen in developing the analytical method for analysis of non-steroidal anti-inflammatory drugs; by E. Holguin-Jenner, J. Crawford, and B. Rust during study visits; and by G. Riordan, I.J. Gray, K. Borkowski, and W.R. Keyes during sample preparation and data analysis. The authors also gratefully acknowledge the donation of the Macroduct® sweat collection system’s Webster Sweat Inducer for use in this study by Wescor, Inc., an Elitech Company.
Funding
This work was supported by the National Institutes of General Medical Sciences [Grant No. T32-GM008799 (K.A.)], the National Institutes of Diabetes and Digestive and Kidney Diseases [Grant No. U24DK097154-01 (J.W.N.)], and the United States Department of Agriculture [Project Nos. 2032-51530-022-00D (J.W.N.) and 6026-51000-010-05S (B.D.P.)]. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIGMS, NIH or USDA. The USDA is an equal opportunity provider and employer.
Abbreviations
- ADH
alcohol dehydrogenase
- COX
cyclooxygenase
- CYP
cytochrome P450
- EC
endocannabinoid
- ECL
endocannabinoid-like compound
- FAAH
fatty acid amide hydrolase
- LOX
lipoxygenase
- NOS
nitric oxide synthase
- NSAID
non-steroidal anti-inflammatory drug
- log P
octanol-water partition coefficient (also known as KOW)
- PUFA
polyunsaturated fatty acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
K.A. and J.W.N. designed the study; K.A. and R.B. enrolled study participants and conducted study visits; K.A., R.B., and J.W.N. generated lipid mediator data; K.A., B.D.P., and J.W.N. performed data analysis and interpretation; and K.A. and J.W.N. wrote the manuscript. All authors reviewed and approved the submitted manuscript.
References
- 1.Mena-Bravo A, Luque de Castro MD. Sweat: a sample with limited present applications and promising future in metabolomics. J Pharm Biomed Anal. 2014;90:139–147. doi: 10.1016/j.jpba.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 2.Calderón-Santiago M, Priego-Capote F, Jurado-Gamez B, Luque de Castro MD. Optimization study for metabolomics analysis of human sweat by liquid chromatography-tandem mass spectrometry in high resolution mode. J Chromatogr A. 2014;1333:70–78. doi: 10.1016/j.chroma.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 3.Calderón-Santiago M, Priego-Capote F, Turck N, Robin X, Jurado-Gamez B, Sanchez JC, Luque de Castro MD. Human sweat metabolomics for lung cancer screening. Anal Bioanal Chem. 2015;407:5381–5392. doi: 10.1007/s00216-015-8700-8. [DOI] [PubMed] [Google Scholar]
- 4.Delgado-Povedano MM, Calderón-Santiago M, Priego-Capote F, Luque de Castro MD. Development of a method for enhancing metabolomics coverage of human sweat by gas chromatography–mass spectrometry in high resolution mode. Anal Chim Acta. 2016;905:115–125. doi: 10.1016/j.aca.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Harker M, Coulson H, Fairweather I, Taylor D, Daykin CA. Study of metabolite composition of eccrine sweat from healthy male and female human subjects by 1H NMR spectroscopy. Metabolomics. 2006;2:105–112. doi: 10.1007/s11306-006-0024-4. [DOI] [Google Scholar]
- 6.Forstrom L, Goldyne ME, Winkelmann RK. Prostaglandin activity in human eccrine sweat. Prostaglandins. 1974;7:459–464. doi: 10.1016/0090-6980(74)90090-2. [DOI] [PubMed] [Google Scholar]
- 7.Agrawal K, Hassoun LA, Foolad N, Pedersen TL, Sivamani RK, Newman JW. Sweat lipid mediator profiling: a noninvasive approach for cutaneous research. J Lipid Res. 2017;58:188–195. doi: 10.1194/jlr.M071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal K, Waller JD, Pedersen TL, Newman JW. Effects of stimulation technique, anatomical region, and time on human sweat lipid mediator profiles. Prostaglandins Other Lipid Mediat. 2018;134:84–92. doi: 10.1016/j.prostaglandins.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959;23:545–549. [PubMed] [Google Scholar]
- 10.De Giovanni N, Fucci N. The current status of sweat testing for drugs of abuse: a review. Curr Med Chem. 2013;20:545–561. doi: 10.2174/0929867311320040006. [DOI] [PubMed] [Google Scholar]
- 11.Adewole OO, Erhabor GE, Adewole TO, Ojo AO, Oshokoya H, Wolfe LM, Prenni JE. Proteomic profiling of eccrine sweat reveals its potential as a diagnostic biofluid for active tuberculosis. Proteomics Clin Appl. 2016;10:547–553. doi: 10.1002/prca.201500071. [DOI] [PubMed] [Google Scholar]
- 12.Raiszadeh MM, Ross MM, Russo PS, Schaepper MA, Zhou W, Deng J, Ng D, Dickson A, Dickson C, Strom M, Osorio C, Soeprono T, Wulfkuhle JD, Petricoin EF, Liotta LA, Kirsch WM. Proteomic analysis of eccrine sweat: Implications for the discovery of schizophrenia biomarker proteins. J Proteome Res. 2012;11:2127–2139. doi: 10.1021/pr2007957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cone EJ, Hillsgrove MJ, Jenkins AJ, Keenan RM, Darwin WD. Sweat testing for heroin, cocaine, and metabolites. J Anal Toxicol. 1994;18:298–305. doi: 10.1093/jat/18.6.298. [DOI] [PubMed] [Google Scholar]
- 14.Harris R, Jones HE, Artis WM. Orally administered ketoconazole: route of delivery to the human stratum corneum. Antimicrob Agents Chemother. 1983;24:876–882. doi: 10.1128/AAC.24.6.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peck CC, Conner DP, Bolden BJ, Almirez RG, Kingsley TE, Mell LD, Murphy G, Hill VE, Rowland LM, Ezra D. Outward transcutaneous chemical migration: implications for diagnostics and dosimetry. Skin Pharmacol. 1988;1:14–23. doi: 10.1159/000210747. [DOI] [PubMed] [Google Scholar]
- 16.Czifra G, Szollosi AG, Toth BI, Demaude J, Bouez C, Breton L, Biro T. Endocannabinoids regulate growth and survival of human eccrine sweat gland-derived epithelial cells. J Invest Dermatol. 2012;132:1967–1976. doi: 10.1038/jid.2012.118. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M. Lipid mediators in life science. Exp Anim. 2011;60:7–20. doi: 10.1538/expanim.60.7. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura H, Murayama T. Role of sphingolipids in arachidonic acid metabolism. J Pharmacol Sci. 2014;124:307–312. doi: 10.1254/jphs.13R18CP. [DOI] [PubMed] [Google Scholar]
- 19.Du H, Chen X, Zhang J, Chen C. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-γ. Br J Pharmacol. 2011;163:1533–1549. doi: 10.1111/j.1476-5381.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 21.Cashman JN. The mechanisms of action of NSAIDs in analgesia. Drugs. 1996;52(Suppl 5):13–23. doi: 10.2165/00003495-199600525-00004. [DOI] [PubMed] [Google Scholar]
- 22.Tegeder I, Muth-Selbach U, Lotsch J, Rusing G, Oelkers R, Brune K, Meller S, Kelm GR, Sorgel F, Geisslinger G. Application of microdialysis for the determination of muscle and subcutaneous tissue concentrations after oral and topical ibuprofen administration. Clin Pharmacol Ther. 1999;65:357–368. doi: 10.1016/s0009-9236(99. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Physical activity. 2015 https://www.cdc.gov/physicalactivity/basics/adults/. Accessed September 30, 2016.
- 24.Wescor Inc. Macroduct Sweat Collection System Model 3700 SYS Instruction/Service Manual. 2004 http://www.wescor.com/translations/Translations/M2551-7A-EN.pdf. Accessed July 11, 2016.
- 25.Huber PJ, Ronchetti EM. The basic types of estimates, Robust Statistics. second. John Wiley & Sons, Inc; Hoboken, NJ: 2009. pp. 53–84. [Google Scholar]
- 26.SAS Institute Inc. JMP(R) 12 Basic Analysis. SAS Institute Inc.; Cary, NC: 2015. Modeling utilities: exploring data for outliers, missingness, and strong predictors; pp. 313–342. [Google Scholar]
- 27.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Series B Methodol. 1964;26(2):211–252. [Google Scholar]
- 28.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. doi: 10.1093/biomet/52.3-4.591. [DOI] [Google Scholar]
- 29.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4, 2015. 2015;67:48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 30.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24:1461–1462. doi: 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- 32.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–63. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 33.Xiao G, Tsai AL, Palmer G, Boyar WC, Marshall PJ, Kulmacz RJ. Analysis of hydroperoxide-induced tyrosyl radicals and lipoxygenase activity in aspirin-treated human prostaglandin H synthase-2. Biochemistry. 1997;36:1836–1845. doi: 10.1021/bi962476u. [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Williams MV, DuBois RN, Blair IA. Cyclooxygenase-2-mediated DNA Damage. J Biol Chem. 2005;280:28337–28346. doi: 10.1074/jbc.M504178200. [DOI] [PubMed] [Google Scholar]
- 35.Markworth JF, Vella L, Lingard BS, Tull DL, Rupasinghe TW, Sinclair AJ, Maddipati KR, Cameron-Smith D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1281–R1296. doi: 10.1152/ajpregu.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAnulty SR, Owens JT, McAnulty LS, Nieman DC, Morrow JD, Dumke CL, Milne GL. Ibuprofen use during extreme exercise: effects on oxidative stress and PGE2. Med Sci Sports Exerc. 2007;39:1075–1079. doi: 10.1249/mss.0b13e31804a8611. [DOI] [PubMed] [Google Scholar]
- 37.Agrawal K, Melliou E, Li X, Pedersen TL, Wang SC, Magiatis P, Newman JW, Holt RR. Oleocanthal-rich extra virgin olive oil demonstrates acute anti-platelet effects in healthy men in a randomized trial. J Funct Foods. 2017;36:84–93. doi: 10.1016/j.jff.2017.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fowler CJ, Stenstrom A, Tiger G. Ibuprofen inhibits the metabolism of the endogenous cannabimimetic agent anandamide. Pharmacol Toxicol. 1997;80:103–107. doi: 10.1111/j.1600-0773.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 39.Aneetha H, O’Dell DK, Tan B, Walker JM, Hurley TD. Alcohol dehydrogenase-catalyzed in vitro oxidation of anandamide to N-arachidonoyl glycine, a lipid mediator: Synthesis of N-acyl glycinals. Bioorg Med Chem Lett. 2009;19:237–241. doi: 10.1016/j.bmcl.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung C, Davies NG, Hoog J-O, Hotchkiss SAM, Smith Pease CK. Species variations in cutaneous alcohol dehydrogenases and aldehyde dehydrogenases may impact on toxicological assessments of alcohols and aldehydes. Toxicology. 2003;184:97–112. doi: 10.1016/S0300-483X(02. [DOI] [PubMed] [Google Scholar]
- 41.Menzel JE, Kolarz G. Modulation of nitric oxide synthase activity by ibuprofen. Inflammation. 1997;21:451–461. doi: 10.1023/A:1027374605731. [DOI] [PubMed] [Google Scholar]
- 42.Rimmerman N, Bradshaw HB, Hughes HV, Chen JS-C, Hu SS-J, McHugh D, Vefring E, Jahnsen JA, Thompson EL, Masuda K, Cravatt BF, Burstein S, Vasko MR, Prieto AL, O’Dell DK, Walker JM. N-palmitoyl glycine, a novel endogenous lipid that acts as a modulator of calcium influx and nitric oxide production in sensory neurons. Mol Pharmacol. 2008;74:213–224. doi: 10.1124/mol.108.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 44.Zou AP, Imig JD, Ortiz de Montellano PR, Sui Z, Falck JR, Roman RJ. Effect of P-450 omega-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol Renal Physiol. 1994;266:F934–F941. doi: 10.1152/ajprenal.1994.266.6.F934. [DOI] [PubMed] [Google Scholar]
- 45.Escalante B, Erlij D, Falck, McGiff J. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 46.Kendall AC, Pilkington SM, Massey KA, Sassano G, Rhodes LE, Nicolaou A. Distribution of bioactive lipid mediators in human skin. J Invest Dermatol. 2015;135:1510–1520. doi: 10.1038/jid.2015.41. [DOI] [PubMed] [Google Scholar]
- 47.Reddy MM, Quinton PM. Rapid regulation of electrolyte absorption in sweat duct. J Membr Biol. 1994;140:57–67. doi: 10.1007/BF00234486. [DOI] [PubMed] [Google Scholar]
- 48.Kendall AC, Nicolaou A. Bioactive lipid mediators in skin inflammation and immunity. Prog Lipid Res. 2013;52:141–164. doi: 10.1016/j.plipres.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Caplan YH, Goldberger BA. Alternative specimens for workplace drug testing. J Anal Toxicol. 2001;25:396–399. doi: 10.1093/jat/25.5.396. [DOI] [PubMed] [Google Scholar]
- 50.Johnson HL, Maibach HI. Drug excretion in human eccrine sweat. J Invest Dermatol. 1971;56:182–188. doi: 10.1111/1523-1747.ep12260784. [DOI] [PubMed] [Google Scholar]
- 51.Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A. HMDB 3.0–The human metabolome database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuster VL, Chi Y, Lu R. The prostaglandin transporter: Eicosanoid reuptake, control of signaling, and development of high-affinity inhibitors as drug candidates. Trans Am Clin Climatol Assoc. 2015;126:248–257. [PMC free article] [PubMed] [Google Scholar]
- 53.Bojesen IN, Hansen HS. Membrane transport of anandamide through resealed human red blood cell membranes. J Lipid Res. 2005;46:1652–1659. doi: 10.1194/jlr.M400498-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Wu CX, Liu ZF. Proteomic profiling of sweat exosome suggests its involvement in skin immunity. J Invest Dermatol. 2018;138:89–97. doi: 10.1016/j.jid.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 55.Ishikawa J, Narita H, Kondo N, Hotta M, Takagi Y, Masukawa Y, Kitahara T, Takema Y, Koyano S, Yamazaki S, Hatamochi A. Changes in the ceramide profile of atopic dermatitis patients. J Invest Dermatol. 2010;130:2511–2514. doi: 10.1038/jid.2010.161. [DOI] [PubMed] [Google Scholar]
- 56.Fujii N, McGinn R, Paull G, Stapleton JM, Meade RD, Kenny GP. Cyclooxygenase inhibition does not alter methacholine-induced sweating. J Appl Physiol. 2014;117:1055–1062. doi: 10.1152/japplphysiol.00644.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rigas B, Levine L. Human salivary eicosanoids: Circadian variation. Biochem Biophys Res Comm. 1983;115:201–205. doi: 10.1016/0006-291X(83. [DOI] [PubMed] [Google Scholar]
- 58.Nadler JL, Yamamoto JV. Diurnal variation and exercise induced changes of prostacyclin in man. Prostaglandins Leukot Med. 1986;22:71–78. doi: 10.1016/0262-1746(86)90023-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


