Abstract
Rationale & Objective
Novel urinary biomarkers have enabled earlier detection of kidney tubular damage, but their prognostic value for adverse cardiovascular outcomes is uncertain. We hypothesized that tubular damage, measured by urine α1-microglobulin (α1m), amino-terminal propeptide of type III procollagen (PIIINP), and neutrophil gelatinase-associated lipocalin (NGAL), would be associated with higher risks for cardiovascular events and mortality among elders.
Study Design
Case-cohort study
Setting & Participants
This study included a randomly selected subcohort (n=502), CVD cases (n=245), and heart failure cases (n=220) from the Health, Aging, and Body Composition (Health ABC) Study.
Predictors
Baseline urine concentrations of α1m, PIIINP, and NGAL
Outcomes
Incident CVD, heart failure, and all-cause mortality
Analytical Approach
Cox proportional hazards models were used to evaluate biomarker associations with each outcome.
Results
At baseline, the mean age was 74 years and eGFR was 73 ml/min/1.73m2. After adjustment for demographics, eGFR, ACR, and other cardiovascular risk factors, each doubling in biomarker was associated with the following adjusted hazard ratios (HRs) for CVD: α1m, 1.51 (95% CI, 1.16-1.96); PIIINP, 1.21 (1.00-1.46); NGAL, 1.12 (1.05-1.20). There were 248 deaths in the subcohort over a median follow-up of 12.4 years. The adjusted associations of each biomarker (HR per doubling) with all-cause mortality were: α1m, 1.29 (95% CI, 1.10-1.51); PIIINP, 1.05 (95%, 0.94-1.18); NGAL, 1.07 (95% CI, 1.02-1.12). The biomarkers did not have statistically significant associations with heart failure after multivariable adjustment.
Limitations
Urine biomarkers were measured at a single time point; no validation cohort available.
Conclusions
Kidney tubular damage is an independent risk factor for CVD and death among elders. Future studies should investigate mechanisms by which renal tubular damage may adversely impact cardiovascular risk.
Keywords: urine biomarker, tubular injury markers, prognostication, cardiovascular disease (CVD), heart failure (HF), mortality, elderly, amino-terminal propeptide of type III procollagen (PIIINP), α1-microglobulin (A1M), amino-terminal propeptide of type III procollagen (PIIINP), neutrophil gelatinase-associated lipocalin (NGAL)
Introduction
Chronic kidney disease (CKD) is an established risk factor for cardiovascular disease (CVD) and heart failure, but the underlying mechanisms are uncertain.1–3 Renal tubular function is essential for volume status regulation, acid-base homeostasis, mineral metabolism, and hormone production.4 On kidney biopsy, the presence of renal tubular atrophy and interstitial fibrosis are strong predictors of kidney disease progression.5,6 However, tubular injury and dysfunction are poorly quantified by traditional measures of kidney health, including estimated glomerular filtration rate (eGFR) and albuminuria.7
In certain settings, urine biomarkers have been useful for the prognostication of CKD incidence and progression;8–12 it is less clear whether or not they can forecast CKD complications, including cardiovascular risk and death. Urine α1-microglobulin (α1m), amino-terminal propeptide of type III procollagen (PIIINP), and neutrophil gelatinase-associated lipocalin (NGAL) are promising markers of renal tubular damage. In kidney biopsy series, urine α1m, PIIINP, and NGAL were reported to correlate significantly with the extent of tubulointerstitial fibrosis.13–17 Higher urine levels of α1m, PIIINP, and NGAL have also been shown to be independent predictors of kidney function decline.8,11,18–20 Some studies have evaluated their prognostic value for CVD, heart failure, and mortality; however, these studies have yielded conflicting results.10,13,14,18–22 Further, no prior study has measured these biomarkers concurrently in order to compare their relative strengths of association with longitudinal outcomes.
The objective of this study was to evaluate the associations of urine α1m, PIIINP, and NGAL with incident CVD, heart failure, and all-cause mortality in an ambulatory cohort of elderly individuals. We hypothesized that tubular damage, as assessed by urine biomarker levels, would be an independent predictor of cardiovascular outcomes and death.
Methods
Study Design and Participants
The Health, Aging, and Body Composition (Health ABC) Study is a National Institute on Aging-sponsored cohort that enrolled 3,075 well-functioning men and women aged 70-79 years from two clinical sites in Memphis, TN, and Pittsburgh, PA. Eligibility required: self-reported ability to walk a quarter mile, climb ten steps, and perform basic activities of daily living without difficulty; the absence of life-threatening illness; and plans to remain in the geographic area for at least 3 years. Informed consent was obtained from all participants, and the study was performed in compliance with the Declaration of Helsinki. Participants underwent a baseline evaluation in 1997-1998 that included a medical history and physical assessment, physical examination, and radiographic tests. Follow-up occurred every 6 months by telephone or through annual visits to clinical centers.
We developed a case-cohort sample of the Health ABC study for measurement of novel kidney injury biomarkers (Figure 1). The case-cohort design uses a subsampling technique in survival data for estimating the relative risk of disease in a cohort study without collecting data from the entire cohort. Among the entire cohort of 3,075 Health ABC participants, the overall event rates for CVD, heart failure, and mortality were 2.06% per year, 1.64% per year, and 3.88% per year, respectively. From the entire cohort, 502 participants were selected as a random subcohort; within the subcohort, 248 (49%) participants died during follow-up, providing ample power for mortality analyses. Additionally, we selected at random 245 cases of CVD (50% of total CVD events) and 220 cases of heart failure (57% of total heart failure events). Among these, 97 CVD cases and 94 heart failure cases originated in the subcohort. This design provided a total of 776 individuals who underwent urine biomarker measurement.
Figure 1.
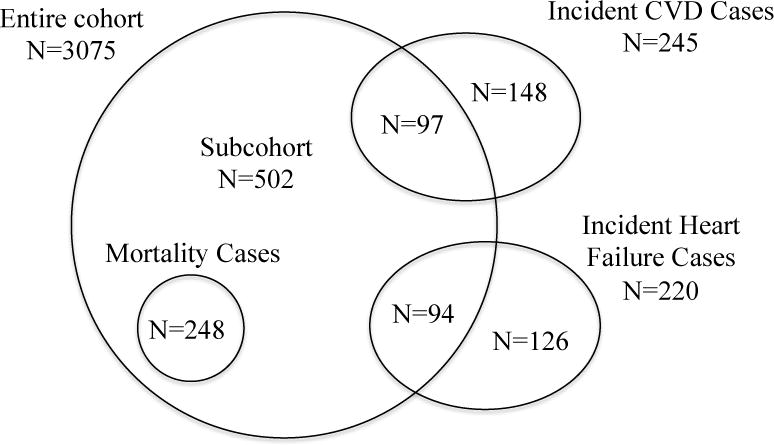
Sampling for the study within the Health ABC Cohort
The study was approved by the institutional review boards at the University of Tennessee Health Science Center and the University of Pittsburgh. The present study was also approved by the University of California, San Francisco, San Francisco Veterans Affairs Medical Center, and Tufts Medical Center committees on human research.
Predictors
Urine α1m, PIIINP, and NGAL were measured concurrently at the Cincinnati Children’s Hospital Medical Center Laboratory from urine specimens collected at the baseline visit. All urine specimens were in continuous storage at −80°C until biomarker measurement without prior freeze-thaw. The laboratory personnel performing the biomarker assays were blinded to clinical information about the participants. Urine α1m was measured by a commercially available assay (Siemens BNII nephelometer, Munich, Germany). The detectable limit of the α1m assay was 0.5 mg/dl. Urine PIIINP was measured by a commercially available ELISA (USCN Life Sciences, Wuhan, Hubei, China). Urine NGAL was assayed using a human-specific commercially available ELISA (AntibodyShop, Grusbakken, Denmark).23 Intra- and inter-assay coefficients of variation for the urine measurements were: α1m, 4.1%/10.3%; PIIINP, <10%/<12%; and NGAL, 2.1%/9.1%.
Outcomes
The outcomes were incident CVD, heart failure, and death. Follow-up was analyzed through 2011. Participants were questioned about hospitalizations for CHD, heart failure or stroke every 6 months. When an event was reported, hospital records were collected and verified by a Health ABC Disease Adjudicator at each site. Incident CVD was defined by either first coronary heart disease (CHD) event and/or stroke after enrollment. CHD was defined by coronary death or any overnight hospitalization in an acute care hospital for acute myocardial infarction. Incident stroke was defined as fatal and nonfatal stroke events. Incident heart failure was defined as the first overnight hospitalization for decompensated heart failure. Heart failure criteria required a diagnosis from a physician and treatment for heart failure. Deaths were ascertained by review of local obituaries, reports to the clinical centers by family members, or via semiannual study contacts. Date of death was taken from the death certificate.24
Covariates
Covariates were assessed at baseline and included: age, sex, race, clinical site, education level, current smoking (defined by current vs former or never), diabetes mellitus (defined by the self-reported use of hypoglycemic agents, fasting plasma glucose >126 mg/dL, or a 2-hour oral glucose tolerance test >200mg/dL), hypertension (defined by either self-report plus use of antihypertensive medications or measured systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg), prevalent heart failure, prevalent CHD (defined as myocardial infarction, angina, or coronary artery bypass), systolic blood pressure, body mass index, serum albumin (measured by colorimetric assay on a Johnson & Johnson Vitros 950 analyzer),25 C-reactive protein (measured in duplicate by ELISA kits from R&D Systems, Inc),26 fasting high- and low-density lipoprotein cholesterol levels (calculated using the Friedewald equation),27 and current statin use. Cystatin C was measured by a particle-enhanced immunonephelometric assay (N Latex Cystatin C) using a BNII Nephelometer (Dade Behring, Inc., Deerfield, IL). Glomerular filtration rate was estimated (eGFR) using the combined CKD-EPI creatinine–cystatin C equation.28 Urine albumin and urine creatinine were measured by immunoturbidimetry and colorimetric enzyme assay, respectively, using a Siemens Dimension Xpand Plus HM clinical analyzer (Siemens, Munich, Germany).
Statistical Analyses
Baseline characteristics of participants by urine biomarker quartiles were compared across α1m quartiles. Individuals with prevalent CVD and heart failure (N=136 and N=17 in random sub-cohort, respectively) were excluded from incident CVD and heart failure analyses, respectively. Spearman coefficients were used to evaluate correlations between the urinary markers and eGFR. We then used separate Cox proportional hazards models to evaluate associations of the urinary biomarkers with incident CVD, heart failure and all-cause mortality. For CVD and heart failure analyses, participants in the subcohort were weighted by the inverse probability of being selected into the subcohort. Participants were censored at death, last available study follow-up, or loss to follow-up. Covariates for multivariable models were selected based on biological plausibility, and included: age, sex, race, study site, education level, and traditional cardiovascular risk factors, including eGFR, urine albumin, urine creatinine, diabetes mellitus, hemoglobin A1c (HbA1c), hypertension, systolic blood pressure, antihypertensive medication use (including use of medications that block the renin-angiotensin-aldosterone system), prevalent CVD, prevalent heart failure, smoking status, body mass index, LDL, HDL, CRP and statin use. Because urine creatinine is susceptible to bias by muscle mass and health status, the biomarker concentrations were analyzed without standardization to urine creatinine; to correct for urine tonicity, we adjusted for urine creatinine in the multivariable models. Due to their right-skewed distributions, biomarker concentrations were analyzed as log-transformed continuous variables (to the base 2), with results presented per doubling, and by quartile, with participants in the lowest quartile comprising the reference group. Urine α1m and PIIINP were undetectable in 31% and 2% of participants, respectively; the lower limit of detection was imputed for undetectable values. Participants with detectable α1m were divided into three categories and they were compared to those with undetectable α1m as the reference group. In sensitivity analyses, we evaluated associations of urine biomarker/creatinine ratios with each outcome. The proportional hazards assumption was satisfied for all models (P values for Schoenfeld residuals>0.20). All analyses were conducted using R (R Core Team, version 3.3.2, Vienna, Austria) and SPSS statistical software (version 16.0.2; SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics of Health ABC sub-cohort
Among the 502 participants included in the random sub-cohort, the mean age was 74 and 48% of participants were women (Table 1). African-Americans comprised 39% of the sub-cohort, and diabetes and hypertension were present in 24% and 65% of participants, respectively. CVD and heart failure were prevalent among 28% and 3% of participants, respectively. The mean eGFR was 73 ml/min/1.73m2 and 22% of participants had an eGFR <60 ml/min/1.73m2, while 19% of participants had a urine albumin-creatinine ratio >30 mg/g. Compared to participants with undetectable urine α1m, participants in the highest category of α1m were more likely to be male or African American, had higher prevalence of diabetes mellitus and CVD, and had higher CRP and lower baseline eGFR. Compared to participants in the lowest quartile of urine PIIINP (Table S1), those in the highest quartile were more likely to be male or African American, had higher prevalence of diabetes mellitus, hypertension, CVD, and CHF, and had higher CRP and lower eGFR. Similarly, participants in the highest quartile of urine NGAL were more likely to be male or African American; have a higher prevalence of diabetes mellitus, CVD, and CKD (defined by eGFR<60 ml/min/1.73m2); and have higher CRP, compared with participants in the lowest NGAL quartile (Table S2).
Table 1.
Baseline Characteristics by Quartile of α1-microglobulin in Health ABC Subcohort (n=502)
| Overall | Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|---|
| (A1M <0.50 mg/dL) | (A1M 0.50–0.90 mg/dL) | (A1M 0.91–1.60 mg/dL) | (A1M >1.60 mg/dL) | ||
| No. of patients | 502 | 156 | 119 | 113 | 114 |
| Age, y | 74 ± 3 | 73 ± 3 | 74 ± 3 | 74 ± 3 | 74 ± 3 |
| Female sex | 243 (48%) | 90 (58%) | 62 (52%) | 56 (50%) | 35(31%) |
| African-American | 195 (39%) | 58 (37%) | 44 (37%) | 41 (36%) | 52 (46%) |
| Site | |||||
| Memphis | 261 (52%) | 76 (49%) | 67 (56%) | 54 (48%) | 64 (56%) |
| Pittsburgh | 241 (48%) | 80 (51%) | 52 (44%) | 59 (52%) | 50 (44%) |
| Education | |||||
| Less than high school | 115 (23%) | 35 (23%) | 32 (27%) | 19 (17%) | 29 (26%) |
| High school graduate | 171 (34%) | 61 (39%) | 38 (32%) | 35 (31%) | 37 (33%) |
| Postsecondary education | 214 (43%) | 59 (38%) | 49 (41%) | 59 (52%) | 47 (42%) |
| Diabetes mellitus | 118 (24%) | 36 (24%) | 18 (15%) | 22 (20%) | 42 (38%) |
| Hemoglobin A1c (%) | 6.40 ± 1.30 | 6.24 ± 0.98 | 6.19 ± 0.93 | 6.41 ± 1.37 | 6.85 ± 1.76 |
| Hypertension | 324 (65%) | 97 (62%) | 75 (63%) | 77 (68%) | 75 (66%) |
| Systolic BP (mm Hg) | 134 ± 21 | 134 ± 21 | 136 ± 21 | 134 ± 21 | 134 ± 20 |
| Diastolic BP (mm Hg) | 70 ± 11 | 69 ± 10 | 70 ± 12 | 72 ± 11 | 72 ± 12 |
| Antihypertensive medication use | 271 (54%) | 81 (52%) | 60 (51%) | 61 (54%) | 69 (61%) |
| RAAS blockade use | 79 (16%) | 25 (16%) | 14 (12%) | 20 (18%) | 20 (18%) |
| Smoking | |||||
| Never | 203 (40%) | 63 (40%) | 47 (40%) | 50 (44%) | 43 (38%) |
| Former | 260 (52%) | 83 (53%) | 63 (53%) | 56 (50%) | 58 (51%) |
| Current | 39 (8%) | 10 (6%) | 9 (8%) | 7 (6%) | 13 (11%) |
| Prevalent CVD | 136 (28%) | 35 (23%) | 29 (25%) | 32 (29%) | 40 (36%) |
| Prevalent Heart Failure | 17 (3%) | 4 (3%) | 2 (2%) | 4 (4%) | 7 (6%) |
| Body Mass Index (kg/m2) | 27.2 ± 4.4 | 27.3 ± 4.8 | 27.6 ± 4.6 | 27.2 ± 3.5 | 26.6 ± 4.6 |
| C-reactive Protein (mg/L) | 1.63 [0.98, 2.95] | 1.54 [1.02, 2.93] | 1.51 [0.98, 2.78] | 1.60 [0.95, 2.81] | 2.03 [1.03, 3.47] |
| Albumin (mg/dL) | 3.99 ± 0.32 | 4.03 ± 0.32 | 3.98 ± 0.31 | 3.98 ± 0.29 | 3.97 ± 0.33 |
| Total cholesterol (mg/dL) | 201 ± 38 | 206 ± 38 | 200 ± 37 | 205 ± 39 | 193 ± 39 |
| LDL cholesterol (mg/dL) | 121 ± 35 | 123 ± 32 | 118 ± 36 | 125 ± 34 | 115 ± 36 |
| HDL cholesterol (mg/dL) | 53 ± 17 | 55 ± 19 | 54 ± 18 | 53 ± 16 | 49 ± 14 |
| Triglycerides (mg/dL) | 122 [90, 166] | 128 [91, 176] | 120 [91, 165] | 114 [86, 160] | 117 [91, 150] |
| Lipid-lowering medication use | 69 (14%) | 22 (14%) | 16 (14%) | 16 (14%) | 15 (13%) |
| eGFR (ml/min/1.73m2) | 73 ± 18 | 77 ± 17 | 74 ± 18 | 73 ± 18 | 66 ± 20 |
| eGFR<60 ml/min/1.73m2 | 112 (22%) | 20 (13%) | 25 (21%) | 23 (20%) | 44 (39%) |
| UACR (mg/g) | 5 [4, 20] | 6 [4, 12] | 6 [4, 17] | 9 [5, 25] | 18 [8, 40] |
| UACR > 30 mg/g | 97 (19%) | 15 (10%) | 21 (18%) | 23 (17%) | 38 (33%) |
Categorical data presented as count (percent); continuous data as median [interquartile range] or mean ± standard deviation.
Abbreviations: A1M, α1-microglobulin; BP, blood pressure: CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate by cystatin C CKD-EPI equation; RAAS blockade, renin-angiotensin-aldosterone system blockade; UACR, urinary albumin-creatinine ratio; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Correlations of urinary biomarkers and eGFR
Urine α1m, PIIINP, and NGAL showed moderately strong inter-correlations (Table S3; r = 0.3 to 0.6, p<0.01) and each marker was positively correlated with urine albumin (r = 0.3 to 0.6; p<0.01). Urine α1m (r = −0.214; p<0.01) and PIIINP (r = −0.123; p<0.01) were negatively correlated with eGFR.
Associations of urine biomarkers with CVD and heart failure
In unadjusted analyses, higher levels of all three biomarkers were associated with higher CVD risk, with α1m having the largest effect size (Table 2). For α1m, the association with CVD was attenuated moderately by adjustment for demographics, eGFR, ACR, and other traditional CVD risk factors, but it retained the largest magnitude of association in relation to the other biomarkers. The hazard ratios for the associations of PIIINP and NGAL with CVD were essentially unchanged by multivariable adjustment, but the association of PIIINP with CVD was no longer statistically significant in final models (p=0.05). When the biomarkers were analyzed by quartile, participants in the highest quartile of urine NGAL had a doubling in CVD risk, compared to those in the lowest quartile, in fully adjusted models. Associations of α1m categories and PIIINP quartiles with CVD were not statistically significant after multivariable adjustment.
Table 2.
Associations of urine biomarkers with incident cardiovascular disease in Health ABC
| Unadjusted HR (95% CI) | Model 1* HR (95% CI) | Model 2** HR (95% CI) | Model 3† HR (95% CI) | ||
|---|---|---|---|---|---|
| Urine A1M | |||||
| Continuous (per doubling) | 1.65 (1.34, 2.03) | 1.55 (1.22, 1.97) | 1.56 (1.21, 2.00) | 1.51 (1.16, 1.96) | |
| Categorical | |||||
| Q1 | (<0.50 mg/dL) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | (0.50–0.90 mg/dL) | 1.09 (0.67, 1.77) | 0.82 (0.48, 1.41) | 1.05 (0.59, 1.89) | 0.99 (0.54, 1.79) |
| Q3 | (0.91–1.60 mg/dL) | 1.38 (0.85, 2.52) | 1.15 (0.68, 1.98) | 1.45 (0.81, 2.61) | 1.35 (0.74, 2.45) |
| Q4 | (>1.60 mg/dL) | 2.33 (1.39, 3.92) | 1.64 (0.90, 3.00) | 1.94 (1.00, 3.75) | 1.66 (0.82, 3.35) |
| Urine PIIINP | |||||
| Continuous (p er doubling) | 1.23 (1.08, 1.41) | 1.16 (0.98, 1.37) | 1.25 (1.04, 1.50) | 1.21 (1.00, 1.46) | |
| Categorical | |||||
| Q1 | (<1.39 ug/L) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | (1.39–2.49 ug/L) | 1.19 (0.72, 1.97) | 0.90 (0.51, 1.58) | 0.82 (0.44, 1.50) | 0.79 (0.43, 1.45) |
| Q3 | (2.50–4.04 ug/L) | 1.11 (0.67, 1.85) | 0.64 (0.34, 1.20) | 0.59 (0.31, 1.15) | 0.55 (0.28, 1.08) |
| Q4 | (>4.04 ug/L) | 2.11 (1.25, 3.55) | 1.52 (0.79, 2.90) | 1.99 (1.01, 3.94) | 1.76 (0.87, 3.55) |
| Urine NGAL | |||||
| Continuous (per doubling) | 1.10 (1.03, 1.17) | 1.10 (1.02, 1.18) | 1.13 (1.05, 1.21) | 1.12 (1.05, 1.20) | |
| Categorical | |||||
| Q1 | (<9.51 ng/mL) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | (9.51–20.84 ng/mL) | 0.94 (0.56, 1.58) | 0.86 (0.48, 1.55) | 0.79 (0.41, 1.51) | 0.83 (0.43, 1.60) |
| Q3 | (20.85–51.75 ng/mL) | 0.87 (0.51, 1.47) | 0.82 (0.43, 1.56) | 0.81 (0.40, 1.64) | 0.86 (0.42, 1.74) |
| Q4 | (>51.75 ng/mL) | 2.25 (1.36, 3.74) | 2.54 (1.34, 4.83) | 2.53 (1.25, 5.15) | 2.62 (1.28, 5.36) |
Abbreviations: A1M, α1-microglobulin; NGAL, neutrophil gelatinase-associated lipocalin; PIIINP, amino-terminal propeptide of type III procollagen.
Adjusted for age, sex, race, study site, education level, and urine creatinine.
Adjusted additionally for eGFR, diabetes mellitus, prevalent heart failure, systolic blood pressure, antihypertensive medication use, statin use, smoking status, body mass index, C-reactive protein, low-density lipoprotein, high-density lipoprotein and hemoglobin A1c.
Adjusted additionally for urine albumin.
We then evaluated associations of urine biomarker levels with incident heart failure (Table 3). In demographic-adjusted models, each doubling of α1m was associated with a 50% higher risk of heart failure (p<0.001), and participants in the highest category of α1m had a doubling in heart failure risk, compared to participants with undetectable α1m. However, after multivariable adjustment, these associations were attenuated and they were no longer statistically significant. Urine PIIINP and NGAL were not associated with incident heart failure events when analyzed as continuous or ordinal variables.
Table 3.
Associations of urine biomarkers with incident heart failure in Health ABC
| Unadjusted HR (95% CI) | Model 1* HR (95% CI) | Model 2** HR (95% CI) | Model 3† HR (95% CI) | ||
|---|---|---|---|---|---|
| Urine A1M | |||||
| Continuous (per doubling) | 1.46 (1.22, 1.73) | 1.50 (1.23, 1.82) | 1.23 (1.00, 1.51) | 1.22 (0.97, 1.53) | |
| Ordinal | |||||
| Range of α1m (mg/dL) | |||||
| C1 | (<0.50 mg/dL) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| C2 | (0.50–0.90 mg/dL) | 1.09 (0.68, 1.75) | 1.00 (0.60, 1.66) | 1.01 (0.60, 1.73) | 0.94 (0.55, 1.61) |
| C3 | (0.91–1.60 mg/dL) | 1.13 (0.70, 1.83) | 1.06 (0.63, 1.79) | 0.92 (0.52, 1.61) | 0.80 (0.45, 1.44) |
| C4 | (>1.60 mg/dL) | 2.09 (1.32, 3.29) | 1.98 (1.15, 3.41) | 1.63 (0.92, 2.91) | 1.32 (0.71, 2.47) |
| Urine PIIINP | |||||
| Continuous (per doubling) | 1.05 (0.94, 1.17) | 0.96 (0.83, 1.11) | 0.91 (0.78, 1.07) | 0.89 (0.76, 1.04) | |
| Categorical | |||||
| Q1 | (< 1.39 ug/L) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | (1.39–2.49 ug/L) | 0.66 (0.41, 1.07) | 0.53 (0.31, 0.90) | 0.48 (0.27, 0.86) | 0.49 (0.28, 0.86) |
| Q3 | (2.50–4.04 ug/L) | 0.92 (0.57, 1.47) | 0.63 (0.35, 1.10) | 0.53 (0.29, 0.96) | 0.49 (0.26, 0.90) |
| Q4 | (>4.04 ug/L) | 1.26 (0.79, 2.02) | 0.94 (0.52, 1.71) | 0.90 (0.48, 1.68) | 0.77 (0.40, 1.48) |
| Urine NGAL | |||||
| Continuous (per doubling) | 1.00 (0.94, 1.06) | 0.99 (0.92, 1.06) | 0.98 (0.91, 1.06) | 0.96 (0.88, 1.04) | |
| Categorical | |||||
| Q1 | (<9.51 ng/mL) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | (9.51–20.84 ng/mL) | 1.07 (0.66, 1.73) | 0.93 (0.55, 1.58) | 0.82 (0.47, 1.44) | 0.78 (0.45, 1.37) |
| Q3 | (20.85–51.75 ng/mL) | 0.94 (0.58, 1.53) | 0.84 (0.48, 1.45) | 0.71 (0.39, 1.30) | 0.63 (0.35, 1.16) |
| Q4 | (>51.75 ng/mL) | 1.19 (0.74, 1.91) | 1.13 (0.65, 1.96) | 0.89 (0.49, 1.62) | 0.77 (0.42, 1.41) |
Abbreviations: A1M, α1-microglobulin; NGAL, neutrophil gelatinase-associated lipocalin; PIIINP, amino-terminal propeptide of type III procollagen.
Adjusted for age, sex, race, study site, education level, and urine creatinine.
Adjusted additionally for eGFR, diabetes mellitus, prevalent cardiovascular disease, systolic blood pressure, antihypertensive medication use, statin use, smoking status, body mass index, C-reactive protein, low-density lipoprotein, high-density lipoprotein, and hemoglobin A1c.
Adjusted additionally for urine albumin.
Associations of urine biomarkers with all-cause mortality
Each doubling in biomarker was associated with higher mortality risk, by 29% for urine α1m and 7% for NGAL, in models adjusting for demographics, eGFR, ACR, and other traditional kidney and cardiovascular risk factors (Table 4). Additional adjustment for urine KIM-1 had little impact on the associations of urine α1m (HR per doubling, 1.28; 95% CI, 1.09-1.50) and NGAL (HR per doubling, 1.06; 95% CI, 1.02-1.11) with mortality. In demographic-adjusted models, each doubling of urine PIIINP was nominally associated with 12% higher mortality risk, but this was not statistically significant (p=0.05), and was attenuated by adjustment for kidney and cardiovascular risk factors. When the biomarkers were modeled as quartiles, the high quartiles were associated with higher mortality risk, by 2.1-fold for α1m and 1.7-fold for PIIINP in demographic-adjusted models, but these associations did not reach statistical significance in fully adjusted models.
Table 4.
Associations of urine biomarkers with all – cause mortality in Health ABC
| Unadjusted HR (95% CI) | Model 1* HR (95% CI) | Model 2** HR (95% CI) | Model 3† HR (95% CI) | ||
|---|---|---|---|---|---|
| Urine A1M | |||||
| Continuous (per doubling) | 1.46 (1.29, 1.65) | 1.47 (1.28, 1.68) | 1.36 (1.17, 1.58) | 1.29 (1.10, 1.51) | |
| Categorical | |||||
| Q1 | (<0.50 mg/dL) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | (0.50–0.90 mg/dL) | 1.18 (0.81, 1.72) | 1.13 (0.77, 1.66) | 1.15 (0.77, 1.70) | 1.07 (0.72, 1.59) |
| Q3 | (0.91–1.60 mg/dL) | 1.44 (1.00, 2.07) | 1.42 (0.97, 2.07) | 1.32 (0.90, 1.94) | 1.19 (0.80, 1.76) |
| Q4 | (>1.60 mg/dL) | 2.12 (1.50, 3.01) | 2.06 (1.39, 3.06) | 1.72 (1.13, 2.62) | 1.45 (0.94, 2.25) |
| Urine PIIINP | |||||
| Continuous (per doubling) | 1.13 (1.03, 1.24) | 1.12 (1.00, 1.25) | 1.10 (0.98, 1.23) | 1.05 (0.94, 1.18) | |
| Categorical | |||||
| Q1 | (<1.39 ug/L) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | (1.39–2.49 ug/L) | 0.84 (0.57, 1.25) | 0.81 (0.54, 1.23) | 0.78 (0.52, 1.19) | 0.77 (0.50, 1.16) |
| Q3 | (2.50–4.04 ug/L) | 1.32 (0.92, 1.91) | 1.24 (0.80, 1.91) | 1.19 (0.76, 1.85) | 1.10 (0.70, 1.73) |
| Q4 | (>4.04 ug/L) | 1.69 (1.18, 2.41) | 1.65 (1.06, 2.57) | 1.69 (1.07, 2.67) | 1.43 (0.88, 2.32) |
| Urine NGAL | |||||
| Continuous (p er doubling) | 1.06 (1.02, 1.11) | 1.07 (1.02, 1.11) | 1.08 (1.03, 1.13) | 1.07 (1.02, 1.12) | |
| Categorical | |||||
| Q1 | (<9.51 ng/mL) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | (9.51–20.84 ng/mL) | 1.14 (0.79, 1.64) | 1.19 (0.80, 1.76) | 1.13 (0.76, 1.69) | 1.07 (0.71, 1.60) |
| Q3 | (20.85–51.75 ng/mL) | 0.96 (0.66, 1.39) | 1.10 (0.72, 1.67) | 1.03 (0.67, 1.59) | 0.94 (0.61, 1.45) |
| Q4 | (>51.75 ng/mL) | 1.28 (0.90, 1.83) | 1.49 (0.99, 2.25) | 1.39 (0.92, 2.10) | 1.24 (0.82, 1.89) |
Abbreviations: A1M, α1-microglobulin; NGAL, neutrophil gelatinase-associated lipocalin; PIIINP, amino-terminal propeptide of type III procollagen.
Adjusted for age, sex, race, study site, education level, and urine creatinine.
Adjusted additionally for eGFR, diabetes mellitus, cardiovascular disease, heart failure, systolic blood pressure, antihypertensive medication use, statin use, smoking status, body mass index, C-reactive protein, low-density lipoprotein, high-density lipoprotein, and hemoglobin A1c.
Adjusted additionally for urine albumin.
Sensitivity analyses
In sensitivity analyses, we evaluated associations of urine biomarker-creatinine ratios with each outcome. Creatinine-standardized α1m (HR per doubling, 1.23; 95% CI, 1.11-1.38) and NGAL (HR per doubling, 1.07; 95% CI, 1.02-1.12) were independently associated with mortality risk in fully adjusted models. Creatinine-standardized NGAL was also associated with higher risk of CVD (HR per doubling, 1.12; 95% CI, 1.05-1.20), but the association of creatinine-standardized α1m with CVD did not reach statistical significance (HR per doubling, 1.12; 95% CI, 0.93-1.34). Creatinine-standardized PIIINP was not associated with CVD (HR per doubling, 1.14; 95% CI, 0.96-1.35) or mortality (HR per doubling, 1.09; 95% CI, 0.97-1.23), and none of the creatinine-standardized biomarkers were associated with heart failure risk.
Discussion
Renal tubular health is critical for solute and water reabsorption, toxin secretion, acid-base regulation, and mineral metabolism.4 Tubular damage is an important risk factor for kidney disease progression, but its prognostic significance for CKD complications, including cardiovascular outcomes and death, has been uncertain. In this study of community-dwelling older adults, we found that higher urine levels of α1m and NGAL were associated with higher risks for CVD events and all-cause mortality, but not with heart failure events. These associations remained statistically significant after adjustment for eGFR, ACR, and traditional kidney and cardiovascular risk factors. There were no statistically significant associations of urine PIIINP with CVD, heart failure, or mortality. Notably, urine α1m appeared to be the strongest predictor of CVD and mortality, in relation to the other markers. These findings support the overall hypothesis that kidney tubule damage is an independent risk factor for CVD and death, complementary to eGFR and ACR.
Our study builds upon prior work that has examined the relationship of kidney tubular health with cardiovascular risk. Among participants of the Health ABC Study, we previously reported that higher urine concentrations of kidney injury molecule 1 (KIM-1), a biomarker of proximal tubular injury, were associated with higher risks for heart failure (HR for high vs low quartile, 1.32; 95% CI, 1.02-1.70) and death (HR for high vs low quartile, 1.28; 95% CI, 1.08-1.52).29,30 In contrast to KIM-1, which is released into urine by injured proximal tubular cells,31 α1m is a low-molecular-weight protein that is freely filtered at the glomerulus and reabsorbed by proximal tubular epithelial cells under healthy conditions.32,33 Urine α1m concentrations have been correlated with the extent of interstitial fibrosis and tubular atrophy on kidney biopsy specimens from persons with drug-induced interstitial nephritis and from kidney transplant recipients.13,14 In a cohort of HIV-infected and uninfected women, higher urine α1m levels were associated with faster kidney function decline.18 By comparison, PIIINP, the amino-terminal propeptide of type III collagen, is released into urine during deposition of type III collagen in the extracellular matrix, and therefore indicates ongoing fibrosis.15 Higher urine PIIINP levels were found to be associated with the severity of tubulointerstitial fibrosis in kidney biopsy series,16,17 and were found to be associated with faster CKD progression in a cohort of elders.19 Finally, urine neutrophil gelatinase-associated lipocalin (NGAL) is predominantly expressed by epithelial cells in the distal tubule.34 Urine NGAL is a sensitive biomarker of acute kidney injury in adults and children,35–37 and elevated urine NGAL levels have been associated with CKD risk.8,10,11,20
Few prior studies have evaluated associations of urine α1m with cardiovascular outcomes or death. In a study of 2,948 Framingham Heart Study participants, O’Seaghdha et al. used a multiplex panel of biomarkers and found that higher urine α1m was associated with higher all-cause mortality risk (HR, 1.26; 95% CI, 1.13-1.40) but not with incident CVD (HR, 1.08; 95% CI, 0.94-1.23).38 Among HIV-infected women, those in the highest α1m tertile had a 1.6-fold mortality risk (95% CI, 1.0-2.6), compared to women in the lowest α1m tertile, in models adjusting for kidney risk factors, baseline eGFR and albuminuria.18 The present study builds upon this prior literature by demonstrating that urine α1m is an independent risk factor for CVD and mortality among elders. Further studies are needed to validate our findings and to determine whether the association between tubular dysfunction and excess cardiovascular risk represents a causal link or a shared pathogenesis.
Our observation that urine NGAL was associated with CVD and death, but not with heart failure, contributes to a growing body of conflicting literature in regard to urine NGAL and clinical outcomes. Liu et al. reported that urine NGAL was associated independently with future ischemic atherosclerotic events, but not with heart failure events or death in the Chronic Renal Insufficiency Cohort (CRIC) Study.20 Helmersson-Karlquist et al. found that urine NGAL was independently associated with cardiovascular and all-cause mortality among community-dwelling Swedish men, whereas Peralta et al. observed no significant association between urine NGAL and mortality among HIV-infected women.10,21 Among patients with heart failure, Damman et al. demonstrated that higher urine NGAL levels were associated with increased risk for all-cause mortality and heart failure-related hospitalizations22. These discordant findings could be explained by differences in study populations, including age, baseline kidney function, and comorbid conditions, but warrant further investigation.
We observed little association between urine PIIINP levels and risks of CVD, heart failure or death, although the association with CVD approached statistical significance. These findings should be interpreted alongside a report from the Cardiovascular Health Study, in which higher urine PIIINP levels were associated with higher risk for death, but not for incident CVD or heart failure.19 Of note, PIIINP was measured by a radioimmunoassay in the Cardiovascular Health Study and with enzyme-linked immunosorbent assay in the current study; therefore, assay methodology may have contributed to the observed differences. Nonetheless, although strongly associated with kidney outcomes, urine markers of fibrosis may not be as relevant for CVD and death.
We observed moderate inter-correlations between the tubular markers and albuminuria, the traditional marker of glomerular and endothelial injury, supporting the presence of shared risk factors. However, the associations of α1m and NGAL with CVD and mortality were minimally attenuated by adjustment for urine albumin in multivariable models. Although the independent associations do not prove causality, our findings do suggest that tubular damage contributes to cardiovascular risk through pathways that are independent of endothelial injury and kidney filtration.
There are several limitations to this study. First, our objective was to explore mechanisms by which kidney damage leads to increased cardiovascular risk, independent of the clinical markers of eGFR and ACR; therefore, this study did not evaluate the utility of these urine biomarkers for global risk prediction or discrimination of CVD outcomes. Second, we did not have access to serum levels of these biomarkers, so we cannot exclude the possibility that higher serum levels among individuals with excess cardiovascular risk contributed to our findings. Third, our findings will require validation in additional cohorts. Because we studied a cohort of elderly individuals, our results may not be generalizable to younger populations. Fourth, because urine biomarker levels were measured at only one time point, we are unable to determine the impact of longitudinal changes in renal tubular health. Fifth, we were unable to ascertain AKI outcomes, as kidney function was measured only four times over the ten-year follow-up period and discharge diagnoses for AKI have not previously been obtained in the Health ABC cohort. Finally, although we adjusted for multiple confounders in our multivariable models, we cannot exclude the possibility of residual confounding.
In this cohort of ambulatory elders, higher urine α1m and NGAL were independently associated with CVD events and all-cause mortality, but not with heart failure events. These findings suggest that kidney tubular damage is an important risk factor for adverse cardiovascular outcomes. Further research is needed to validate these findings and to determine the mechanisms underlying these associations
Supplementary Material
Table S1: Baseline characteristics by quartile of PIIINP in Health ABC subcohort.
Table S2: Baseline characteristics by quartile of NGAL in Health ABC subcohort.
Table S3: Spearman correlations of urinary markers and eGFR.
Acknowledgments
Support: The Health, Aging, and Body Composition Study is supported by the National Institute on Aging (NIA) contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; and National Institute of Nursing Research grant R01-NR012459. This study was also supported by the NIA grant 5R01AG027002-08 to Drs. Shlipak and Sarnak; by the American Heart Association grant EIA 18560026 to Dr. Ix; and by the National Institute of Diabetes and Digestive and Kidney Diseases grant 1K23DK109868-01A1 to Dr. Jotwani. Funders did not have a role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions: Research idea and study design: MJS, MGS, RK, JHI; data acquisition: SRC, MB; data analysis/interpretation: VJ, RK, OMG, CRP, JHI, MJS, MGS; statistical analysis: RK; supervision or mentorship: MJS, MGS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Financial Disclosure: The authors declare that they have no relevant financial interests.
The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003 Oct 28;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Chronic Kidney Disease Prognosis C. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waheed S, Matsushita K, Sang Y, et al. Combined association of albuminuria and cystatin C-based estimated GFR with mortality, coronary heart disease, and heart failure outcomes: the Atherosclerosis Risk in Communities (ARIC) Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012 Aug;60(2):207–216. doi: 10.1053/j.ajkd.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner BM, Rector FC. Brenner & Rector’s the kidney. 8th. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 5.Howie AJ, Ferreira MA, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association – European Renal Association. 2001 Jun;16(6):1163–1169. doi: 10.1093/ndt/16.6.1163. [DOI] [PubMed] [Google Scholar]
- 6.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. American journal of kidney diseases: the official journal of the National Kidney Foundation. 1992 Jul;20(1):1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 7.Rule AD, Amer H, Cornell LD, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010 May 04;152(9):561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhavsar NA, Kottgen A, Coresh J, Astor BC. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2012 Aug;60(2):233–240. doi: 10.1053/j.ajkd.2012.02.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jungbauer CG, Uecer E, Stadler S, et al. N-acteyl-ss-D-glucosaminidase and kidney injury molecule-1: New predictors for long-term progression of chronic kidney disease in patients with heart failure. Nephrology (Carlton) 2016 Jun;21(6):490–498. doi: 10.1111/nep.12632. [DOI] [PubMed] [Google Scholar]
- 10.Peralta CA, Katz R, Bonventre JV, et al. Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012 Dec;60(6):904–911. doi: 10.1053/j.ajkd.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith ER, Lee D, Cai MM, et al. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD) Nephrol Dial Transplant. 2013 Jun;28(6):1569–1579. doi: 10.1093/ndt/gfs586. [DOI] [PubMed] [Google Scholar]
- 12.Shlipak MG, Scherzer R, Abraham A, et al. Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr. 2012 Dec 15;61(5):565–573. doi: 10.1097/QAI.0b013e3182737706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amer H, Lieske JC, Rule AD, et al. Urine high and low molecular weight proteins one-year post-kidney transplant: relationship to histology and graft survival. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013 Mar;13(3):676–684. doi: 10.1111/ajt.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Yang L, Su T, Wang C, Liu G, Li XM. Pathological significance of a panel of urinary biomarkers in patients with drug-induced tubulointerstitial nephritis. Clin J Am Soc Nephrol. 2010 Nov;5(11):1954–1959. doi: 10.2215/CJN.02370310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soylemezoglu O, Wild G, Dalley AJ, et al. Urinary and serum type III collagen: markers of renal fibrosis. Nephrol Dial Transplant. 1997 Sep;12(9):1883–1889. doi: 10.1093/ndt/12.9.1883. [DOI] [PubMed] [Google Scholar]
- 16.Ghoul BE, Squalli T, Servais A, et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol. 2010 Feb;5(2):205–210. doi: 10.2215/CJN.06610909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teppo AM, Tornroth T, Honkanen E, Gronhagen-Riska C. Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation. 2003 Jun 27;75(12):2113–2119. doi: 10.1097/01.TP.0000066809.60389.48. [DOI] [PubMed] [Google Scholar]
- 18.Jotwani V, Scherzer R, Abraham A, et al. Association of Urine alpha1-Microglobulin with Kidney Function Decline and Mortality in HIV-Infected Women. Clin J Am Soc Nephrol. 2015 Jan 7;10(1):63–73. doi: 10.2215/CJN.03220314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ix JH, Biggs ML, Mukamal K, et al. Urine Collagen Fragments and CKD Progression-The Cardiovascular Health Study. J Am Soc Nephrol. 2015 Oct;26(10):2494–2503. doi: 10.1681/ASN.2014070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu KD, Yang W, Anderson AH, et al. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney international. 2013 May;83(5):909–914. doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmersson-Karlqvist J, Larsson A, Carlsson AC, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with mortality in a community-based cohort of older Swedish men. Atherosclerosis. 2013 Apr;227(2):408–413. doi: 10.1016/j.atherosclerosis.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Damman K, Masson S, Hillege HL, et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J. 2011 Nov;32(21):2705–2712. doi: 10.1093/eurheartj/ehr190. [DOI] [PubMed] [Google Scholar]
- 23.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008 May;3(3):665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003 Nov 11;108(19):2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 25.Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, et al. Serum albumin concentration and heart failure risk The Health, Aging, and Body Composition Study. American heart journal. 2010 Aug;160(2):279–285. doi: 10.1016/j.ahj.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koster A, Stenholm S, Alley DE, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010 Dec;18(12):2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarnak MJ, Katz R, Newman A, et al. Association of urinary injury biomarkers with mortality and cardiovascular events. Journal of the American Society of Nephrology: JASN. 2014 Jul;25(7):1545–1553. doi: 10.1681/ASN.2013070713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Driver TH, Katz R, Ix JH, et al. Urinary kidney injury molecule 1 (KIM-1) and interleukin 18 (IL-18) as risk markers for heart failure in older adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis. 2014 Jul;64(1):49–56. doi: 10.1053/j.ajkd.2014.01.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002 Jul;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 32.Akerstrom B, Logdberg L, Berggard T, Osmark P, Lindqvist A. alpha(1)-Microglobulin: a yellow-brown lipocalin. Biochim Biophys Acta. 2000 Oct 18;1482(1–2):172–184. doi: 10.1016/s0167-4838(00)00157-6. [DOI] [PubMed] [Google Scholar]
- 33.Weber MH, Verwiebe R. Alpha 1-microglobulin (protein HC): features of a promising indicator of proximal tubular dysfunction. Eur J Clin Chem Clin Biochem. 1992 Oct;30(10):683–691. [PubMed] [Google Scholar]
- 34.Paragas N, Qiu A, Zhang Q, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nature medicine. 2011 Feb;17(2):216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005 Apr 2–8;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 36.Parikh CR, Jani A, Mishra J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006 Jul;6(7):1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 37.Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008 Jun 3;148(11):810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Seaghdha CM, Hwang SJ, Larson MG, Meigs JB, Vasan RS, Fox CS. Analysis of a urinary biomarker panel for incident kidney disease and clinical outcomes. J Am Soc Nephrol. 2013 Nov;24(11):1880–1888. doi: 10.1681/ASN.2013010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Baseline characteristics by quartile of PIIINP in Health ABC subcohort.
Table S2: Baseline characteristics by quartile of NGAL in Health ABC subcohort.
Table S3: Spearman correlations of urinary markers and eGFR.


