Abstract
Limited information about self-injurious behavior (SIB) is known for children and adolescents with Autism Spectrum Disorder (ASD) who require intensive behavioral health interventions. We examined risk-factors for SIB in 302 individuals with ASD (ages 4–20) admitted to six specialized psychiatric inpatient units. Seventy-four percent were reported by a caregiver to display SIB, however, only 25% were observed to engage in daily SIB during hospitalization. Those exhibiting SIB across environments had significantly higher ratings on caregiver questionnaires of SIB severity. Tree-structured classification was used to develop and validate two predictive models, one indicating which inpatient youth with ASD are likely to have SIB and a second indicating which individuals with SIB at home are likely to continue in an inpatient setting.
Keywords: Autism Spectrum Disorder (ASD), Autism Inpatient Collection (AIC), Self-injurious behavior (SIB), Psychiatric hospitalization
Introduction
Self-injurious behavior (SIB) is a significant concern for children and adolescents with Autism Spectrum Disorder (ASD) and is defined as non-accidental self-inflicted acts causing damage to or destruction of body tissue and carried out without suicidal ideation or intent (Yates 2004). For individuals with ASD and intellectual disability (ID), SIB often involves behaviors such as head banging/slapping as well as self-biting, hair pulling, skin-picking and scratching (Furniss and Biswas 2012). An estimated 15–50% of individuals with ASD exhibit some form of SIB. For example, Baghdadli et al. (2003) found that 50% of a sample of 222 young children (ages 6 and under) with ASD was reported to exhibit SIB, with 14.6% engaging in severe SIB. Similarly, Richards et al. (2012) compared SIB rates among three groups: individuals with ASD (n = 149), Fragile X (n = 123) and Down syndrome (n = 49). Fifty percent of those with ASD had reportedly exhibited SIB within the past month, 2.67 times more likely than those with Down syndrome, but fairly similar to the rate in Fragile X. Finally, Lecavalier (2006) reported rates of SIB in a sample of 487 non-clinically referred children and adolescents with Pervasive Developmental Disorders (ages 3–21 years) by obtaining behavior rating scales from both parents and teachers. Only the frequency of behaviors rated as “moderate” or “severe” was reported. Individual symptom rates ranged from 4.8 to 15.9% of the sample; however, an overall rate of SIB (taking into account all of the possible SIB symptoms) was not provided. Among the most commonly reported behaviors, 15.9% of the sample were rated as moderate or severe on “Hits or Slaps Self” by both parents and teachers. The next most frequently reported behaviors were “Gouges/Eats Inedible Objects” (reported to occur in 12.2% of the sample by parents and 11.5% by teachers) and “Physically Harms Self” (reported to occur in 11.0% of the sample by parents and 10.3% by teachers). Determining an accurate estimate of the rate of SIB in individuals with ASD is challenging, due to study differences in the definition of SIB as well as cohort differences (such as the severity of ASD, community versus clinical samples, age range and cognitive level) (Richards et al. 2012).
Research on potential risks for the development of SIB in the ASD population has examined a wide range of possible factors. For example, Richman et al. (2013) examined data collected from 617 individuals from the National Database of Autism Research (age range 3–35 years, mean 11.21 years) and found that higher rates of impulsivity and stereotypy were most predictive of SIB (even after controlling for ID and ASD severity). Baghdadli et al. (2003) found that younger age, perinatal conditions (e.g., presence of a genetic syndrome), more severe ASD symptoms and greater delays in daily living skills were most predictive of SIB in a sample of 222 young children with ASD (age range 2–7 years). Similarly, Matson and Rivet (2008a) documented a significant relationship between ASD severity and SIB severity. A recent study of 180 young children (ages 4–48 months) at risk for intellectual and developmental disabilities (identified via mass screening and an interdisciplinary evaluation) found that those at-risk for ASD displayed greater rates of SIB (as well as aggression and stereotyped behavior) than children with Down syndrome or those with atypical development but without higher ASD risk (Schroeder et al. 2014). However, gender, age, intellectual ability and communication level were not found to be related to SIB frequency among those children at-risk for ASD. In a study of the psychometric characteristics of the Institute for Basic Research (IBR) Modified Overt Aggression Scale, Cohen et al. (2010) found that in a sample of over 2000 adults with ID, females with ASD as well as those with severe to profound ID were more likely to engage in SIB. Others have documented that individuals with both ASD and ID have higher rates of SIB than those with ID alone (Matson and Rivet 2008b; Rojahn et al. 2010; Smith and Matson 2010). This was confirmed by a meta-analysis of risk markers for challenging behaviors in individuals with ID which found that those with ASD and ID were significantly more likely to display SIB than those with ID alone (McClintock and Oliver 2003). In addition, individuals with ASD functioning within the severe/profound range of ID were also more likely to exhibit SIB than those with mild/moderate ID. Finally, Esbensen et al. (2009) studied a sample of over 700 children and adults with ASD and found that SIB was less severe and frequent among older than younger individuals. In addition, females and individuals with ID were more likely to display SIB.
The majority of studies conducted in the past 20–25 years examining factors related to SIB in the ASD population have focused on community samples. However, prior research indicates that SIB is one of the primary reasons for psychiatric hospitalization in ASD (Siegel et al. 2012). It is possible that those who require more intensive care, such as an admission to psychiatric inpatient hospitalization, have more frequent or severe SIB and different risk factors for SIB. Although likely, this has not been examined and it is also unknown whether the factors associated with SIB would be similar in community and hospitalized samples. We present the results of a naturalistic study of a large sample of children and adolescents with ASD who were admitted for psychiatric inpatient treatment. An analysis of both the rates and types of SIB as well as factors that might predict the presence of SIB was conducted. It was hypothesized that those individuals who exhibited SIB both at home and at least daily in the hospital (“Home and Hospital SIB”) would have significantly higher ratings on measures of irritability and hyperactivity than those whose SIB was reported to occur at home but less than once a day in the hospital (“Home SIB”). In addition, it was hypothesized that, consistent with the ASD/SIB literature, age, lower Nonverbal Intelligence Quotient (NVIQ), lower adaptive functioning, higher ASD symptom severity and higher rates of inattention and hyperactivity symptoms would be most predictive of SIB severity for this inpatient population. Finally, an exploratory hypothesis was that higher rates of SIB would be reported among children and adolescents with ASD who were admitted to inpatient psychiatric units than previously published rates in community samples.
Methods
Subjects
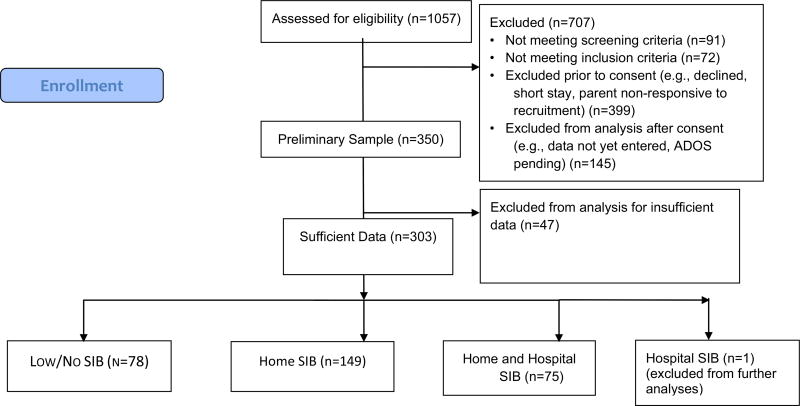
Children and adolescents with ASD admitted to six specialized psychiatric hospital inpatient units that treat ASD and other neurodevelopmental disorders were enrolled through the Autism Inpatient Collection (AIC). The six research sites included Western Psychiatric Institute and Clinic (Pittsburgh, PA), Cincinnati Children’s Hospital (OH), Children’s Hospital Colorado (Aurora CO), Spring Harbor Hospital (Portland, ME), Shepphard Pratt Health System (Baltimore, MD) and Bradley Hospital (Providence, RI). The full methods of the AIC have been published previously (Siegel et al. 2015). Briefly, children aged 4–20 years old with a score of ≥ 12 on the Social Communication Questionnaire (SCQ; Rutter et al. 2003) or with high suspicion of ASD from the inpatient clinical treatment team were eligible for enrollment. ASD diagnoses were confirmed by administration of the Autism Diagnostic Observation Schedule-2 (ADOS-2; Lord et al. 2012) by a study examiner trained to research reliability standards on the ADOS-2, and all subjects met or exceeded ADOS-2 cut-offs for ASD. Exclusion criteria included not having a parent available who was not proficient in English or the child with ASD being a prisoner. Figure 1 summarizes the enrollment process.
Fig. 1.
Consort flow diagram
Subjects were divided into the following four groups: “Low/No SIB,” “Home SIB,” “Hospital SIB,” and “Home and Hospital SIB.” Subjects were considered to have “Low/ No SIB” if they did not have a score of 2 or more (moderate impairment) for any form of SIB as measured by caregiver report on the Repetitive Behavior Scale—Revised (RBSR) (Bodfish et al. 1999; Lam and Aman 2007). The “Home SIB” group consisted of individuals who were given a severity score of 2 or greater on one or more SIB items of the RBSR by the caregiver, but were not determined by the inpatient unit’s behavioral specialist (psychologist or board certified behavior analyst) to have at least one daily occurrence of SIB during the inpatient stay. The “Hospital SIB” group included individuals who were not given a severity score of 2 or greater on one or more SIB items of the caregiver completed RBSR but who engaged in daily SIB on the inpatient unit. The final group, “Home and Hospital SIB,” included individuals with both parent reported SIB as well as observed daily SIB on the inpatient unit during their stay. Stays ranged from 5 to 47 days (average length of stay 25.6 days).
The home-reported SIB on the RBSR reflected the caregiver’s observation of SIB during the month prior to inpatient admission. At all the study sites, SIB target behaviors were identified at the time of admission. Operationalized definitions of SIB were developed by the unit’s behavior specialist and the occurrences were recorded by the direct care staff during the stay. Behaviors falling under the category of SIB included any actions that were likely to cause harm to the child and were initiated by the child, such as punching, scratching or abrading oneself. Typically, behaviors were selected that had been endorsed on the RBSR by the caregiver, although additional behaviors could be added if observed by unit staff. A large number of direct care staff over different shifts worked with each child and recorded data over the course of a hospitalization. SIB occurrence was recorded dichotomously (“yes/no”) based on varied intervals of observation across a variety of settings in the hospital. The unit behavioral specialist utilized his/her observations and SIB occurrence data to determine if the subject had daily SIB during the hospitalization. Due to the large number of staff conducting observations, inter-rater reliability for direct care staff was not obtained. The analyses in the current study involved determining only if a child engaged in at least one episode of SIB per day.
Measures
IQ and Adaptive Behavior
Subjects were administered the Leiter International Performance Scale—Third Edition (Leiter-3; Roid et al. 2013) and Vineland Adaptive Behavior Scales-2 (Sparrow et al. 2005). Due to the anticipated number of children with limited expressive language skills, the Leiter-3, a non-verbal test of cognitive functioning, was administered to all study participants as an estimate of NVIQ. The Vineland-2 is a comprehensive questionnaire of adaptive functioning that was completed by parents. Analyses of the Vineland focused on the total score, where higher scores indicated higher overall adaptive functioning.
Aberrant Behavior Checklist—Community (ABC-C) (Parent) (Aman et al. 1985a)
The ABC-C is a standardized scale comprising 58 items for assessing problem behavior in children and adults with developmental disabilities. It was completed by caregivers within 10 days of admission. The scale was empirically derived from ratings on approximately 1000 individuals, resulting in five subscales: (I). Irritability (15 items), (II) Lethargy/Social Withdrawal (16 items), (III) Stereotypic Behavior (7 items), (IV) Hyperactivity (16 items), and (V) Inappropriate Speech (4 items). Items are rated on a 4-point Likert scale, ranging from (0) “not at all a problem” to (3) “the problem is severe in degree.” The psychometric characteristics of the ABC appear to be very reliable with the ASD patient population (Aman et al. 1985b; Rojahan et al. 2003). For purposes of the current analysis, two items on the Irritability subscale relating to SIB were excluded. If an individual had up to 20% of items missing on any single subscale, these items were imputed using the mean of the observed subscale items. This allowed for us to calculate the subscale total even if some items were missing.
Repetitive Behavior Scale-Revised (RBSR) (Bodfish et al. 1999; Lam and Aman 2007)
The RBSR is a 44-item, parent-completed questionnaire that measures repetitive behaviors in children, adolescents, and adults with ASD. Items are rated on a 0–3 scale (0— behavior does not occur, 1—behavior occurs and is a mild problem, 2—behavior occurs and is a moderate problem, 3—behavior occurs and is a severe problem). Internal consistency for the RBSR subscales ranges from .78 to .91 with subscale inter-rater reliability ranging from .57 to .73 (Lam and Aman 2007). For the current study, only the 8-item SIB subscale was used for the purposes of these analyses. A subject was considered to exhibit SIB if any of the 8 items was given a rating of ≥ 2.
ADOS-2 Comparison Scores
The ADOS-2 (ADOS-2; Lord et al. 2012) comparison score for participants who were administered Modules 1, 2, and 3 were used as a measure of autism severity. The ADOS-2 comparison score was designed to indicate an individual’s level of ASD symptomology compared to others diagnosed with ASD who are of the same age and language level.
Demographics
Information was collected on subjects’ age, race and gender as well as family SES (income).
Verbal Ability
Two verbal ability categories were created, minimally verbal and verbal, based on which ADOS-2 (Lord et al. 2012) module was administered, using this instrument’s established language guidelines for selecting the ADOS-2 module to administer. Minimally verbal was defined as requiring administration of an ADOS-2 Module 1 (for pre-verbal/single words) or Module 2 (phrase speech). Participants were considered verbal based on meeting ADOS-2 criteria for the administration of a Module 3 or 4 (verbally fluent adolescents and adults).
Statistical Analysis
In the full sample and within SIB groups, descriptive statistics for clinical and demographic characteristics were calculated using means and standard deviations for ordered variables and numbers and proportions for categorical variables. To test for differences across SIB groups, ANOVA F-tests were calculated for continuous variables, Chi square or Fisher’s exact tests were calculated for categorical variables, and likelihood-ratio Chi square test statistics from generalized linear models with a log link were calculated for count variables. We controlled for multiple comparisons across all demographic and clinical characteristics (Tables 1, 2, 3) using false discovery rate correction (Benjamini and Hochberg 1995). To assess clinical (rather than statistical) significance, Cohen’s d effect sizes with 95% confidence intervals were calculated for post-hoc pairwise group comparisons. If the characteristic was categorical, we converted the effect size to Cohen’s d’s for comparability (Gleser and Olkin 2009). As a sensitivity analysis, we further assessed whether differences across groups remained after considering site by fitting mixed effects models that included site as a random effect and group as a fixed effect. For continuous characteristics, linear mixed effects models were used. For categorical and count characteristics, generalized linear mixed effects models with a logit and log link, respectively, were used.
Table 1.
Subject demographics and non-verbal IQ in full sample and within SIB groups
| Mean (SD) or %(N) |
Full (N = 302) | Low/No SIB (“N”; N = 78) |
Home SIB (“H”; N = 149) |
Home and Hospital SIB (“HH”; N = 75) |
Statistic (p-value) |
ES (95% CI) HH vs. H |
ES (95% CI) HH vs. N |
ES (95% CI) H vs. N |
|---|---|---|---|---|---|---|---|---|
| Demographic | ||||||||
| Age, %(N) | 12.9 (3.4) | 13.22 (3.42) | 12.97 (3.43) | 12.51 (3.30) | 0.87 (0.422) | − 0.14 (− 0.42, 0.14) | − 0.21 (− 0.53, 0.11) | − 0.07, (− 0.35, 0.20) |
| Female, %(N) | 21.19% (64) | 16.67% (13) | 20.81% (31) | 26.67% (20) | 2.32 (.314) | 0.14 (− 0.15, 0.41) | 0.24 (− 0.07, 0.57) | 0.11 (− 0.16, 0.39) |
| Caucasian, %(N) | 81.79% (247) | 82.05% (64) | 80.54% (120) | 84.00% (63) | 0.41 (.816) | 0.09 (− 0.18 0.40) | 0.05 (− 0.25, 0.39) | − 0.04 (− 0.32, 0.23) |
| Hispanic or Latino, %(N) (N = 285) | 6.32% (18) | 2.86% (2) | 7.14% (10) | 8.00% (6) | FE (.413) | − 0.11 (− 0.41, 0.16) | − 0.07 (− 0.41, 0.25) | 0.04 (− 0.23, 0.33) |
| Income | 3.91 (2.55) | 4.54 (2.70) | 3.66 (2.44) | 3.77 (2.55) | 2.85 (0.060) | 0.04 (− 0.25, 0.34) | − 0.29 (− 0.64, 0.05) | − 0.35 (− 0.64, − 0.05) |
“FE” instead of a statistic indicates that a Fisher’s Exact test was used to compare groups because of small cell sizes (this test does not generate a test statistic)
Table 2.
Percent and N in each SIB group by site
| Low/No SIB (N = 78) | SIB Home (N = 149) | SIB Home and Hospital (N = 75) |
|
|---|---|---|---|
| Site 1 (N = 69) | 28.99 (20) | 43.48% (30) | 27.54% (19) |
| Site 2 (N = 35) | 5.71% (2) | 48.57% (17) | 45.71% (16) |
| Site 3 (N = 30) | 26.67% (8) | 53.33% (16) | 20.00% (6) |
| Site 4 (N = 53) | 20.75% (11) | 41.51% (22) | 37.74% (20) |
| Site 5 (N = 77) | 32.47% (25) | 55.84% (43) | 11.69% (9) |
| Site 6 (N = 38) | 31.58% (12) | 55.26% (21) | 13.16% (5) |
Table 3.
Summary of ADOS, SCQ and ABC subscales in full sample and within SIB groups
| Clinical characteristics | Full (N = 302) | Low/No SIB (“N”; N = 78) |
Home SIB (“H”; N = 149) |
Home and Hospital SIB(“HH”; N = 75) |
Statistic (p-value) |
ES (95% CI) HH vs. H |
ES (95% CI) HH vs. N |
ES (95% CI) H vs. N |
|---|---|---|---|---|---|---|---|---|
| Nonverbal IQ (N = 252) | 75.15 (28.74) | 86.25 (28.42) | 75.91 (26.84) | 58.13 (26.06) | 16.21 (< .0001) | − .67 (− 1.0, − .33) | − 1.02 (− 1.41, − .64) | − .38 (− .67, − .08) |
| ADOS-2 Comp Score, N = 254 | 7.87 (1.74) | 7.88 (1.59) | 7.86 (1.60) | 7.88 (2.10) | 0.00 (0.9961) | .01 (− .29, .31) | 0.0 (− .36, .36) | − .01 (− .33, .31) |
| Minimally verbal (ADOS-2 Mod Cat 1 or 2), N = 298 | 51.34 (153) | 53.24 (41) | 52.74 (77) | 46.67 (35) | 0.88 (0.64) | .10 (− .18, .39) | .12 (− .20, .44) | .02 (− .26, .30) |
| Social Communication Questionnaire (SCQ), N = 277 | 23.50 (6.96) | 21.69 (7.66) | 23.05 (6.84) | 26.25 (5.59) | 8.49 (.0003) | 0.5 (.20, .79) | 0.68 (.33, 1.02) | 0.19 (− .10, .48) |
| ABC Irritability (no SIB items), N = 287 | 24.42 (8.36) | 19.33 (8.85) | 25.67 (7.81) | 27.39 (6.35) | 22.87 (< .0001) | 0.23 (− .06, .53) | 1.04 (.68, 1.39) | 0.78 (.48, 1.07) |
| ABC Lethargy, N = 287 | 15.10 (8.16) | 12.34 (7.1) | 15.6 (8.23) | 17.14 (8.43) | 7.02 (0.0011) | 0.19 (− .11, .48) | 0.62 (.28, .96) | 0.41 (.13, .70) |
| ABC Ste-reotypy, N = 287 | 7.86 (5.56) | 4.65 (4.16) | 8.14 (5.34) | 10.81 (5.62) | 26.16 (< .0001) | 0.49 (.20, .79) | 1.26 (.89, 1.62) | 0.70 (.41, .99) |
| ABC Hyper-activity, N = 288 | 28.47 (10.7) | 24.15 (10.7) | 29.61 (10.78) | 30.88 (9.19) | 9.24 (.0001) | 0.12 (− .17, .42) | 0.67 (.33, 1.01) | 0.51 (.22, .79) |
| ABC Inappropriate Speech, N = 286 | 5.26 (3.65) | 4.36 (3.59) | 5.75 (3.43) | 5.21 (4.01) | 3.66 (0.027)* | − 0.15 (− .44, .14) | 0.22 (− .11, .56) | 0.40 (.11, .68) |
p-Value not significant at α = .05 level after Benjamini–Hochberg false discovery rate adjustment for multiple comparisons
We used tree-structured classification (Therneau et al. 2015) to develop clinically practical predictive models for identifying: (1) which youth with home SIB are likely to have “Home and Hospital SIB,” and (2) which youth are likely to have “Home SIB” and/or “Home and Hospital SIB.” For a given sample and a set of possible predictors, tree-structured classification uses a data-driven approach to iteratively evaluate each possible split point on each predictor and select the one that best divides the sample into two subsamples, within which the outcomes (here, probability of SIB) are more similar to one another. For both categorical and continuous predictors, this splitting value is selected empirically. For example, given Age as a predictor, the algorithm would test age < 14, age < 15, age < 16, and so on. This process is repeated on each subsequent sample until the largest tree possible is grown. The tree is then “pruned back” to avoid overfitting based on a complexity parameter selected as that which provides the most parsimonious model whose error is no more than one standard error above the error of the best model (Hastie et al. 2009). To add further stability to the tree model structure, we also required a minimum of 10 individuals in each subsample.
For the tree predicting any SIB, we considered all possible predictors except RBSR-based items (because SIB was defined based on RBSR) and included all 302 subjects. For the tree predicting hospital SIB if home SIB is present, we considered all possible predictors in Tables 1, 3 and 4 and used only the N = 224 with home SIB. After growing the trees, we estimated the potential predictive accuracy in a new sample by iteratively fitting tree models to 5 sites and testing the model in the 6th omitted site. That is, for each of 6 iterations, we fit a tree model using 5 sites and validated the model using the 6th omitted site. For each omitted site on which the model was validated, the accuracy, true positive rate, and true negative rate of the tree were calculated. All analyses were performed using R version 3.3.1 (R Core Team 2016). Trees were developed using the rpart package in R (Therneau et al. 2015).
Table 4.
RBSR Scale results based on Home SIB and Home and Hospital SIB groups
| RBSR Scale
| |||||
|---|---|---|---|---|---|
| %(N) or mean (SD) | Any SIB (N = 224) | Home SIB only (N = 149) |
Home and Hospital SIB (N = 75) |
Stat (p-value) | ES (95% CI) |
| Hits self with body part (RBSR1) | 65.18 (146) | 55.03 (82) | 85.33 (64) | 18.87 (< .0001) | − 0.68 (− 0.98, − 0.42) |
| Hits self against surface or object (RBSR2) | 62.95 (141) | 55.03 (82) | 78.67 (59) | 10.95 (0.001) | − 0.51 (− 0.80, −0.24) |
| Hits self with object (RBSR3) | 29.91 (67) | 26.85 (40) | 36 (27) | 1.58 (0.209) | − 0.20 (− 0.48, 0.09) |
| Bits self (RBSR4) | 40.62 (91) | 34.9 (52) | 52 (39) | 5.36 (0.021) | − 0.35 (− 0.63, −0.07) |
| Pulls (RBSR5) | 33.04 (74) | 30.2 (45) | 38.67 (29) | 1.26 (0.262) | − 0.18 (− 0.46, 0.1) |
| Rubs or Scratches self (RBSR6) | 43.3 (97) | 42.28 (63) | 45.33 (34) | 0.09 (0.77) | − 0.06 (− 0.34, 0.22) |
| Inserts Finger or Object (RBSR7) | 14.35 (32) | 12.84 (19) | 17.33 (13) | 0.49 (0.482) | − 0.11 (− 0.38, 0.17) |
| Skin Picking (RBSR8) | 44.64 (100) | 46.98 (70) | 40.00 (30) | 0.72 (0.396) | 0.14 (− 0.14, 0.42) |
| Number of RBSR items scored ≥ 2 | 3.35 (1.87) | 3.05 (1.83) | 3.93 (1.8) | 11.39 (.0007) | − 0.49 (− 0.77, − 0.20) |
| Total RBSR Score | 9.78 (5.01) | 8.85 (4.94) | 11.6 (4.67) | 15.97 (.0001) | − 0.57 (− 0.85, − 0.28) |
Results
A total of 303 subjects had sufficient data to be included in the analysis. Of these, 78 (25.7%) subjects did not display SIB at home or in the hospital (“Low/No SIB” group), 149 (49.2%) were determined to exhibit SIB only at home (“Home SIB” group), and 75 (24.8%) exhibited SIB both at home and in the hospital (“Home and Hospital SIB” group). One subject had no reported SIB at home, but was observed to engage in SIB while in the hospital (“Hospital SIB” group). The single “Hospital SIB” subject was not included in our analyses. Thus, our final analytic sample consisted of 302 subjects. The 204 subjects with either Home or Hospital SIB (as reported by parents or staff) represent approximately two-thirds (67.5%) of the sample (see Fig. 1, CONSORT diagram).
Table 1 summarizes the demographic data on enrolled subjects. In addition to providing information on age, gender, and socio-economic status (income) for the entire sample, the table also summarizes demographic differences among the “Low/No SIB,” “Home SIB” and “Home and Hospital SIB” groups. No significant differences across the groups were found. Table 2 provides further description of the distribution of the three groups, where it was noted that group distribution (“Home SIB Home,” “Home and Hospital SIB”, “Low/No SIB”) differed by site ( χ2 = 27.9, df = 10, p = 0.002). Most notably, Site 2 (N = 35) had relatively few individuals with “Low/No SIB” (5.7%, N = 2) but more individuals with “Home and Hospital SIB” (N = 45.7%, N = 16). Conversely, Site 5 (N = 77) consisted of fewer individuals with “Home and Hospital SIB” (11.7%, N = 9) but more individuals with only “Home SIB” (55.8%, N = 43) or “Low/ No SIB” (32.5%, N = 25).
The results of comparisons between SIB groups on NVIQ as well as the ADOS, SCQ and ABC subscales are presented in Table 3. A significant difference was found across the three groups on NVIQ, in which the “Home and Hospital SIB” group mean NVIQ was almost 30 points (d (95% CI) = − 1.02 (− 1.41, − .64)) lower than the “Low/No SIB” group and over 10 points lower than the “Home SIB” group (d (95% CI) = − .67 (− 1.0, − .33)). The “Home SIB” group also had a lower NVIQ than the “No SIB” group (d (95% CI) = − .38 (− .67, − .08)). As hypothesized, the “Home and Hospital SIB” group had more severe social and communication deficits than the other two groups based on the SCQ. Specifically, the “Home and Hospital SIB” group had significantly higher scores on the SCQ than both the “Home SIB” (d (95% CI) = 0.50 (.20, .79)) and “Low/No SIB” groups (d (95% CI) = 0.68 (.33, 1.02)). However, the mean ADOS-2 Comparison Score and percent of minimally-verbal and verbal participants were fairly consistent across the three groups. The “Home and Hospital SIB” group had slightly fewer participants with fluent verbal speech, but the difference was not statistically significant. In terms of externalizing behaviors, both SIB groups were found to exhibit significantly higher scores than the “Low/No SIB” group on ABC Irritability (minus the two SIB items), Hyperactivity, and Lethargy subscales. However, the “Home SIB” and “Home and Hospital SIB” groups did not differ meaningfully on these subscales. All three groups were differentiated on the ABC Stereotypy subscale, with the “Home and Hospital SIB” group exhibiting the greatest stereotypy behavior, followed by the “Home SIB” and finally the “Low/ No SIB” group.
Table 4 summarizes the RBSR Scale results for the two SIB groups only. The two most frequently endorsed types of SIB were “Hitting Oneself with a Body Part” or “Hitting Oneself on a Surface or Object,” occurring in approximately two-thirds of those with any SIB. The remaining RBSR items were endorsed by approximately 30–45% of individuals with SIB, with the exception of “Inserting Finger or Object,” which was only reported among 14% of the individuals with SIB. Significant differences between the “Home SIB” and the “Home and Hospital SIB” groups were noted for three RBSR items: “Hits Self with Body Part,” “Hits Self against Surface or Object” and “Bites Self.” In addition, both the total number of RBSR items with severity scores ≥ 2 and the Total RBSR Score were also significantly higher in the “Home and Hospital SIB” group in comparison to the “Home SIB” group.
Finally, in our sensitivity analyses that included site as a random effect, the exact same characteristics from Tables 1, 3 and 4 showed significant differences across groups. As such, these findings are robust in this regard.
Tree-Structured Modeling
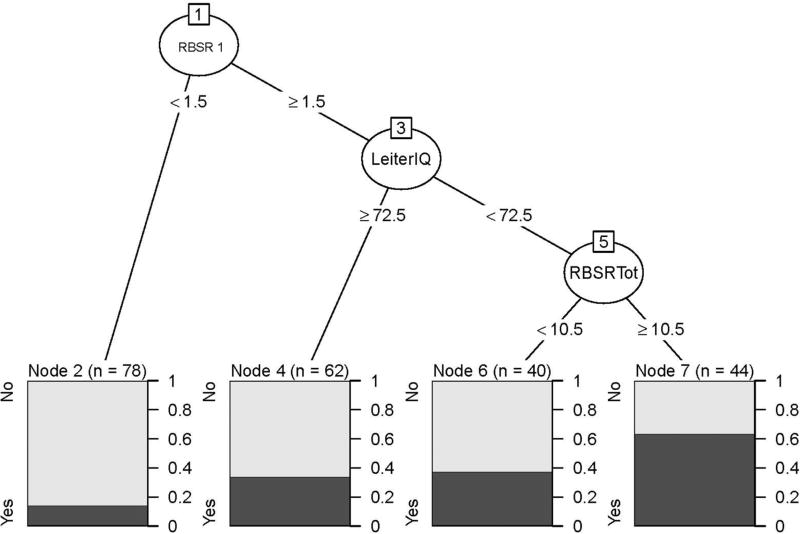
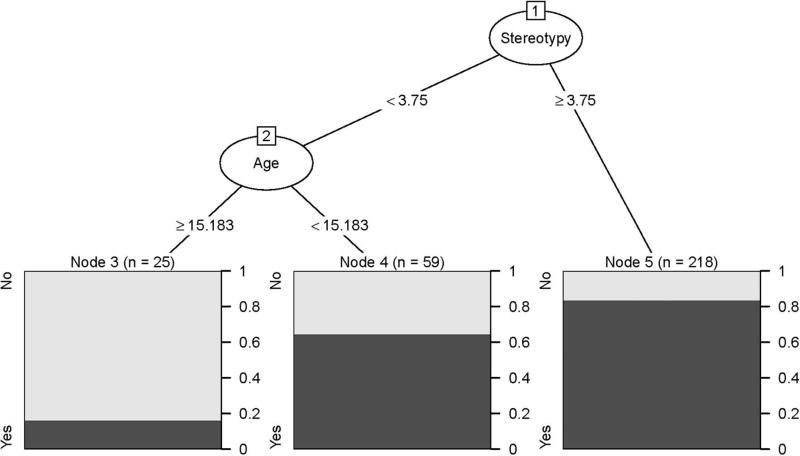
The first model aimed to predict which youth with ASD are likely to have hospital SIB given that they had home SIB. As shown in Fig. 2, the tree model empirically identified four subgroups (1) RBSR ≤ 1 (N = 78; 14.1% with Hospital SIB); (2) RBSR > 1 and Leiter IQ ≥ 72.5 (N = 62, 33.9% with Hospital SIB); (3) RBSR > 1, Leiter IQ < 72.5, and RBSR total < 10.5 (N = 40, 37.5% with Hospital SIB); (4) RBSR > 1, Leiter IQ < 72.5, and RBSR total ≥ 10.5 (N = 44, 63.6% with hospital SIB). The second model we fit (Fig. 3) aimed more generally to predict which youth with ASD in an inpatient setting were likely to have any SIB. The tree model empirically identified three subgroups: (1) Stereotypy < 3.75 and age ≥ 15.2 (N = 25, 16% with SIB); (2) Stereotypy < 3.75 and age < 15.8 (N = 59, 64.4% with SIB); and (3) Stereotypy ≥ 3.75 (N = 218, 83.5% with SIB).
Fig. 2.
Tree-structured model for predicting which youth with home SIB are likely to have hospital SIB
Fig. 3.
Predicting which youth with ASD in an inpatient setting are likely to have any SIB (Home SIB or Home and Hospital SIB)
Table 5 shows results from our cross-validation, which provides estimates for the predictive accuracy of the models if they were to be used at a new site. The model in Fig. 2 (“Which youth with SIB at home are likely to have SIB in the hospital?”) is excellent at predicting which youth will not have hospital SIB (true negative rate = 79.9). However, it was not accurate at predicting which youth will have SIB in the hospital (true positive rate = 23.7). Conversely, the model in Fig. 3 (“Which youth are likely to have SIB at home and/or hospital?”) is extremely accurate at indicating which youth will have SIB (true positive rate = 92.5) but was not accurate at predicting which youth will not have SIB (true negative rate = 29.1).
Table 5.
Cross-validation results estimating predictive accuracy of tree models at a new site
| Site | Which youth with SIB at home are likely to have SIB at the Hospital (Fig. 2) |
Which youth are likely to have SIB at home and/or hospital? (Fig. 3) |
||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Accuracy | True positive rate | True negative rate |
Accuracy | True positive rate | True negative rate |
|
| 1 | 59.18 | 14.79 | 86.67 | 71.01 | 93.88 | 15 |
| 2 | 51.52 | 6.25 | 94.12 | 91.43 | 93.94 | 50 |
| 3 | 54.55 | 50 | 56.25 | 73.33 | 90.91 | 25 |
| 4 | 54.76 | 30 | 77.27 | 83.02 | 97.62 | 27.27 |
| 5 | 65.38 | 0 | 79.07 | 71.43 | 94.23 | 24 |
| 6 | 76.92 | 40 | 85.71 | 68.42 | 84.62 | 33.33 |
| Site mean | 60.38 | 23.67 | 79.85 | 76.44 | 92.53 | 29.10 |
Discussion
The current study examined the rate and possible predictors of SIB in a psychiatric inpatient population of children and adolescents with ASD. As expected, the rate of SIB reported either at home only or at both home and in the hospital (67.5%) was higher than that reported in previously published community samples. While the range of reported SIB in the general ASD population has varied considerably across studies of community samples, estimates have generally ranged between 15 and 50%. This should not be considered a surprise, as SIB and externalizing behaviors (such as aggression and tantrums) are among the most frequent reasons for hospitalization in the ASD population (Siegel et al. 2012).
A unique feature of this study is that naturalistic data were paired with data obtained from caregiver self-report on the occurrence of SIB in the home. It is the combination of these two sources of data that led to some of the novel results that have not been reported before. Two-thirds of the sample had parental reports of SIB at home, whereas only 24.8% of the sample (only about one-third of those with reported SIB) were observed to exhibit at least one instance daily of SIB while in the hospital. Upon closer examination, it was found that those exhibiting SIB across both the home and hospital environments had significantly higher scores on the RBSR (indicating more severe SIB). This group also had significantly lower scores on a measure of cognitive functioning, suggesting that lower IQ is associated with SIB that is observed outside of the home environment and also is associated with more severe SIB. The association between lower IQ and SIB is consistent with prior findings from community samples (e.g., Richman et al. 2013).
It was also hypothesized that that those individuals who exhibited SIB both at home and in the hospital would have significantly higher ratings on measures of irritability and hyperactivity than those whose SIB was only observed at home. Interestingly, this was not found to be the case. The findings clearly indicate that individuals with SIB (either at home or home and hospital) have significantly higher ratings on scales of externalizing behavior (such as the ABC Irritability and Hyperactivity subscales) than subjects without SIB. Conversely, those with SIB in both the home and hospital (in comparison to those with SIB only at home) had significantly higher total scores on the RBSR as well as a significantly higher number of RBSR items rated as at least moderately interfering. This suggests that children whose parents rate them higher on the RBSR are more likely to exhibit observable SIB outside of the home.
Another study hypothesis was that, consistent with the ASD/SIB literature, age, lower IQ, higher ASD symptom severity and higher rates of ADHD symptoms would be most predictive of SIB severity for this inpatient population. However, neither ASD severity nor age were found to be associated with the presence/absence of SIB and no differences between the “Home SIB” and “Home and Hospital SIB” groups were noted on these two variables. Conversely, NVIQ significantly differed across all three groups, with the “Home and Hospital SIB” group mean NVIQ being approximately 30 points lower than the “No SIB” group and 10 points lower than the “Home SIB group.” Some prior studies had also suggested that females with ASD were more likely to engage in SIB (e.g., Cohen et al. 2010). However, no gender differences were noted in the current sample. It is possible that the characteristics of an inpatient cohort, which include a greater number of individuals with SIB, differ from samples drawn from the community.
Finally, the results of the tree-structured classification models provide some possible guidance to practitioners serving the ASD population in more restrictive settings, such as psychiatric inpatient units. Based on our internal cross-validation, the model predicting the presentation of SIB in the hospital setting is expected to be very accurate at indicating which youth with ASD are not at risk. Conversely, the model predicting the presence of any SIB was very accurate at indicating which youth are at risk. However, potential predictive accuracy varied by depending on the site that was used for validation and models require further validation on an inpatient setting to better assess their true accuracy in an external sample. However, results provide valuable insight for caregivers in psychiatric hospital settings in terms of identifying patients with ASD who present higher risk for engaging in severe forms of SIB in the hospital setting. Knowing these risk factors can alert providers to proactively identify protective equipment that may be required to manage such a patient to avoid patient and staff injury.
Limitations
While this study adds to our understanding of SIB in ASD by including a more severe cohort, aspects of the study design should be considered when interpreting the findings. In general, it is important to keep in mind that this was a naturalistic observational study. An advantage of this approach is that it provides an estimate of what would be likely in real-world clinical practice, in that caregivers rated general concerns about their child’s behavior whereas inpatient staff might notice or define concerns differently based on their observations or expertise. However, given the naturalistic design, conditions were not controlled and differences across settings were likely. First, there were some significant differences across sites in regard to both the number of subjects recruited and the relative rates of SIB which could have impacted the findings. In addition, sites differed in terms of average length of stay, which could have significantly impacted the opportunities available to observe SIB. Second, parent reports of SIB were based upon a standardized questionnaire rather than actual observed rates, while inpatient staff used direct observation. Parents were also asked to take into account the prior 30 day period in their ratings, while staff was documenting behavior as it occurred. While direct observation is felt to be a more accurate means of assessing rates of SIB, inpatient staff were unlikely to have reliably documented all incidents of SIB. This could have been a particular problem among those subjects exhibiting fairly low rates of self-injury, as staff may have failed to note such incidents. Inpatient units also have different levels of structure and contingencies than home settings. In other words, the children’s schedules and amount of structured activities likely differed between home and hospital which may play a role in the occurrence of SIB. Similarly, there likely were differences between how staff and parents responded to incidents of SIB. This, along with the possibility of a “honeymoon” period could also account for some of the differences in observed SIB rates in the two environments. Another study limitation is that many of the children may have been placed on medication or that more intensive behavioral intervention may have been provided shortly after entering the units, which could have significantly lowered daily rates of SIB.
In summary, study results suggest that children with ASD who are admitted to inpatient psychiatric units may present somewhat differently than community ASD cohorts with regard to SIB. First, unlike prior research, there were no apparent gender effects and no association between SIB and high rates of hyperactivity or irritability. Also, almost two-thirds of children with reported SIB at the time of admission failed to display SIB on a regular basis while in the hospital. Finally, the most accurate predictor of SIB in the hospital was high scores on the parent RBSR and a NVIQ below 72 (essentially, functioning with intellectual disability). Hospital staff should, in most cases, know if a child has ID (based upon parent report or school records) and can easily use this information in conjunction with a parent RBSR score to determine a risk-level for SIB in the hospital setting. Future research should focus on identifying those individuals who are at greatest risk for developing SIB. Through careful monitoring of the appearance of symptoms, interventions can be put into place as early as possible in hopes of avoiding the need for more intensive treatment, such as psychiatric inpatient hospitalization. Additional future directions might involve examination of the possible relationship between reported SIB at home and other forms of problem behaviors (e.g., aggression). Similarly, differences between natural contingencies in the home environment and the more structured contingencies in place on the inpatient units might have affected the differential occurrence of SIB across these two settings and is an area that might also be examined more carefully. Finally, further examination of the impact of SIB on admission length could provide important information regarding the “costs” of SIB (e.g., costs of medical treatment related to any injuries, daily inpatient hospital rate, medication costs).
Acknowledgments
The ADDIRC is made up of the co-investigators: Matthew Siegel, MD (PI) (Maine Medical Center Research Institute; Tufts University), Craig Erickson, MD (Cincinnati Children’s Hospital; University of Cincinnati), Robin L. Gabriels, PsyD (Children’s Hospital Colorado; University of Colorado), Desmond Kaplan, MD (Sheppard Pratt Health System), Carla A. Mazefsky, PhD (Western Psychiatric Institute and Clinic; University of Pittsburgh), Eric M. Morrow, MD, PhD (Bradley Hospital; Brown University), Giulia Righi, PhD (Bradley Hospital; Brown University), Susan L. Santangelo, ScD (Maine Medical Center Research Institute; Tufts University), and Logan Wink, MD (Cincinnati Children’s Hospital; University of Cincinnati). Collaborating investigators and staff: Jill Benevides, BS, Carol Beresford, MD, Carrie Best, MPH, Katie Bowen, LCSW, Briar Dechant, BS, Joanne Dixon, PhD, Tom Flis, BCBA, LCPC, Holly Gastgeb, PhD, Angela Geer, BS, Louis Hagopian, PhD, Benjamin Handen, PhD, BCBA-D, Adam Klever, BS, Martin Lubetsky, MD, Kristen MacKenzie, BS, Zenoa Meservy, MD, John McGonigle, PhD, Kelly McGuire, MD, Faith McNeill, BA, Ernest Pedapati, MD, Christine Peura, BA, Joseph Pierri, MD, Christie Rogers, MS, CCC-SLP, Brad Rossman, MA, Jennifer Ruberg, LISW, Cathleen Small, PhD, Kahsi A. Pedersen, PhD, Nicole Stuckey, MSN, RN, Barbara Tylenda, PhD, Mary Verdi, MA, Jessica Vezzoli, BS, Deanna Williams, BA, and Diane Williams, PhD, CCC-SLP. We gratefully acknowledge the contributions of the coordinating site advisory group: Donald L. St. Germain, MD and Girard Robinson, MD, and our scientific advisory group: Connie Kasari, PhD., Bryan King, MD, James McCracken, MD, Christopher McDougle, MD, Lawrence Scahill, MSN, PhD, Robert Schultz, PhD and Helen Tager-Flusberg, PhD, the input of the funding organizations and the families and children who participated.
Funding This study was funded by the Simons Foundation Autism Research Initiative (SFARI #296318 to M.S.) and the Nancy Lurie Marks Family Foundation.
Footnotes
Author Contributions: MT, CM, KP and RG conceived of the parent study on which this manuscript is based, collaborated in the coordination of the study and supervised the data collection. BH conceived of the study, participated in its design and drafted the manuscript. MW performed the statistical analysis. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest Dr. Handen received support from the National Institute for Aging (R01AG031110-03A1; 1 R01 AG051406-01), Autism Speaks, Roche and Eli Lilly. Dr. Mazefsky receives support from the National Institute of Child Health and Human Development (R01HD079512; K23HD060601). Drs. Gabriels, Pedersen, Wallace have no conflicts of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- Aman MG, Singh NN, Stewart AW, Field CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency. 1985a;89:485–491. [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the Aberrant Behavior Checklist. American Journal of Mental Deficiency. 1985b;89:492–502. [PubMed] [Google Scholar]
- Baghdadli A, Pascal C, Grisi S, Aussiloux C. Risk factors for self-injurious behaviours among 222 young children with autistic disorders. Journal of Intellectual Disability Research. 2003;47(8):622–627. doi: 10.1046/j.1365-2788.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- Bodfish JW, Symons F, Lewis M. The Repetitive Behavior Scales: A test manual. Morganton, NC: Western Carolina Center Research Reports; 1999. [Google Scholar]
- Cohen IL, Tsiouris JA, Flory MJ, Kim SY, Freedland R, Heaney G, et al. A large scale study of the psychometric characteristics of the IBR Modified overt aggression scale: Findings and evidence for increased self-destructive behaviors in adult females with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2010;40:599–609. doi: 10.1007/s10803-009-0908-z. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Seltzer MM, Lam KS, Bodfish JW. Age-related differences in restricted repetitive behaviors in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:57–66. doi: 10.1007/s10803-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furniss F, Biswas AB. Recent research on aetiology, development and phenomenology of self-injurious behaviour in people with intellectual disabilities: A systematic review and implications for treatment. Journal of Intellectual Disability Research. 2012;56:453–475. doi: 10.1111/j.1365-2788.2012.01534.x. [DOI] [PubMed] [Google Scholar]
- Gleser LJ, Olkin I. Stochastically dependent effect sizes. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta analysis. New York: Russell Sage Foundation; 2009. pp. 357–376. [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction. 2. New York: Springer Science + Business Media LLC; 2009. [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, efforts and subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders. 2006;36:1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism diagnostic observation schedule: A standardized observation of communicative and social behavior. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- Matson JL, Rivet TT. The effects of severity and autism and PDD-NOS symptoms on challenging behaviors in adults with intellectual disabilities. Journal of Developmental and Physical Disabilities. 2008a;20:41–51. [Google Scholar]
- Matson JL, Rivet TT. Characteristics of challenging behaviours in adults with autistic disorder, PDD-NOS, and intellectual disability. Journal of Intellectual and Developmental Disability. 2008b;33:323–329. doi: 10.1080/13668250802492600. [DOI] [PubMed] [Google Scholar]
- McClintock K, Oliver C. Risk markers associated with challenging behaviours in people with intellectual disabilities: A meta-analytic study. Journal of Intellectual Disability Research. 2003;47:405–416. doi: 10.1046/j.1365-2788.2003.00517.x. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2016. http://www.R-project.org/ [Google Scholar]
- Richards C, Oliver C, Nelson L, Moss J. Self-injurious behavior in individuals with autism spectrum disorder and intellectual disability. Journal of Intellectual Disability Research. 2012;56(5):476–489. doi: 10.1111/j.1365-2788.2012.01537.x. [DOI] [PubMed] [Google Scholar]
- Richman DM, Barndard-Brak L, Bosch A, Thompson S, Grubb L, Abby L. Predictors of self-injurious behavior exhibited by individuals with autism spectrum disorder. 2013;223(5):429–439. doi: 10.1111/j.1365-2788.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ, Pomplun M, Koch C. Leiter International Performance Scale. 3. Torrance, CA: Western Psychological Services; 2013. [Google Scholar]
- Rojahan J, Aman MG, Matson JL, Mayville E. The Aberrant Behavior Checklist and the Behavior Problems Inventory: Convergent and divergent validity. Research in Developmental Disabilities. 2003;24:391–404. doi: 10.1016/s0891-4222(03)00055-6. [DOI] [PubMed] [Google Scholar]
- Rojahn J, Wilkins J, Matson JL, Boisjoli J. A comparison of adults with intellectual disabilities with and without ASD on parallel measures of challenging behavior: The Behavior Problems Inventory-01 (BPI-01) and Autism Spectrum Disorders-Behavior Problems for intellectually disabled adults (ASD-BPA) Journal of Applied Research in Intellectual Disabilities. 2010;23:179–185. [Google Scholar]
- Rutter M, Bailey A, Lord C. The social communication questionnaire manual. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schroeder ST, Marquis JG, Reese RM, Richman DM, Mayo-Ortega L, Oyama-Ganiko R, et al. Risk factors for self-injury, aggression, and stereotyped behavior among young children at risk for intellectual and developmental disabilities. American Journal on Intellectual and Developmental Disabilities. 2014;119(4):351–370. doi: 10.1352/1944-7558-119.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Doyle K, Chemelski B, Payne D, Ellsworth B, Harmon J, Robbings D, Milligan B, Lubetsky M. Specialized inpatient psychiatry units for children with autism and developmental disorders: A United States survey. Journal of Autism and Developmental Disorders. 2012;42:1863–1869. doi: 10.1007/s10803-011-1426-3. [DOI] [PubMed] [Google Scholar]
- Siegel M, Smith KA, Mazefsky C, Gabriels RL, Erickson C, Kaplan D, et al. The autism inpatient collection: Methods and preliminary sample description. Molecular Autism. 2015 doi: 10.1186/s13229-015-0054-8. [DOI] [PMC free article] [PubMed]
- Smith KRM, Matson JL. Behavior problems: Differences among intellectually disabled adults with co-morbid autism spectrum disorders and epilepsy. Research in Developmental Disabilities. 2010;31:1062–1069. doi: 10.1016/j.ridd.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland Adaptive Behavior Scales. 2. San Antonio, TX: Pearson; 2005. [Google Scholar]
- Therneau T, Atkinson B, Ripley B. Rpart: Recursive Partitioning and Regression Trees. R package version 4.1–10. 2015 https://CRAN.R-project.org/package=rpart.
- Yates TM. The developmental psychopathology of self-injurious behavior: Compensatory regulation in posttraumatic adaptation. Clinical Psychology Review. 2004;24:35–74. doi: 10.1016/j.cpr.2003.10.001. [DOI] [PubMed] [Google Scholar]